Nontoxic clostridium perfringens and clostridium septicum fusion protein vaccine and production method thereof
A technology of Clostridium perfringens and fusion protein, applied in vaccines, multivalent vaccines, veterinary vaccines, etc., can solve problems such as incomplete antigenicity of antigenic proteins, biosafety risks, and impact on immunogenicity, so as to avoid Effects of antigenic protein immunogenicity, maintenance of immunogenicity, and effect of reducing biosafety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] ——Construction and identification of Escherichia coli BL / EC strain
[0059] 1. Gene synthesis
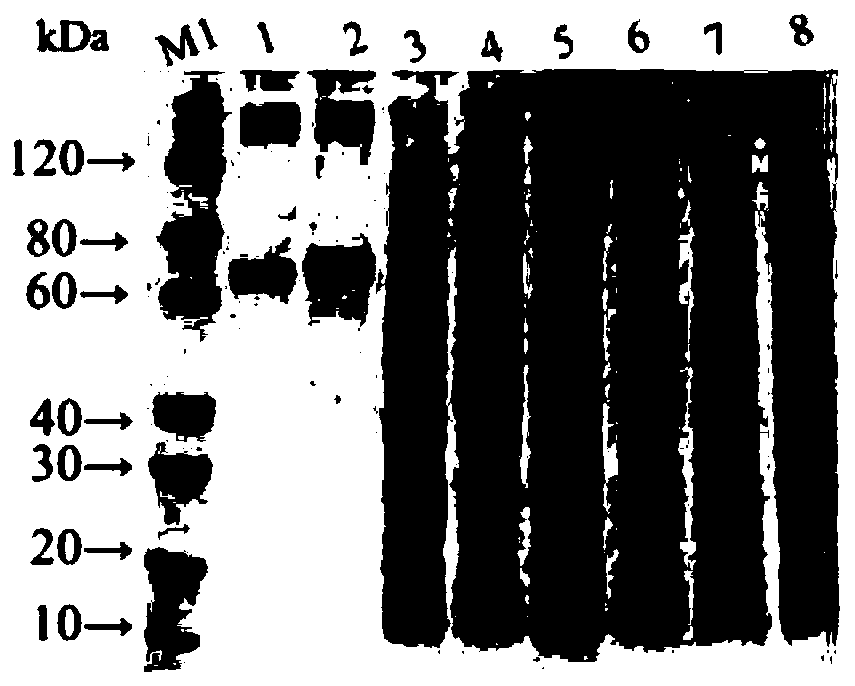
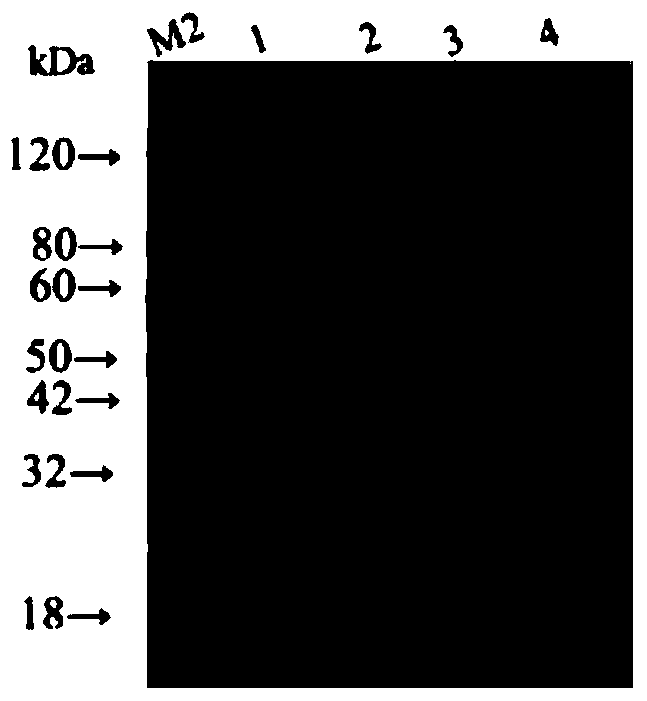
[0060] The application is based on the natural gene sequences of Clostridium perfringens ETX and Clostridium putrefaction CSA, after codon optimization, the ETX coding gene containing 3 amino acid mutations (Y30A, H106P and Y196A), the ETX coding gene containing 4 amino acid mutations ( C54L, N264A, H269A and W310A) and 11 amino acid deleted CSA coding genes were tandem. At the same time, the gene encoding the 6×His tag was added to the C-terminus of the fusion protein. The gene sequence GETX was synthesized by chemical synthesis m3 CSA M4△11 , containing a total of 2166 nucleotides, the corresponding protein is called rETX m3 CSA M4△11 . The specific nucleic acid sequence is shown in SEQ ID No.1, and the amino acid sequence is shown in SEQ ID No.2.
[0061] Sequence 1:
[0062]
[0063]
[0064]
[0065] Sequence 2:
[0066]
[0067]
[0068] 2. Expre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com