Recombinant human source collagen type-III alpha 1 chain and application thereof
A collagen and collagen technology, applied in the field of genetic engineering, can solve problems such as different codon preferences, failure to meet purity requirements, insufficient protein purity, etc., to optimize secondary structure, avoid low translation efficiency, eliminate codons and develop The effect of clip structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
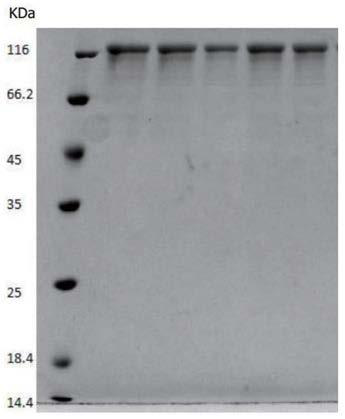
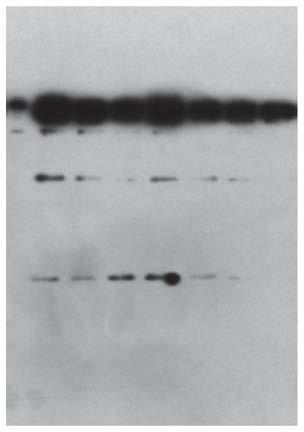
[0075] The full length of the recombinant human type III collagen is 1082 amino acids, with a 6His tag at the amino end and a Strep tag at the carboxyl end. The amino acid sequence of the recombinant human type III collagen is shown in SEQ ID NO.1.
[0076] The preparation method is as follows:
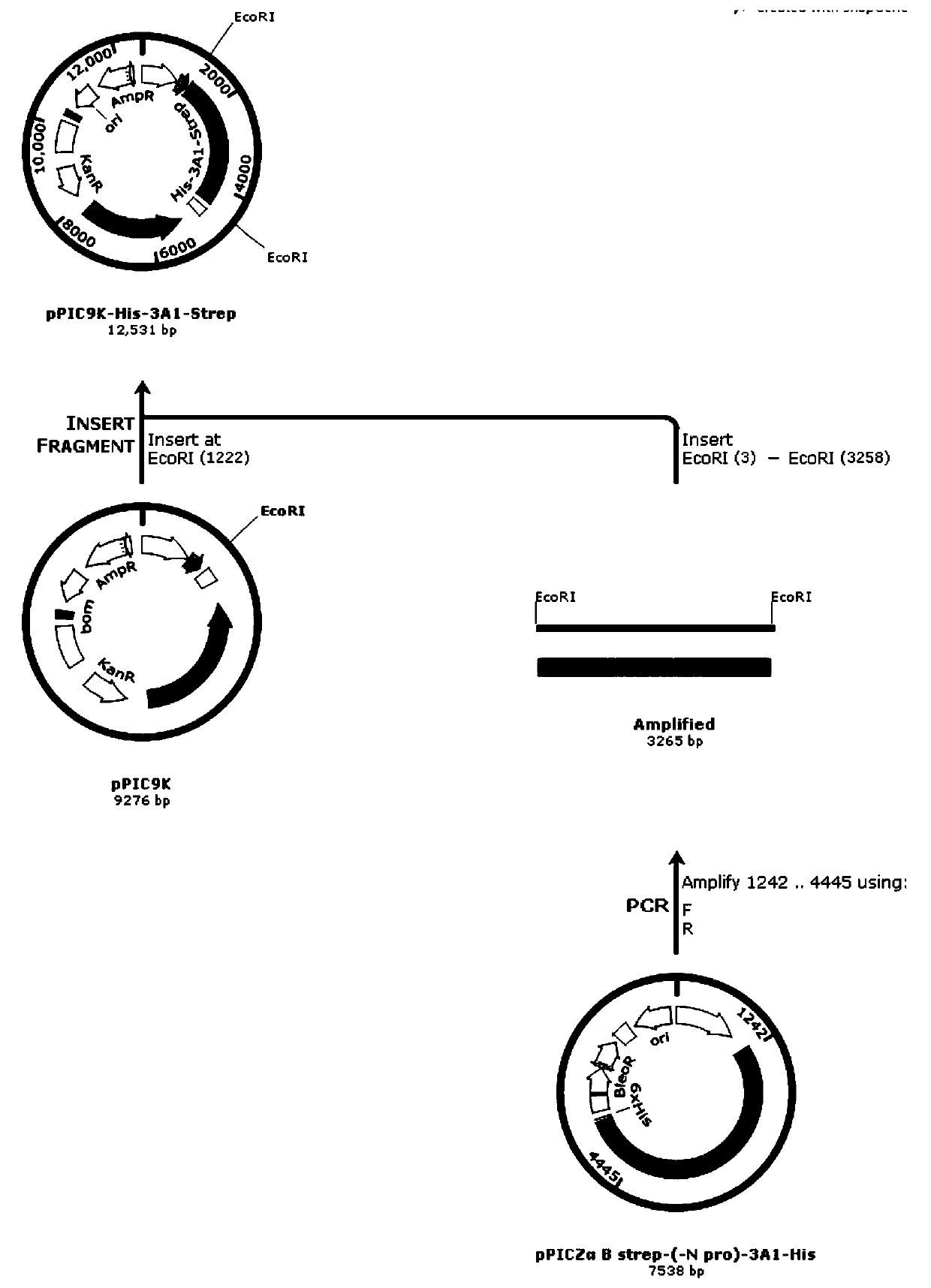
[0077] 1. Construction of recombinant expression vectors (such as figure 1 shown)
[0078] 1.1 Synthesis of optimized genes
[0079] According to the gene sequence NM_000090.3 of type III collagen registered in Genebank, gene codons were optimized according to the codon usage frequency of Pichia pastoris, codons with low usage rates were eliminated, and the synonymous transformation method was used to eliminate EcoRI (GAATTC), Restriction sites such as Not I (GCGGCCGC) have removed 2 GGTAAG splicing sites and 4 GGTGAT splicing sites. The optimized type III collagen gene sequence is shown in SEQ ID NO.2. The optimized type III collagen gene sequence Artificially synthesized by Nanji...
Embodiment 2
[0171] Example 2 Preparation of Collagen Skin Care Products for External Use
[0172] According to the following mass ratio, dissolve each raw material with water, and stir well to form a colorless, odorless and transparent moisturizing skin care essence.
example 1
[0173] Example 1, the recombinant human type III collagen prepared in Example 1 of the present invention 0.1%; glycerol 1%; butanediol 1%; sodium hyaluronate 0.1%; carbomer 0.5%; betaine 1%; silk peptide 0.05%; β-glucan 0.2%; jojoba ester 0.1%; dipotassium glycyrrhizinate 0.3%; triethanolamine 0.1%; the rest is purified water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| capacitance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com