Recombinant human collagen and application thereof
A technology of human collagen and collagen, which is applied in the field of genetic engineering, can solve the problems of unsuitable long-fragment peptide chain full-length detection, complicated purification process operation, and difficulty in obtaining collagen products, so as to avoid the limitation of utilization efficiency, Elimination of codons and hairpin structures, high biocompatibility and biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The full length of the recombinant human collagen is 1056 amino acids, with a Flag tag at the N-terminal and a 6His tag at the C-terminal. The amino acid sequence of the recombinant human collagen is shown in SEQ ID NO.1.
[0065] The preparation method is as follows:
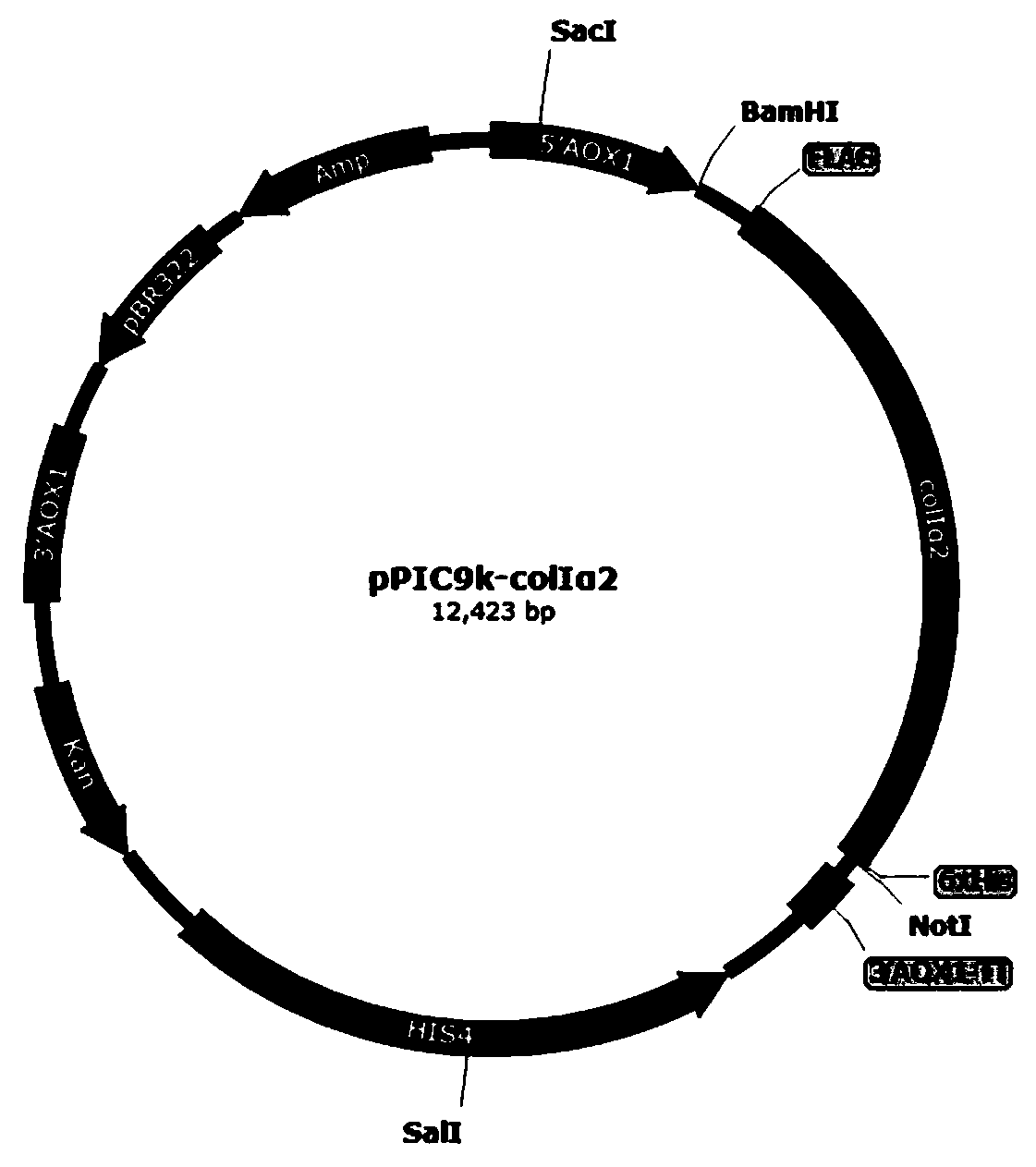
[0066] 1. Construction of recombinant plasmids
[0067] 1.1 Synthesis of optimized genes
[0068] According to the amino acid sequence of human type I collagen α2 chain mature peptide and its corresponding gene sequence registered in Genbank, referring to the preferred codons of Pichia pastoris, the DNASTAR software was used to optimize the gene without changing the original amino acid sequence of collagen ColIα2 Sequence, and removed the restriction endonuclease sites such as ApaI, Bgl II, BamH I, EcoR I, Not I, Sac I, Sal I, the obtained gene sequence is shown in SEQ ID NO.2, and then provided by Nanjing GenScript Synthetic Technology Co., Ltd.
[0069] 1.2 Construction of recombinant plasmids by PC...
Embodiment 2
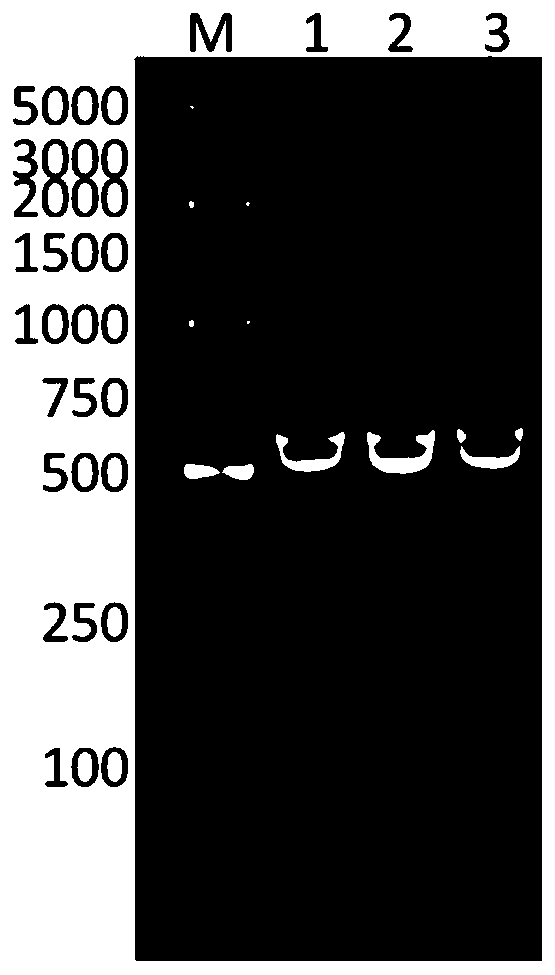
[0118] Example 2 Identification of Recombinant Human Collagen
example 1
[0120] 1. Western blotting detection
[0121] 1) Take the fermentation supernatant and carry out SDS-PAGE electrophoresis until the bromophenol blue completely runs out from the lower edge of the separation gel;
[0122] 2) Take out the gel from the glass plate and soak it in the transfer buffer;
[0123] 3) Take a PVDF membrane with the same size as the gel, soak it in anhydrous methanol for 10 seconds, transfer it to the membrane transfer buffer and continue soaking for later use;
[0124] 4) Place the sponge, filter paper, PVDF membrane, gel, filter paper, and sponge pre-soaked in the transfer buffer in the transfer folder in order to ensure that there are no air bubbles between each layer;
[0125] 5) Put the transfer clip into the electroporation tank, the membrane is close to the positive electrode, the glue is close to the negative pole, and the electroporation tank is placed in an ice water bath, 450mA transfer for 2h;
[0126] 6) Take out the PVDF membrane, soak it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com