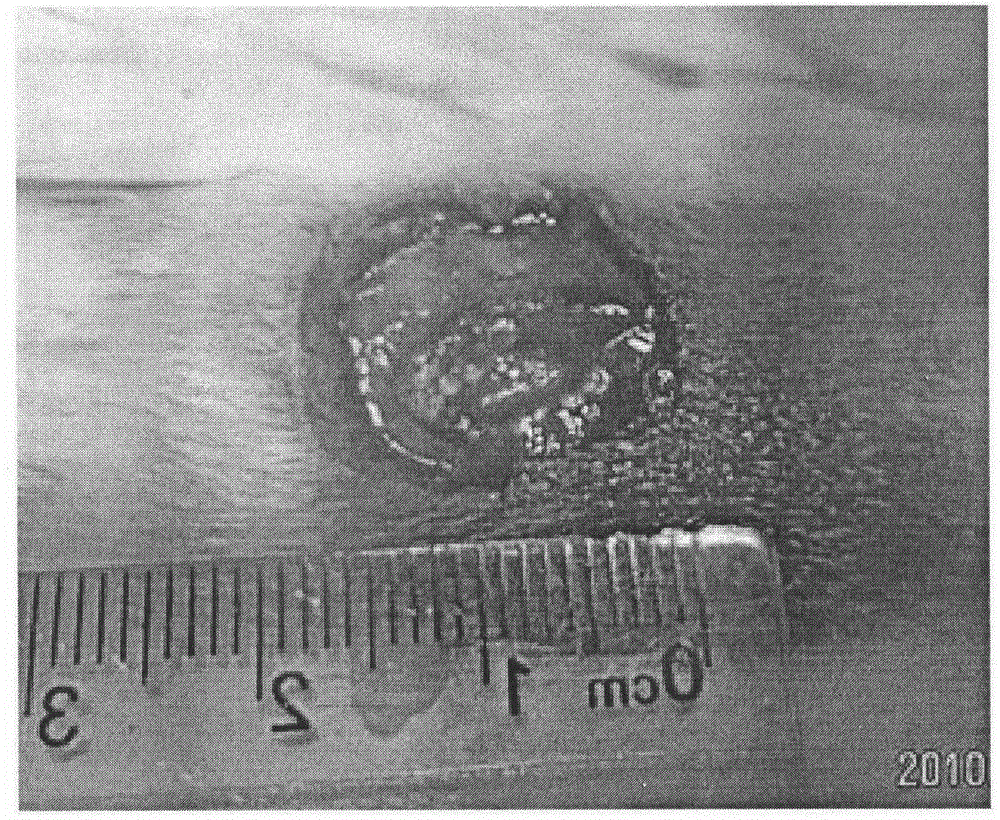
Medical dressing with bioactivity and preparation method of medical dressing
A bioactive and growth factor technology, applied in the fields of biomedical engineering and materials science, can solve problems such as failure efficacy and cell wound deterioration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] a. Preparation of the outer layer porous polymer material film: Take a part of the mass of the polymer material and dissolve it in 15 mL of chloroform solution, add six parts of the mass of salt particles with a particle size of 50-100 μm after the dissolution, and stir After uniformity, pour it into a pre-prepared mold and place it at room temperature for 24 hours; after the solvent evaporates, take the material out of the mold and place it in deionized water at 37°C for 3 hours on a shaker, take it out and dry it to obtain the outer porous polymer material layer;
[0024]b. Preparation of stem cell growth factor: use DMEM medium (complete medium) containing 10% fetal bovine serum, at 37°C, 5%CO 2 Stem cells were routinely cultured under certain conditions until the cell confluence reached about 90% and the cell concentration reached 1-10×10 6 , absorb the culture solution containing stem cell growth factors, centrifuge, discard the precipitate and take the supernatant...
Embodiment 1
[0031] a. Preparation of the outer layer porous polymer material film: Dissolve 2g of polylactic acid (PLA) in 15mL of chloroform solution, add 12g of salt particles with a particle size of 50μm after the dissolution, stir well and pour into the pre-prepared Spread the film into a film with a thickness of 1 mm in a mold, and place it at room temperature for 24 hours; after the solvent evaporates, take the material out of the mold and place it in deionized water at 37 ° C for 3 hours on a shaker, and then dry it to obtain the porous polymer material of the outer layer layer;
[0032] b. Preparation of stem cell growth factor: use DMEM medium (complete medium) containing 10% fetal bovine serum, at 37°C, 5%CO 2 Under certain conditions, the remaining embryonic stem cells derived from artificial insemination for IVF operation were routinely cultured until the cell confluence reached about 90% and the cell concentration reached 1×10 6 , absorb the culture solution containing stem ...
Embodiment 2
[0038] a, the preparation of the outer layer porous polymer material film: 15g polyglycolic acid (PGA) is dissolved in the chloroform solution of 15mL, after dissolving, add 90g of the salt particle that the particle diameter is 60 μm, stir and pour into pre- In the prepared mold, spread it into a film with a thickness of 0.5 mm, and place it at room temperature for 24 hours; after the solvent evaporates, take the material out of the mold and place it in deionized water at 37 ° C for 3 hours on a shaker, and then dry it to obtain the outer layer of porous polymer. Glycolic acid (PGA) layer;
[0039] b. Preparation of stem cell growth factor: use DMEM medium (complete medium) containing 10% fetal bovine serum, at 37°C, 5%CO 2 Embryonic stem cells derived from bovine artificial insemination were routinely cultured under certain conditions until the cell confluence reached about 90% and the cell concentration reached 2×10 6 , absorb the culture solution containing stem cell grow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com