Composition for treatment of and method of monitoring hepatitis C virus using interferon-tau
a technology of interferon and hepatitis c virus, which is applied in the direction of antivirals, drug compositions, peptide/protein ingredients, etc., can solve the problems of difficult in vitro culture of virus, difficult to tolerate ribavirin in combination with ifn-,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
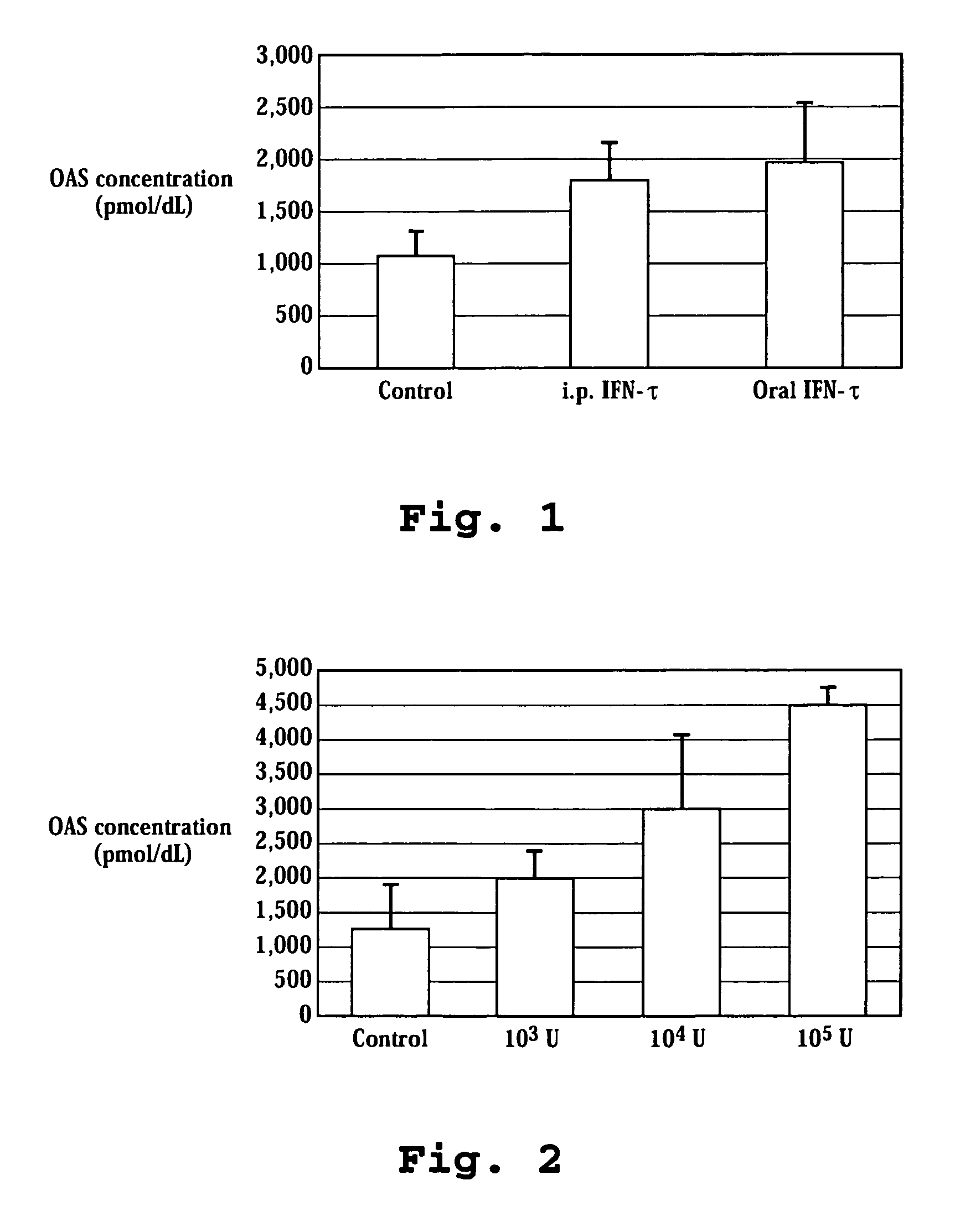
Induction of OAS with Orally and Intraperitoneally Administered Ovine IFN-τ to Mice
[0131] OvIFN-τ (4.99×108 units / mg protein; Pepgen Corp., California or Biological Process Development Facility, Dept. of Food Science and Technology, University of NE-Lincoln, Lincoln, Nebr.; SEQ ID NO:4) was dissolved in 10% maltose solution to prepare ovIFN-τ Solution. The use of OvIFN-τ (SEQ ID NO:2) is also contemplated in the present invention. Two hundred microliters of ovIFN-τ solution was orally administered to ICR mice (average body weight approximately 30 g, 6 weeks of age, female) using a 20 gauge disposable oral sound (Fuchigami, Kyoto) to inject directly to an upper part of the stomach (gastric administration; GA).
[0132] For intraperitoneal administration (I.P.), 100 microliters of ovIFN-τ solution was used. Sample injection to an upper part of a stomach was confirmed by administration of a dye. Twenty-four hours after the administration, the mouse was anesthetized with Nembutal. Blood ...
example 2
Dose-Dependent Induction of OAS by Oral Administration of IFN-τ in Mice
[0134] Using the same procedure as Example 1, OvIFN-τ was orally administered in units of 0, 103, 104, or 105 to an ICR mouse. Twelve hours after oral administration, whole blood was taken from a mouse heart and an OAS activity of whole blood was determined. As shown in FIG. 2, the OAS activity in whole blood increased in a dose dependent manner.
example 3
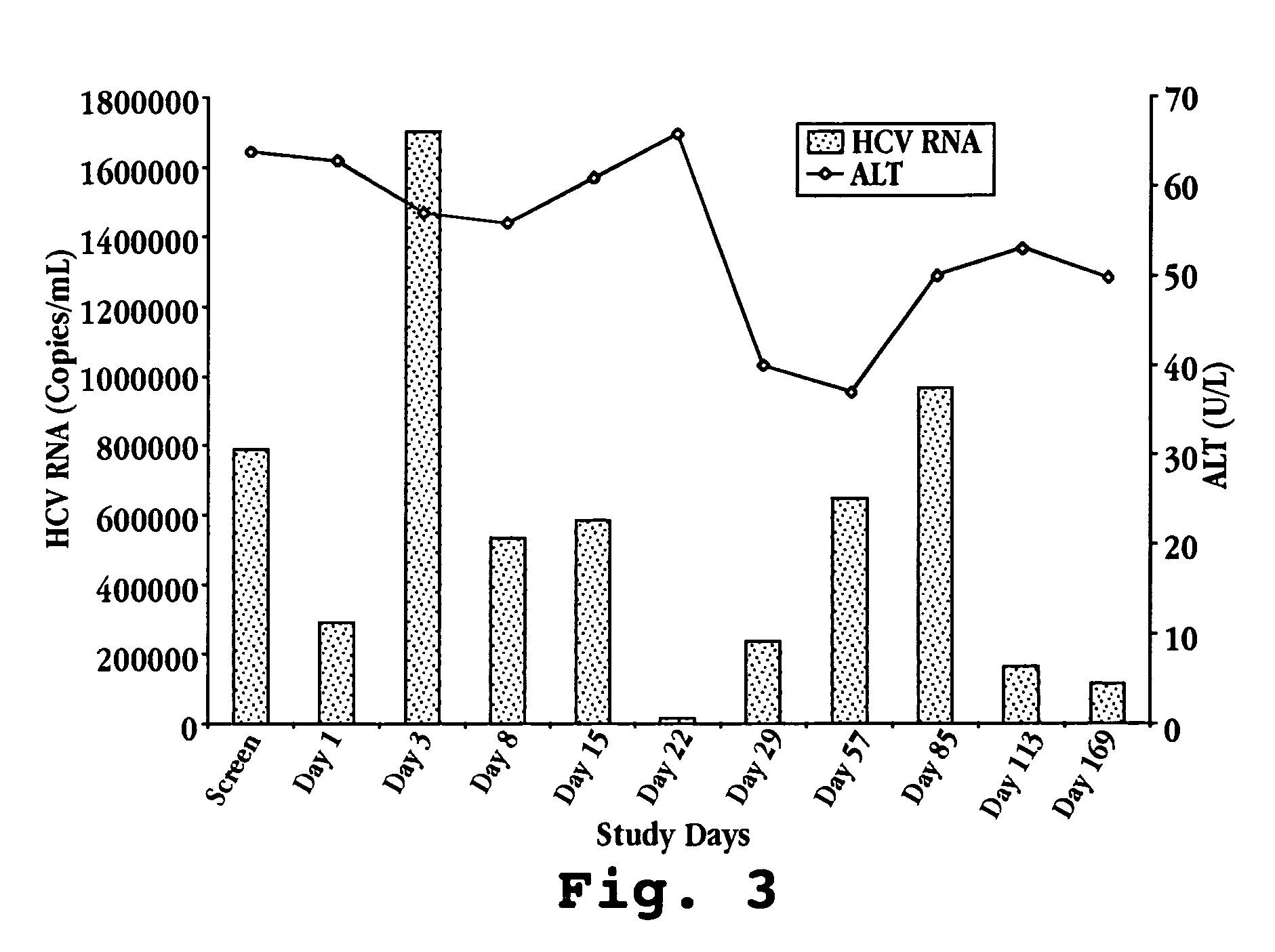
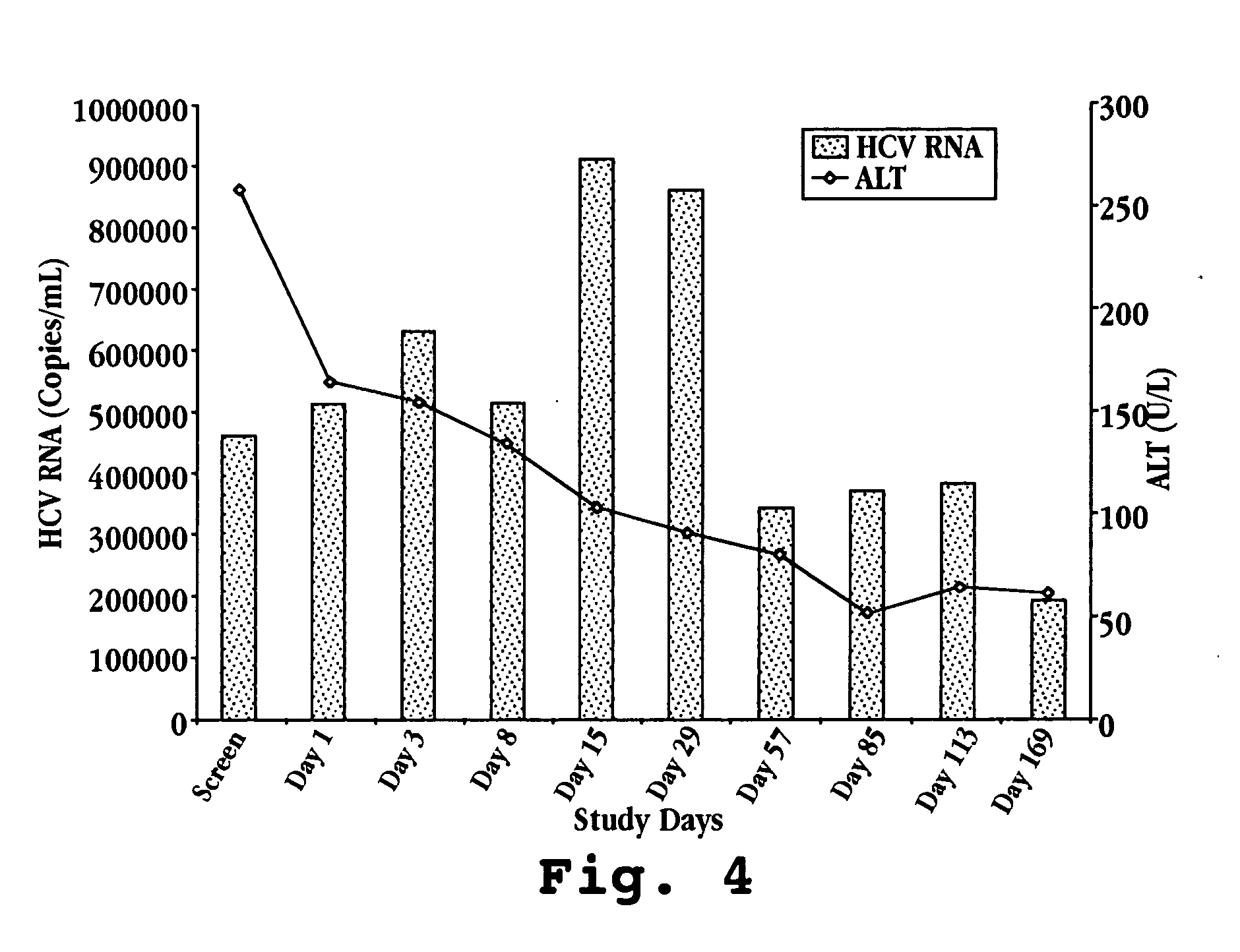
Reduced ALT, Reduced HCV Viral Titer, and Induction of OAS by Oral Administration of IFN-τ in Human Patients
[0135] A. IFN-τ Preparation
[0136] On day one, one bottle of Ov-IFN-τ (SEQ ID NO:4) is removed from the refrigerator and the patient self-administers the proper volume of test material according to Table 2. Ov-IFN-τ (SEQ ID NO:2) may also be prepared and administered in the same manner.
TABLE 2Recombinant Ov-IFN-τ Patient Dose AdministrationNumber ofOv-IFN-τVolume (ml)Total DailyDose GroupPatients(mg / ml)per Dose (TID)Dose (ml)I61.00.331.0II61.01.03.0III61.03.09.0IV61.05.015.0
[0137] B. Patient Dosing Instructions
[0138] The patient keeps all vials of test material and syringes in the refrigerator maintained at 2 to 8 degrees centrigrade. Prior to the self-administration of medication, the patient removes one vial and one syringe from the refrigerator. The patient removes the cap from the tip of the syringe, places the tip of the syringe into the bottle of medication and withd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| OD | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com