Method for detecting biological activity of swine interferon alpha
A technology of biological activity and porcine interferon, which is applied in the field of detection of porcine interferon α biological activity, can solve problems such as difficulty in development and increase in use costs, and achieve the effects of improving accuracy, improving repeatability, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This embodiment provides a method for detecting the biological activity of porcine interferon α, which is an application for detecting the biological activity of recombinant porcine interferon α, and the detection method in this embodiment can also be used for natural porcine interferon correspondingly The activity detection of α, it comprises the steps:
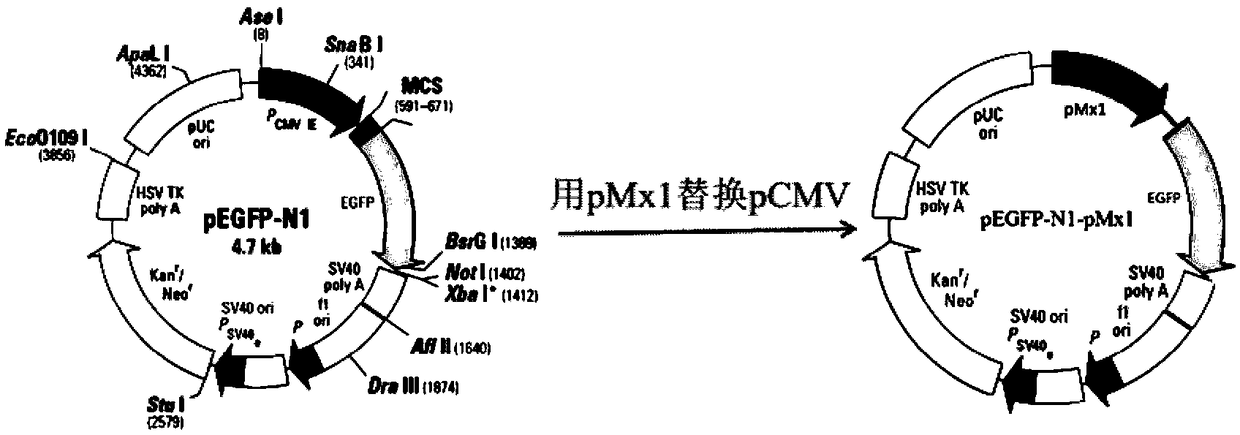
[0049] According to the gene sequence of the porcine Mx1 protein published in Genebank, the promoter region containing the ISRE response element at the 5' end was selected, PCR primers were designed, and DNA was extracted from pk-15 cells by the phenol-chloroform-isoamyl alcohol method as a template. Use above-mentioned PCR primer and Ex-Taq enzyme to carry out PCR amplification (underlined part is upstream and downstream primer position);
[0050]
[0051] The product obtained by PCR amplification was double-digested by AseI and AgeI and then recovered and purified by gel cutting (introduce the AseI restriction si...
Embodiment 2
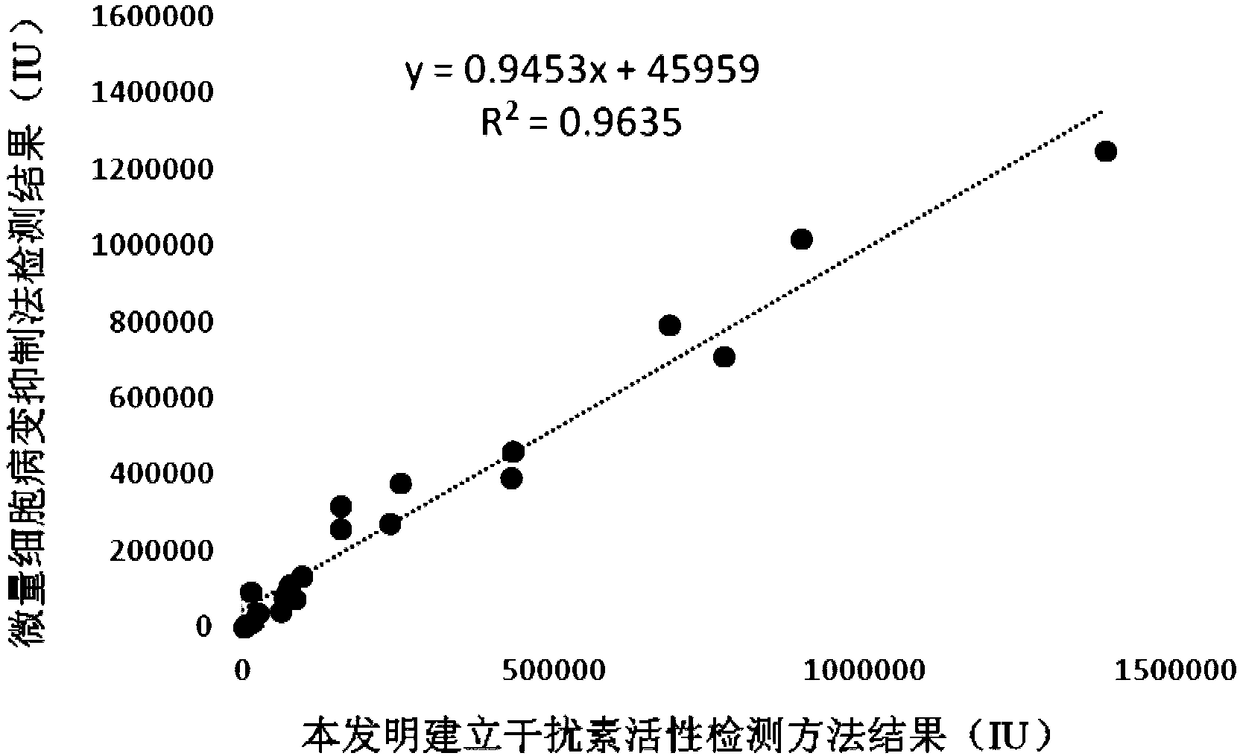
[0061] This example provides a comparative correlation experiment between the detection results of the method for detecting the biological activity of porcine interferon α of the present invention and the detection results of the micro-cytopathic inhibition method.
[0062] EGFP reporter gene method and trace cytopathic inhibition method were used to detect 20 recombinant porcine interferon-alpha samples at the same time, and linear regression was used to analyze whether the results of the two methods were correlated.
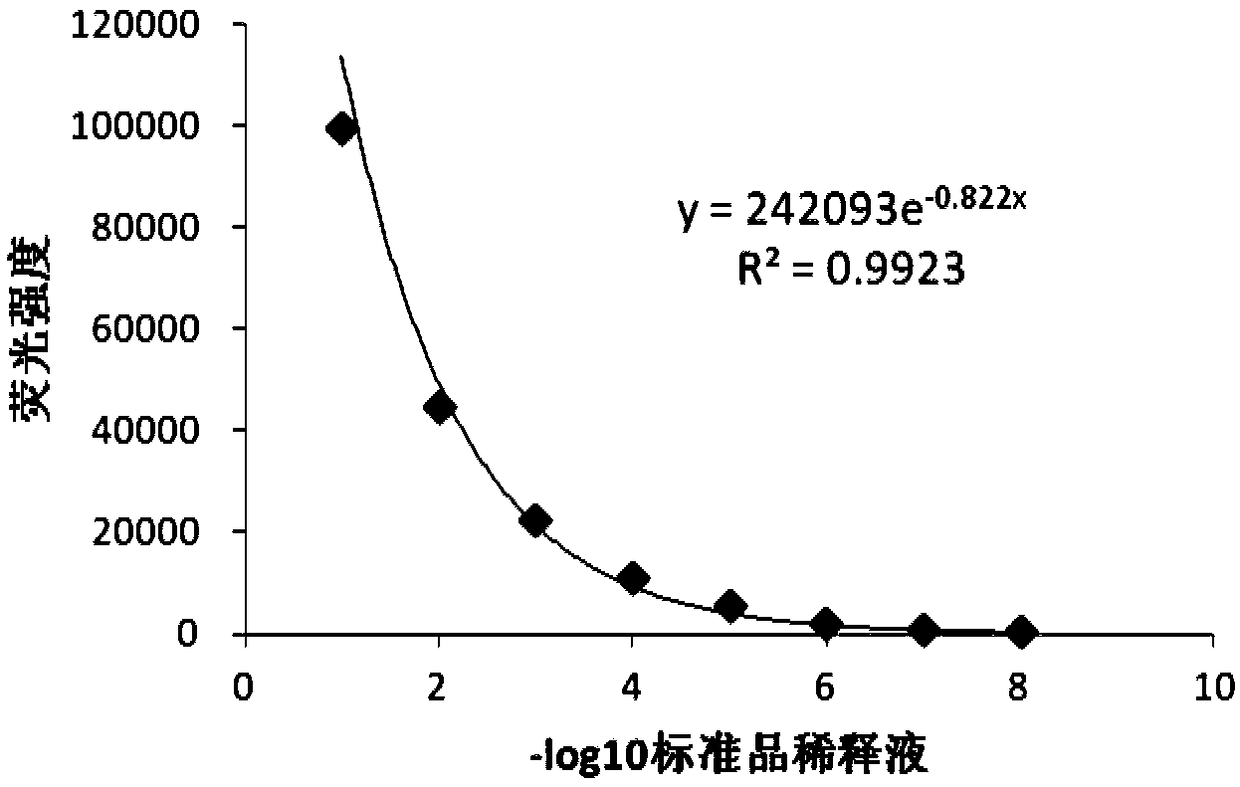
[0063] The detection process of the EGFP reporter gene method is shown in Example 1.
[0064] The micro-cytopathic inhibition method is operated as follows: take 1 mL of porcine interferon-α specimen, dilute it with complete medium 1:100, and then dilute it with complete medium; inoculate pk-15 subculture to 96-well cell culture plate, Wells were inoculated with 100 μl cell suspension (2×10 5 each / mL); then add 100 μl of recombinant porcine interferon α in dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com