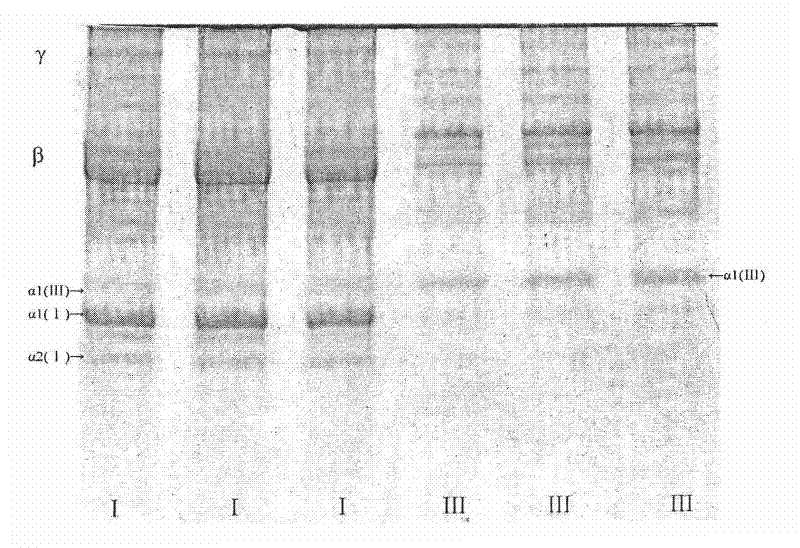
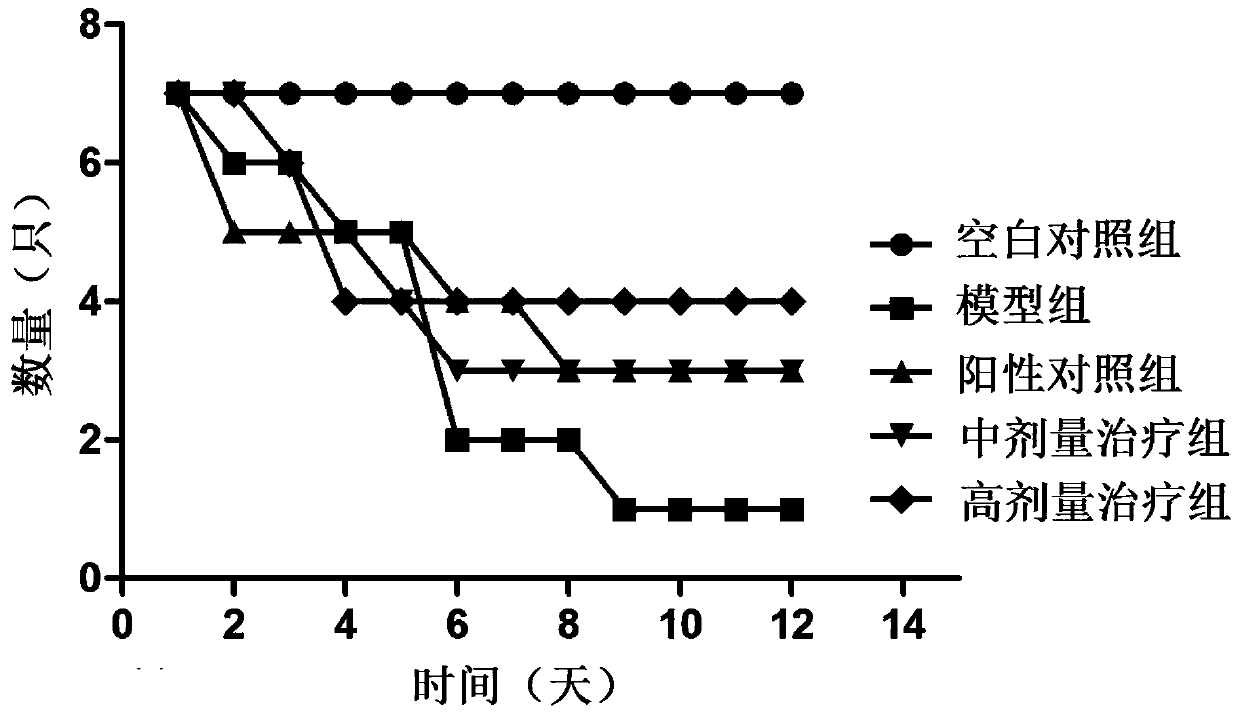
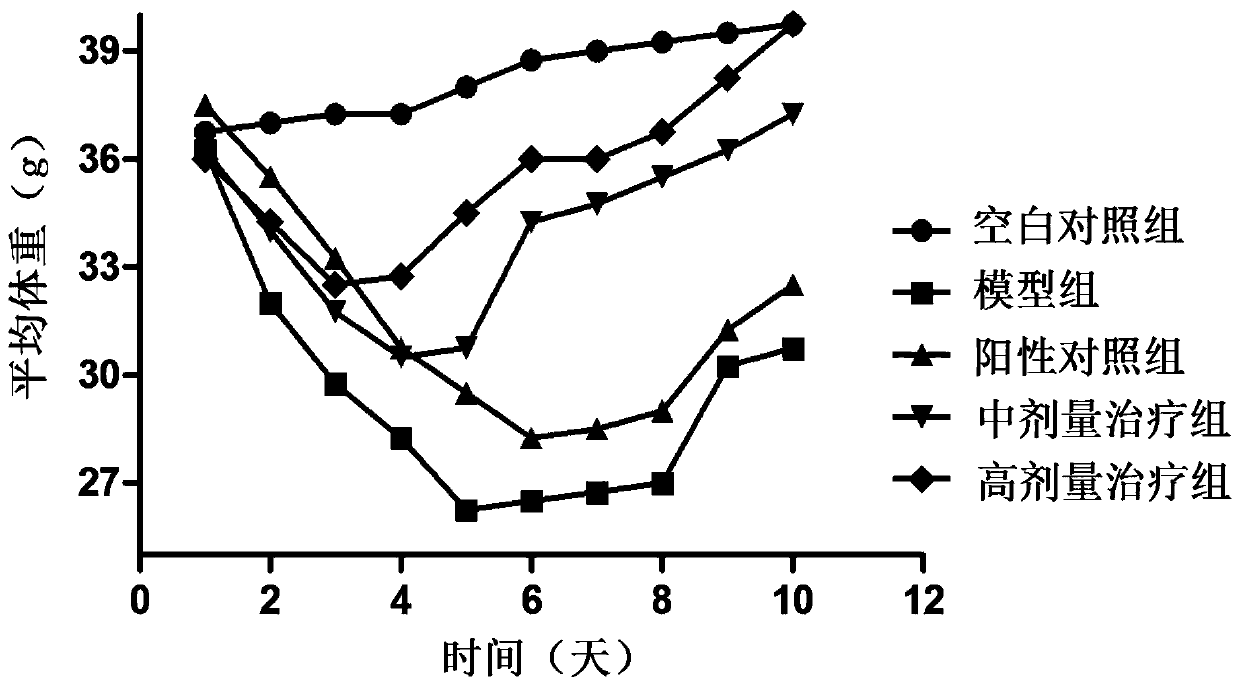
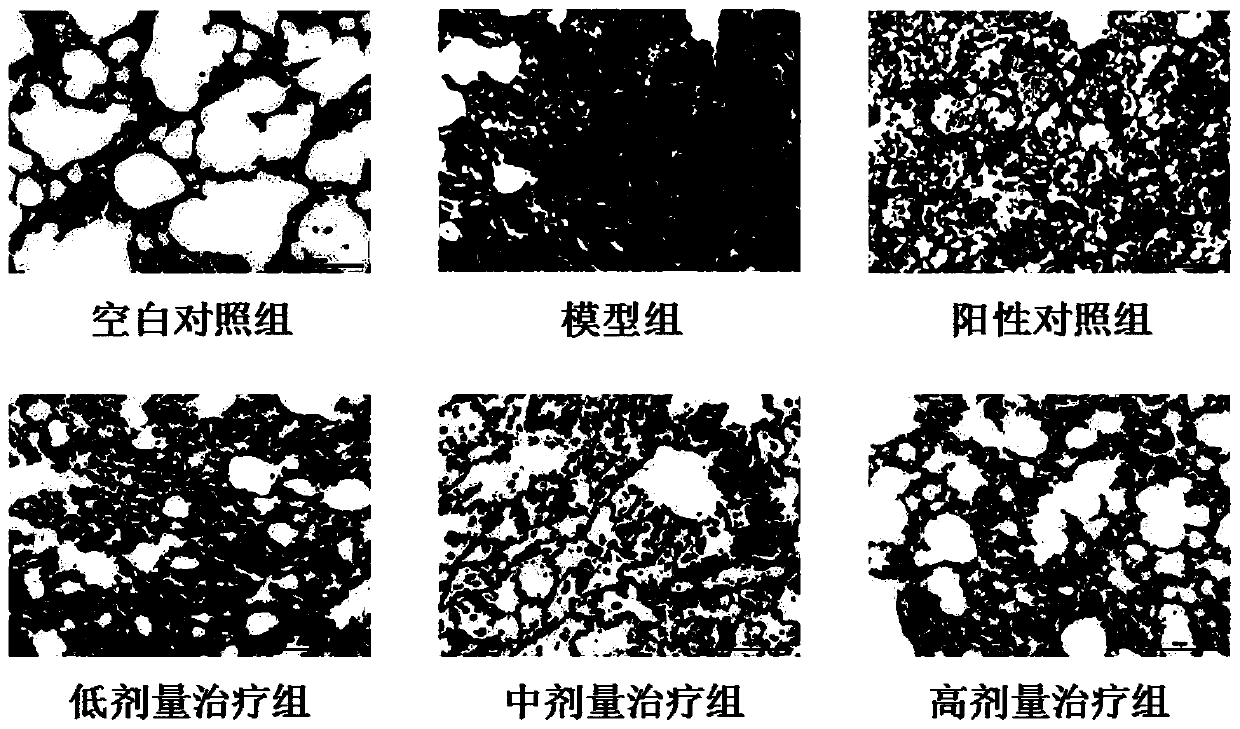
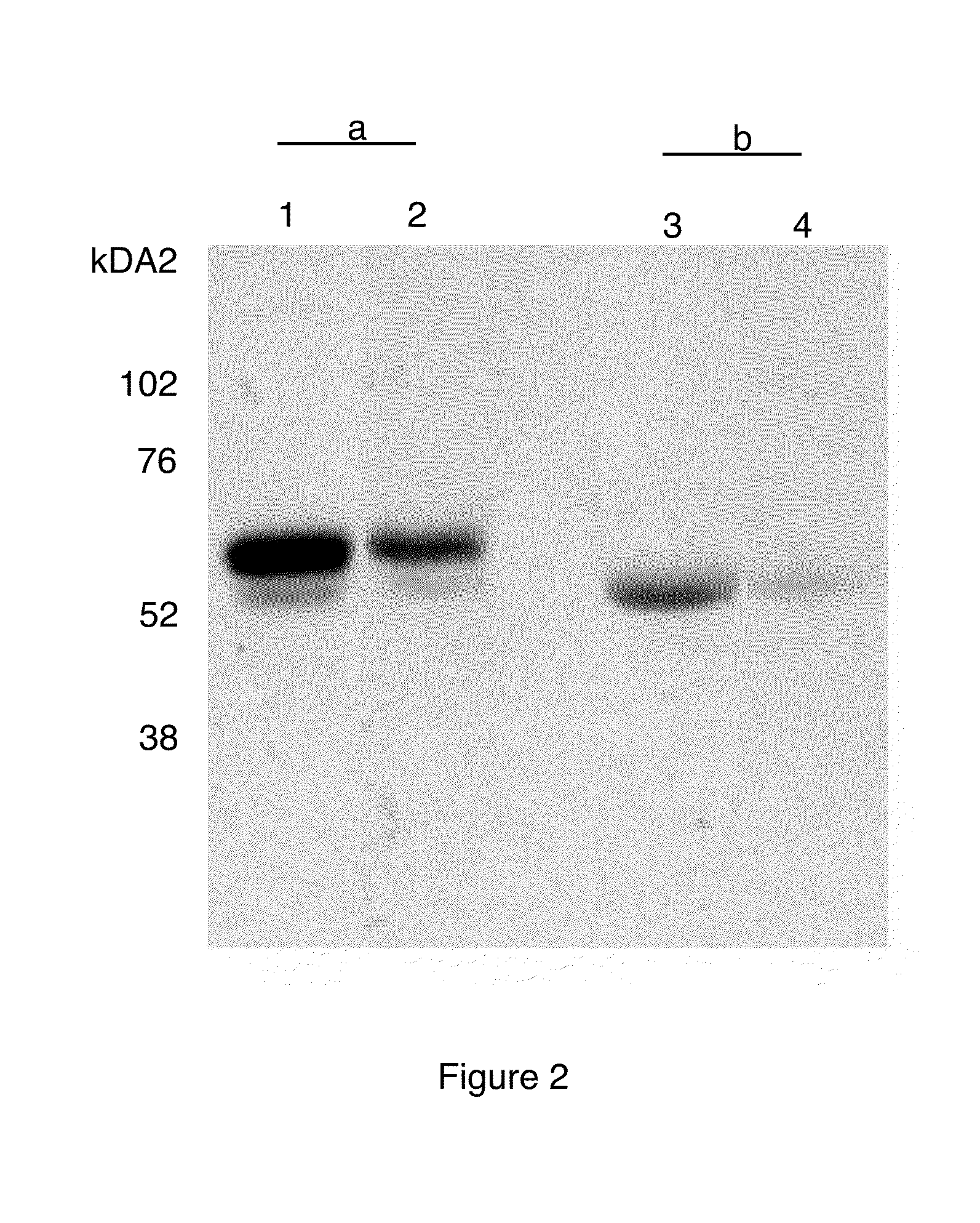
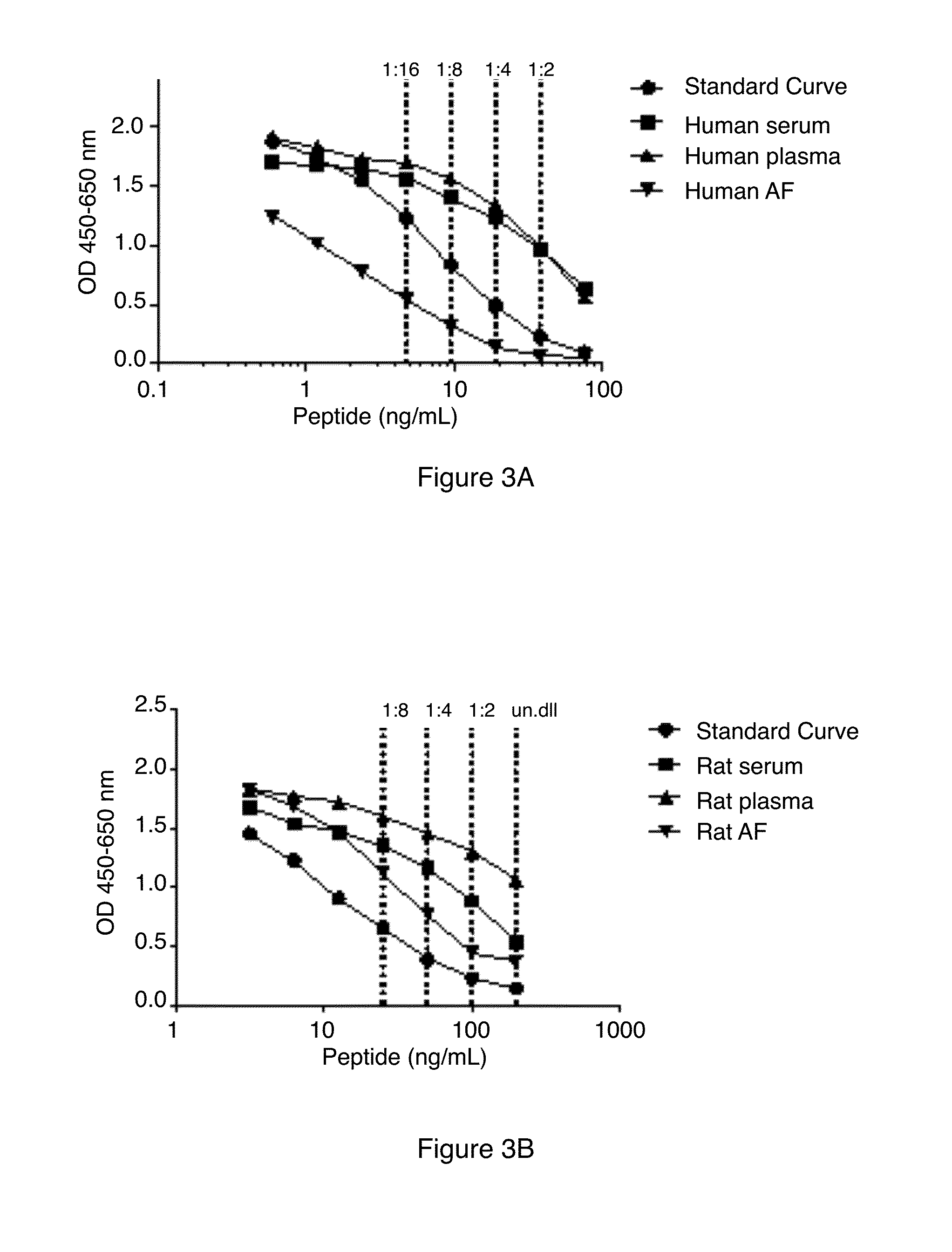
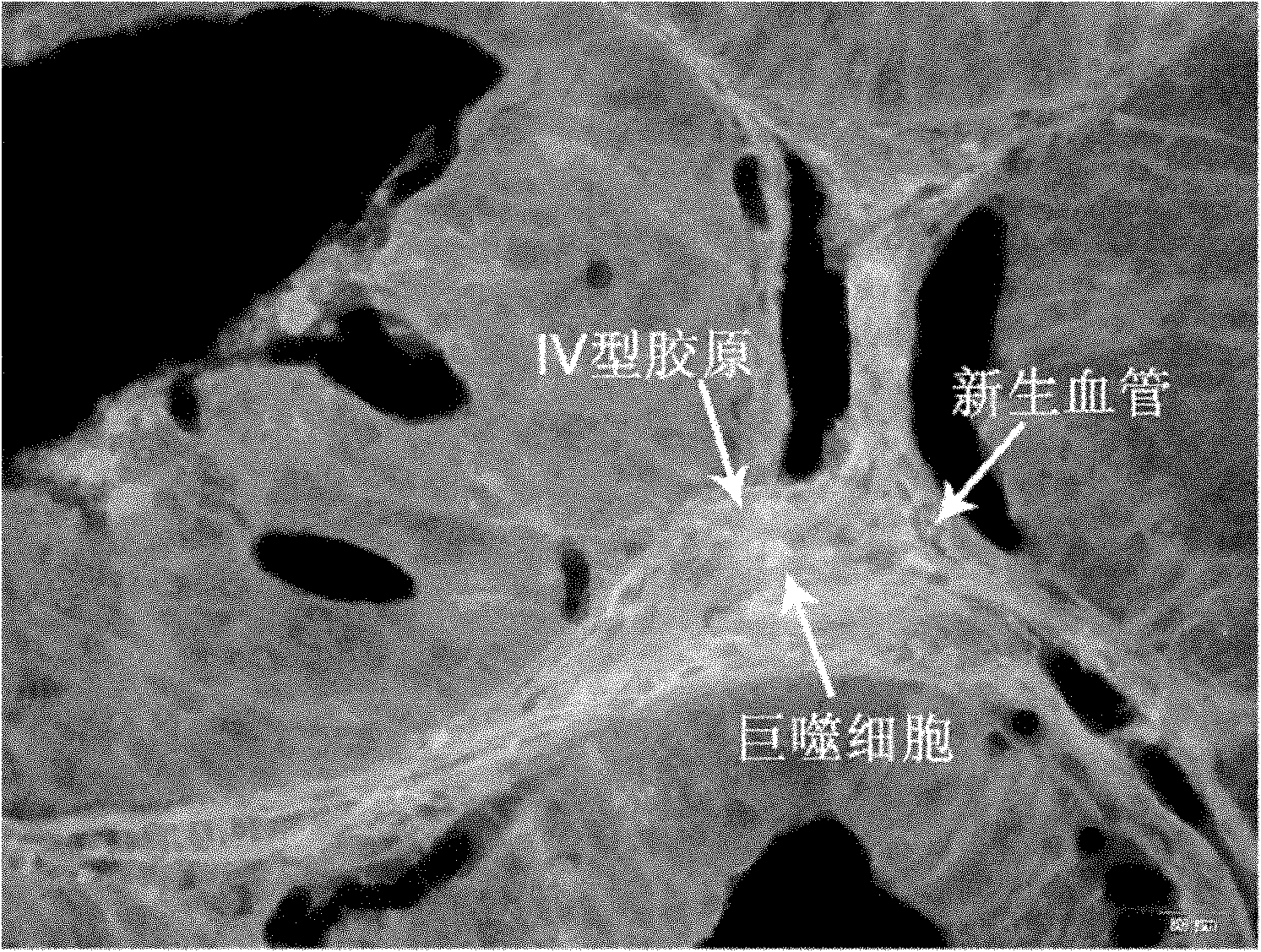Patents
Literature
39 results about "Collagen Type III" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A fibrillar collagen consisting of three identical alpha1(III) chains that is widely distributed in many tissues containing COLLAGEN TYPE I. It is particularly abundant in BLOOD VESSELS and may play a role in tissues with elastic characteristics.
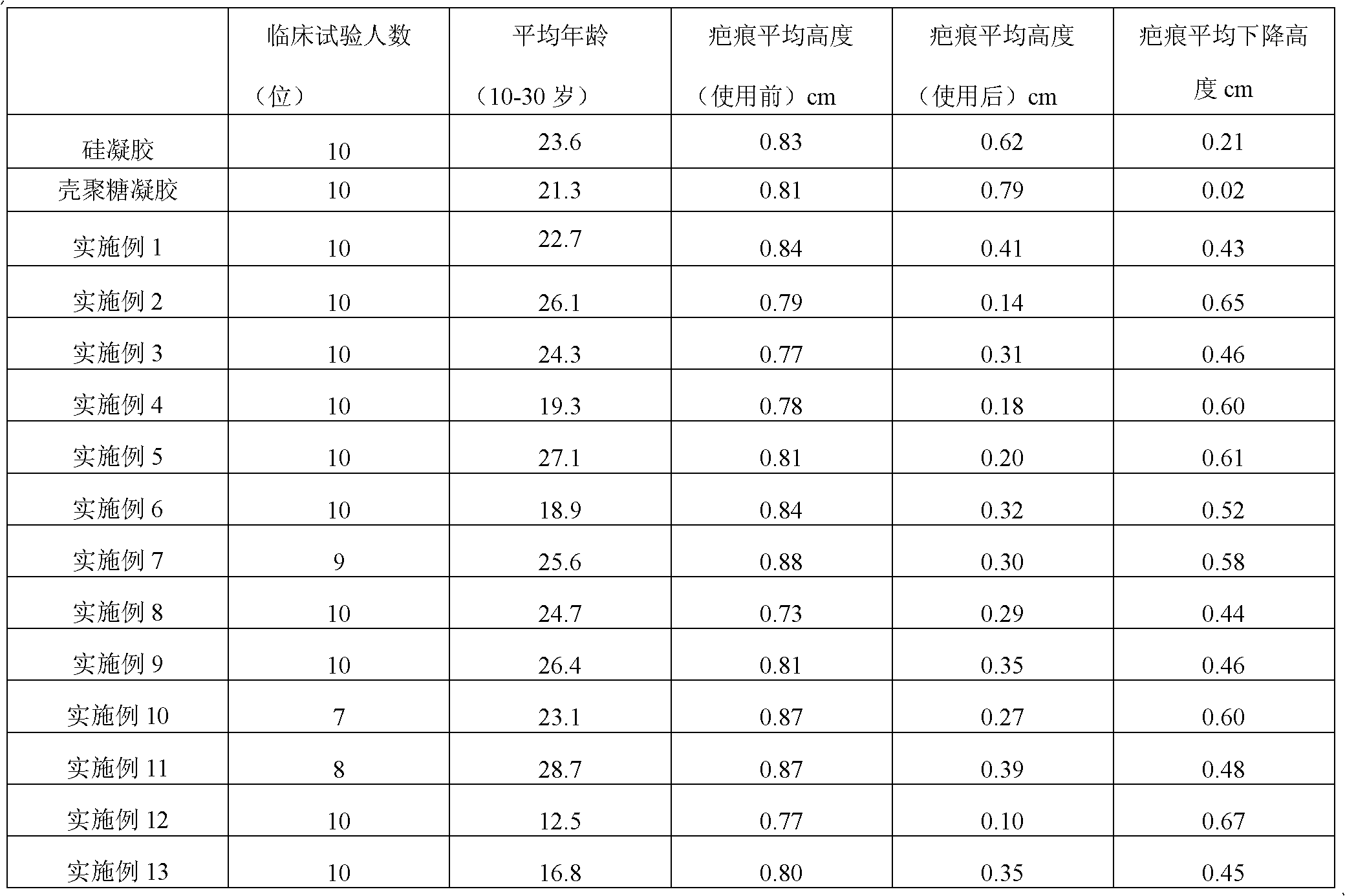
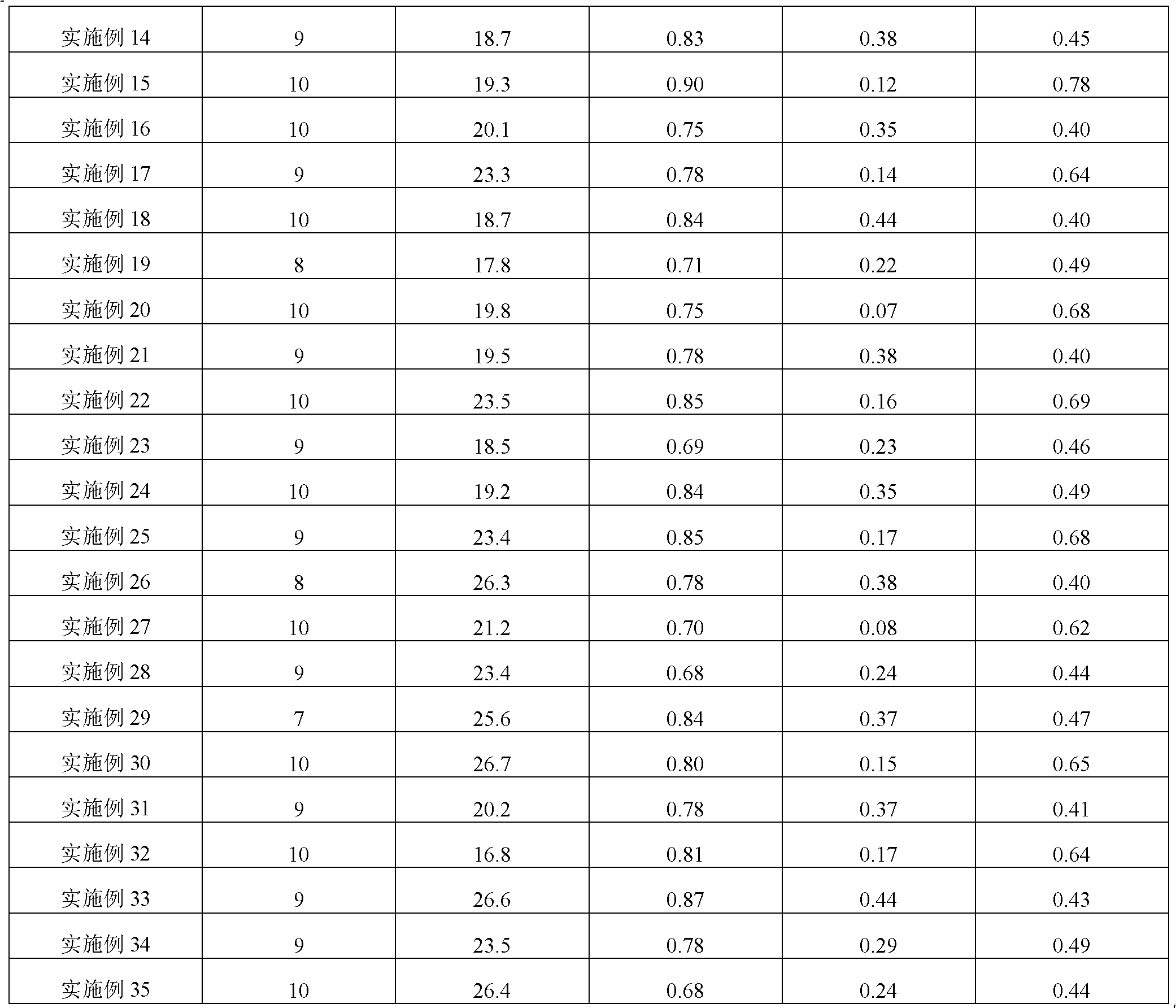
Scar repair material
The invention discloses a scar repair material which is prepared by the following method: dissolving 0.5-5 parts by weight of water-soluble chitosan or chitosan the deacetylation degree of which is 75-97% into 10-100 parts by weight of water or an acetic acid aqueous solution the mass concentration of which is 1-5% while stirring; adding 1-3 parts by weight of polyoxyethylene Sorbitan lauric acid monoester which has emulsifying action and 5-10 parts by weight of humectant glycerol to obtain a transparent gelatinous solution; adding 20-90 parts by weight of polydimethylsiloxane into the gelatinous solution under homogeneity; and evenly mixing to obtain the scar repair material. The scar repair material disclosed in the invention can actively and passively moisturize skin, has the efficacy on actively resisting and inhibiting bacteria, has the action on quickening the healing of skin wound surface, has better repair and protection actions on tiny flaws and infection on the scar surface as being used on the scar surface, and can inhibit the secretion of collagen type I in cheloid skin so as to lower the ratio of collagen type I / collagen type III.
Owner:TIANJIN JIASHITANG SCI & TECH
Cardiac fibroblast-derived extracellular matrix and injectable formulations thereof for treatment of ischemic disease or injury
InactiveUS20160354447A1Effective treatmentReduce injuriesAntibacterial agentsPowder deliveryDiseaseExtremity injury
Compositions and methods using an engineered cardiac fibroblast-derived 3-dimensional extracellular matrix (ECM) are disclosed. The ECM includes the structural proteins fibronectin, collagen type I, collagen type III, and elastin, and from 60% to 90% of the structural proteins present in the engineered extracellular matrix are fibronectin. The compositions, which can be used to treat cardiac disease or ischemic disease or injury, are injectable compositions, where the ECM is diced into small fragments or lyophilized into a powder. The disclosed methods include a method of treating ischemic disease or injury by contacting a cell free patch made from the ECM with the injured tissue, without the concomitant delivery of therapeutic cells, and a method of treating ischemic limb injury by contacting a patch, either by itself or seeded with therapeutic cells, with the injured limb tissue.
Owner:WISCONSIN ALUMNI RES FOUND
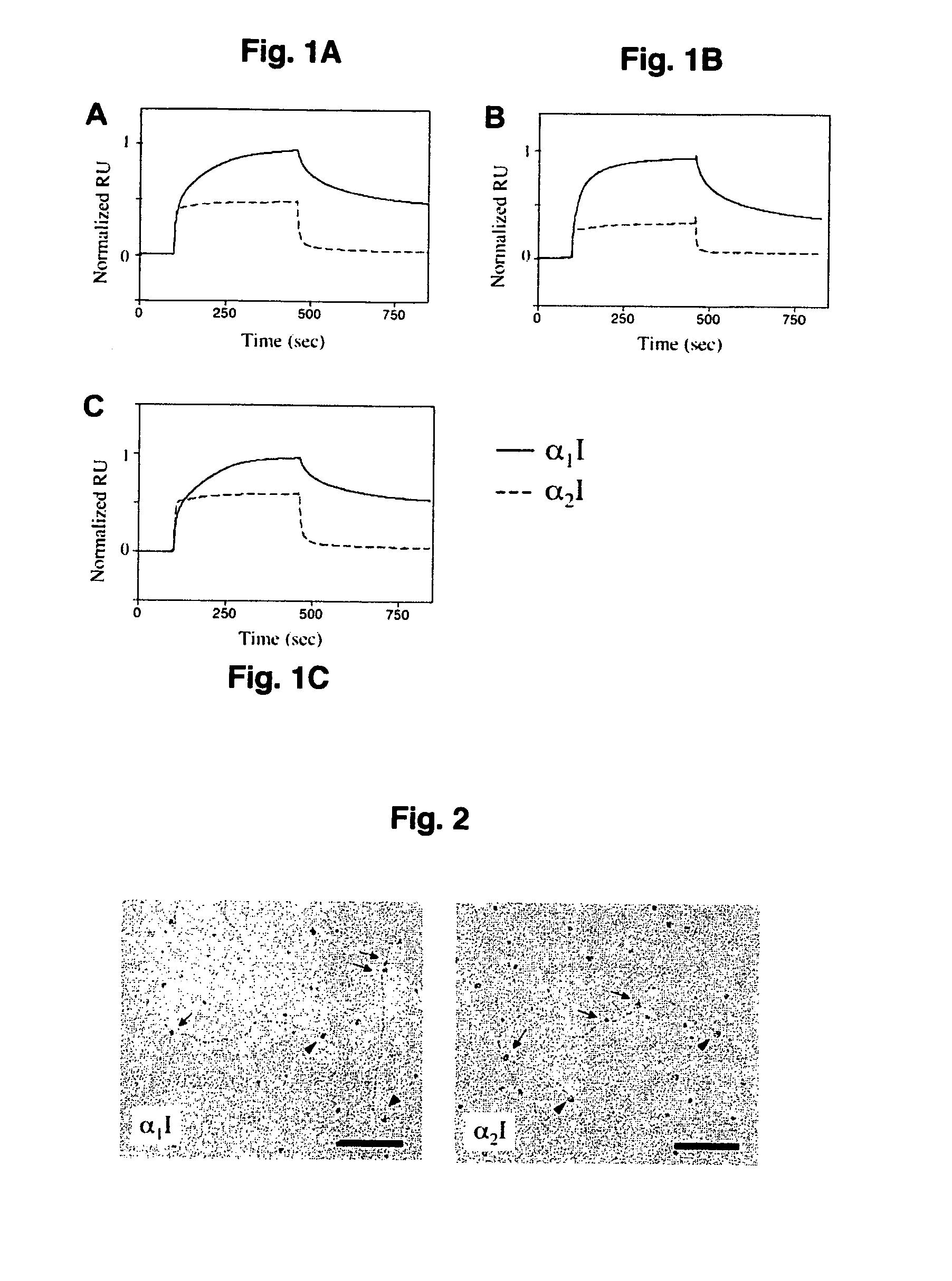
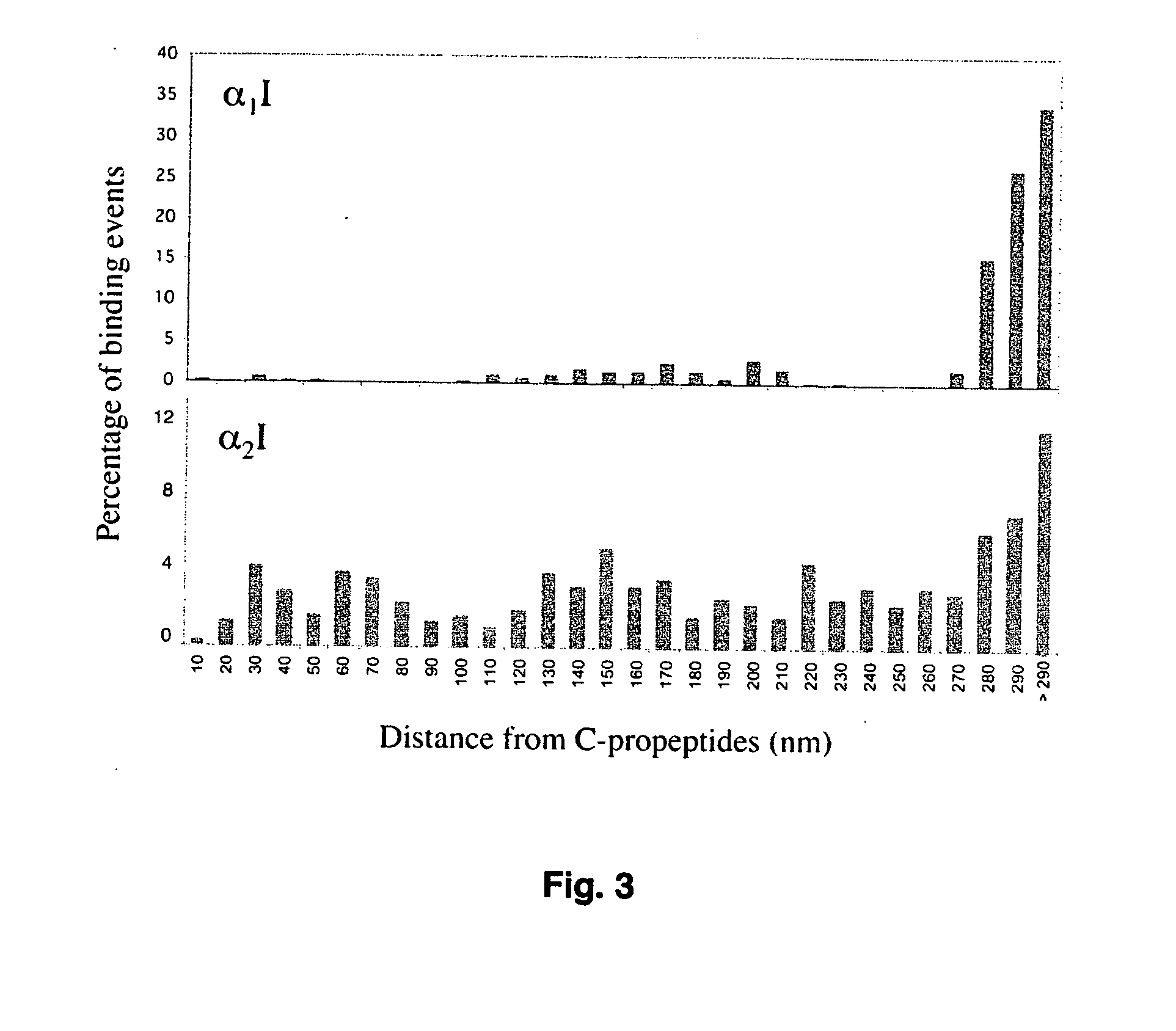
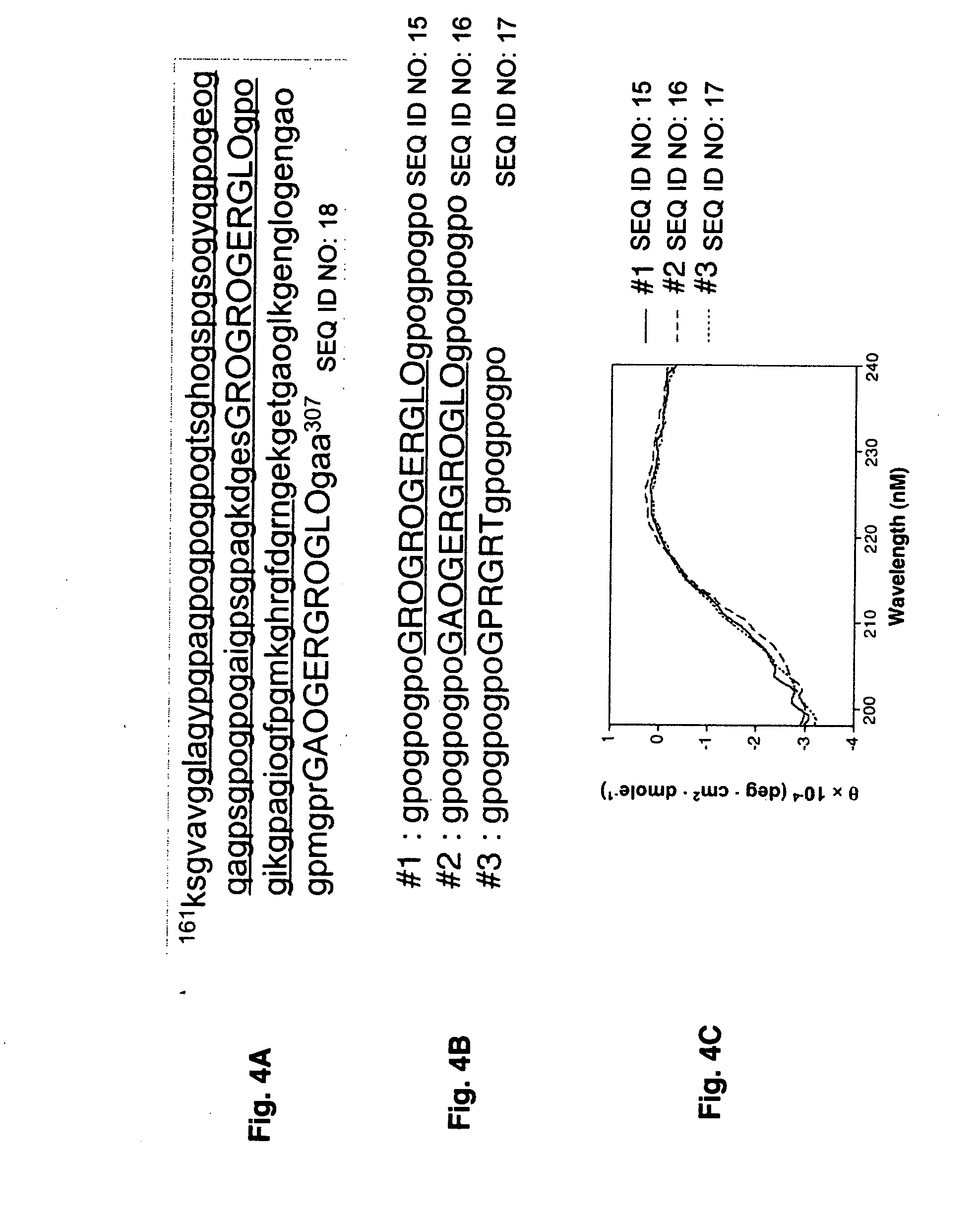
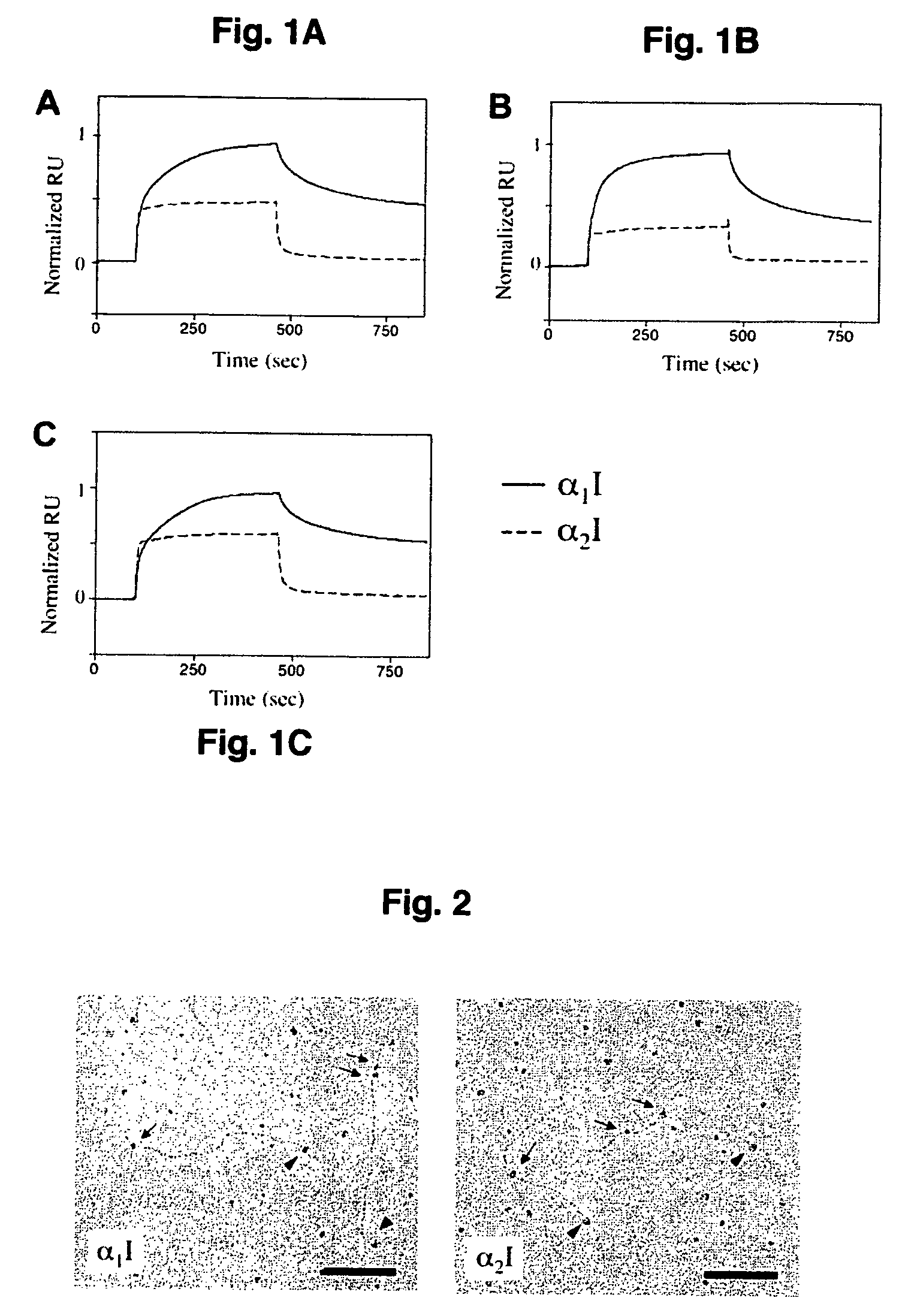
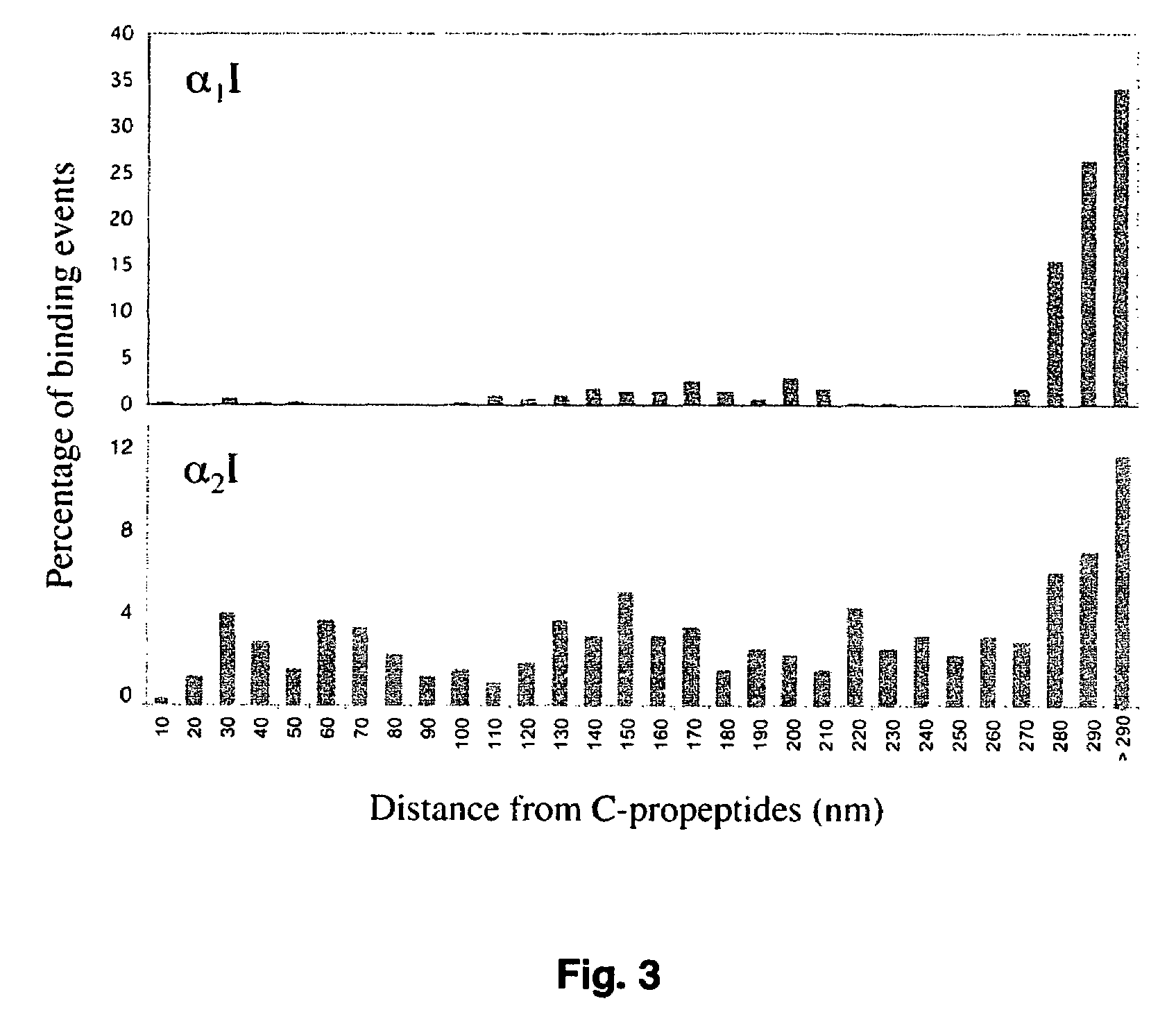
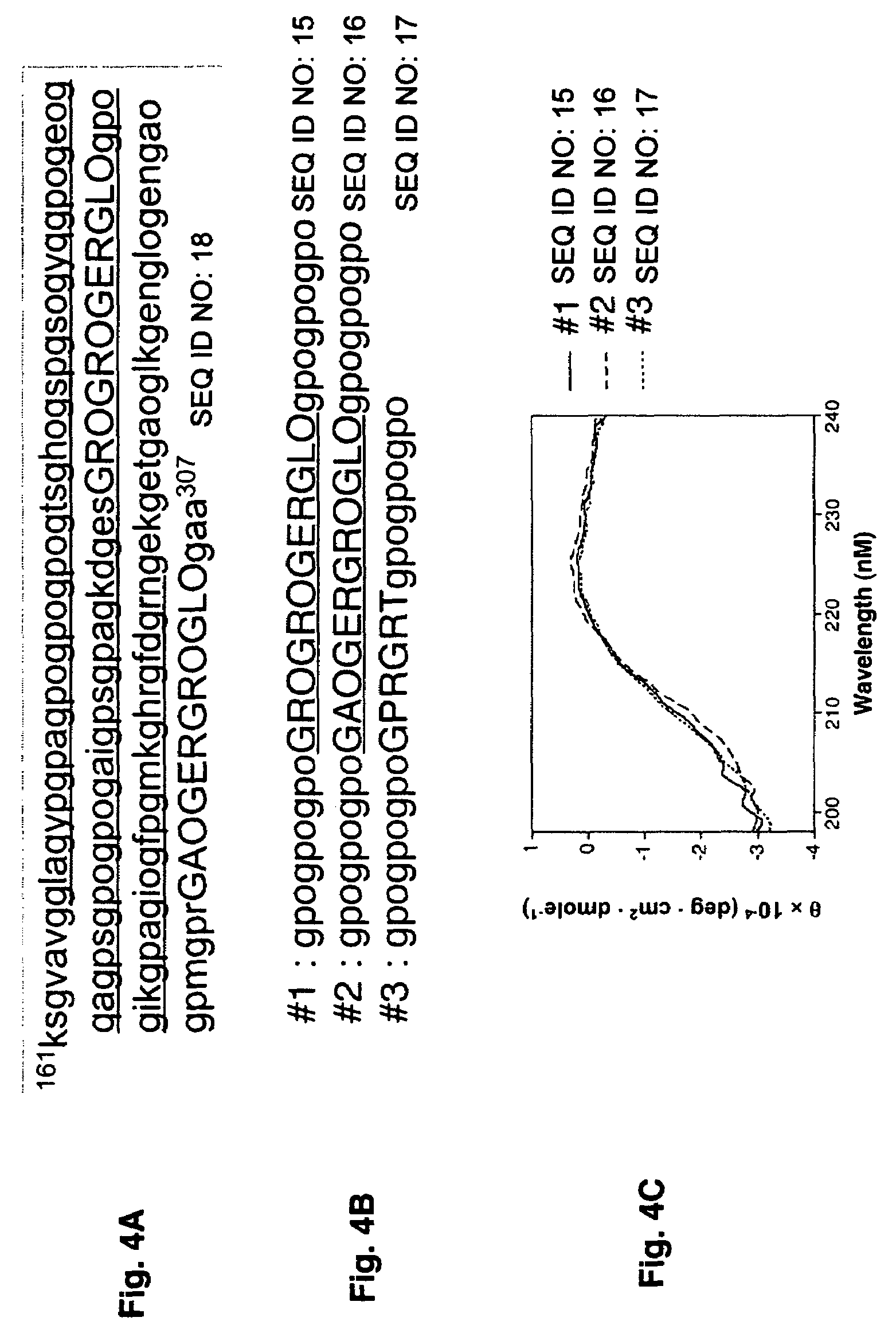
Specific binding sites in collagen for integrins and use thereof
The present invention identified a high affinity binding sequence in collagen type III for the collagen-binding integrin I domains. Provided herein are the methods used to characterize the sequence, the peptides comprising this novel sequence and the use of the peptides in enabling cell adhesion. Also provided herein are methods to identify specific integrin inhibitors, sequences of these inhibitors and their use in inhibiting pathophysiological conditions that may arise due to integrin-collagen interaction.
Owner:TEXAS A&M UNIVERSITY
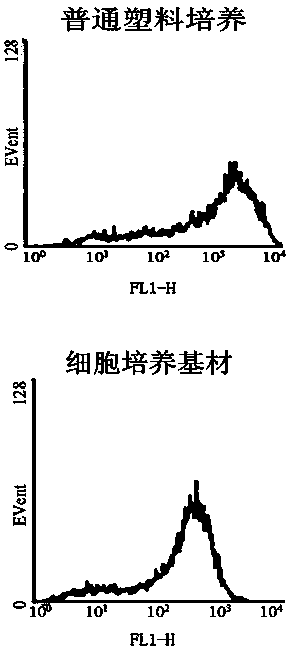
Cell culture substrate, and preparation method and application thereof
InactiveCN103497892AHigh amplification efficiencyMaintain immunophenotypeCell culture supports/coatingSkeletal/connective tissue cellsCell culture mediaCollagen Type III
The invention belongs to the technical field of biological material of tissue engineering. The invention discloses a cell culture substrate, a preparation method thereof, and an application thereof. According to the invention, umbilical cord fibroblast cell self-secretion substrate is adopted; and through decellularization treatment and drying and sterilization, a natural tissue engineering material is obtained. The material comprises collagen type I, collagen type III, fibronectin, laminin, decorin, and the like, and can be used in in-vitro amplification of target mesenchymal stem cells. With the cell culture substrate provided by the invention, mesenchymal stem cell in-vitro amplification efficiency can be substantially improved, special immune phenotype of stem cells can be maintained, and stem cell active oxygen content can be substantially reduced. Also, raw material source is wide, and no ethic and moral problem is caused.
Owner:SUN YAT SEN UNIV
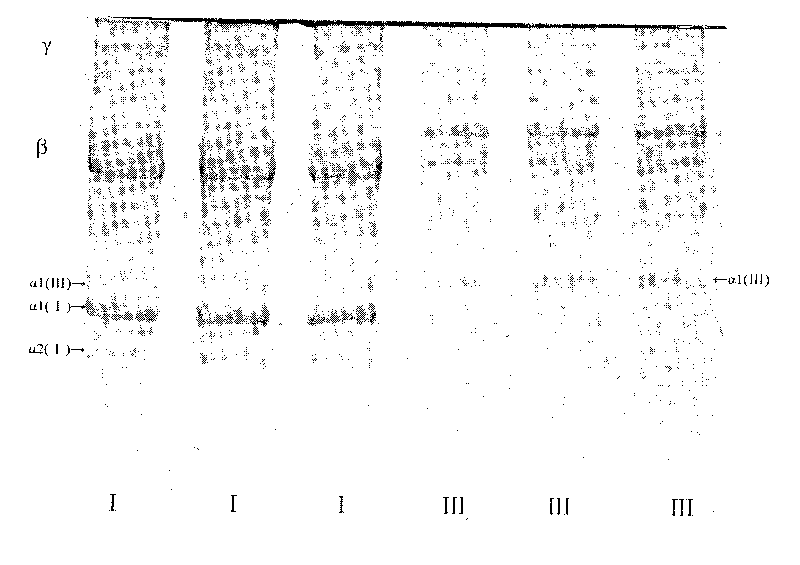
Method for preparing medical III type collagen
InactiveCN101717804ARich sourcesLow priceConnective tissue peptidesPeptide/protein ingredientsWater bathsSalting out
The invention discloses a method for preparing a medical III type collagen, which is characterized by comprising the following steps of: pre-treating fresh newborn kip as a raw material and then extracting a mixture of I type and II type collagens by using a pepsin degradation limiting method and a salting out method; dissolving the mixture with 1.5 mol / L NaCl and 0.05mol / L Tris buffer solution the pH value of which is 7.5 for 2-5 times to remove most of I type collagen; heating the mixture with 2-6mol / L guanidine hydrochloride and 0.05mol / L Tris-HCl buffer solution the pH value of which is 7.5 in a water bath with the temperature of 45-95 DEG C for 30-60 minutes to modify the collagens; reducing the temperature and the concentration of the guanidine hydrochloride; renaturing the II type collagen after 2-6 hours; and centrifuging the mixture at high speed to obtain a III type collagen.
Owner:SICHUAN UNIV
Method for simultaneously labelling collagen type IV, macrophage and neovascularisation of tumors
The invention discloses a method for simultaneously labelling collagen type IV, macrophage and neovascularisation of tumors. The method comprises the following steps: 1. immobilizing tissue sections on a plus microscope slide processed by polylysine; 2. dewaxing the tissue sections in dimethylbenzene; 3. carrying out antigen retrieval; 4. washing the sections; 5. carrying out confining; 6. addingprimary antibodies; 7. washing the sections; 8. carrying out confining in the same way as the step 5; 9. using the fragment antigen-binding F(ab')2-QDs525 of goat anti-mouse immunoglobulin G labelledby quantum dot QDs525, the fragment antigen-binding F(ab')2-QDs585 of goat anti-rabbit immunoglobulin G labelled by quantum dot QDs585 and the fragment antigen-binding F(ab')2-QDs655 of rabbit anti-goat immunoglobulin G labelled by quantum dot QDs655 as secondary antibodies and dropwise adding the mixture after removing the confining liquid; 10. washing the sections; and 11. sealing the sections:preparing buffered glycerol with glycerol and 10ml of tris buffered saline (TBS) and storing the sections after sealing the sections with the buffered glycerol. The method is used for efficiently, accurately, rapidly and simultaneously detecting various components in tumor tissue microenvironment.
Owner:WUHAN UNIV
Scar repair material
ActiveCN102406966AImprove repair effectPromote wound healingSurgeryCollagen Type IIIWater soluble chitosan
The invention discloses a scar repair material. A preparation method comprises the following steps of: dissolving water-soluble chitosan in water or dissolving chitosan with the deacetylation degree of 75 to 97 percent in an acetic acid aqueous solution under stirring, adding polyoxyethylene sorbitan monolaurate with an emulsifying effect and glycerin with a moisturizing effect, and uniformly mixing to obtain a transparent gel-like solution; and adding polydimethylsiloxane into the gel-like solution under homogenizing, and uniformly mixing to obtain the scar repair material. The scar repair material can actively and passively moisture skin, has a hydration effect on scar skin, has a good repair effect, has an effect of resisting and inhibiting bacteria, has a good effect of resisting and inhibiting the bacteria on the surfaces of scars and outside the scars, has an effect of accelerating skin wound healing, can have a good effect of repairing and protecting fine cracks on the surfacesof the scars, and can inhibit the secretion of collagen type I in keloid skin to reduce the proportion of the collagen type I or collagen type III.
Owner:TIANJIN JIASHITANG SCI & TECH
Methods for diagnosing and evaluating non-alcoholic steatohepatitis
ActiveCN108138232AOrganic active ingredientsMicrobiological testing/measurementOncologyCollagen Type III
Owner:GENFIT SA
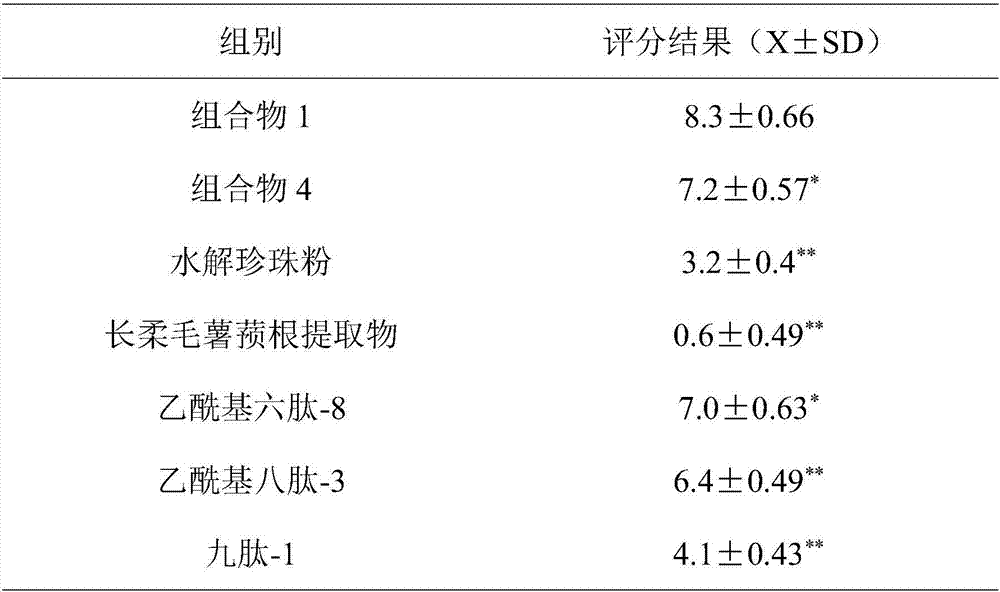
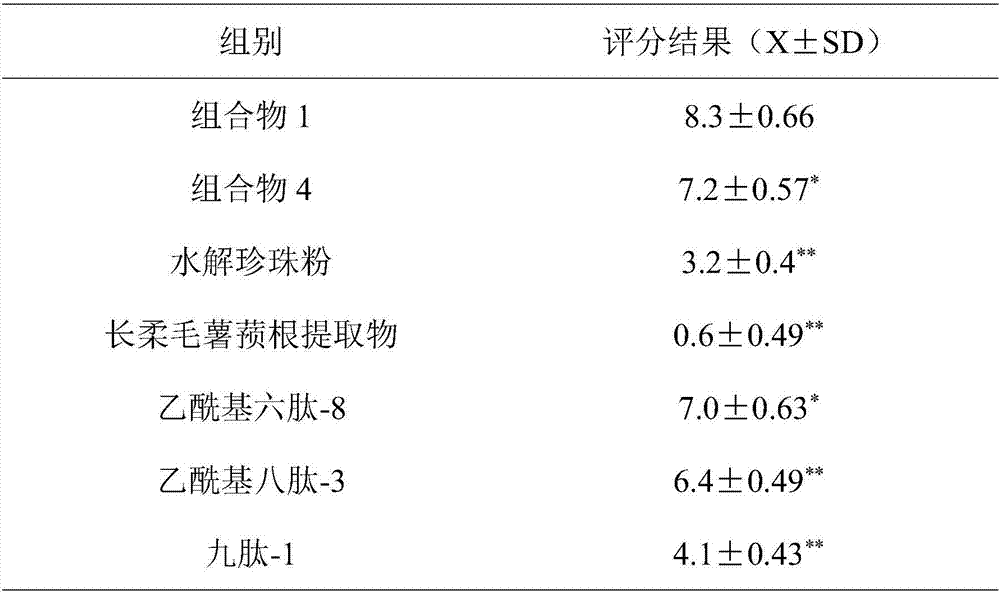
Anti-aging composition and preparation and application thereof
ActiveCN107441017ASignificant anti-aging effectEasy to removeCosmetic preparationsToilet preparationsCollagen Type IIIHuman skin fibroblast
The invention belongs to the field of cosmetics and particularly relates to an anti-aging composition and preparation and application thereof. The anti-aging composition is prepared from the following ingredients in parts by weight: 1 to 3 parts of hydrolyzed pearl powder, 1 to 3 parts of dioscorea villosa root extract, 0.5 to 2 parts of acetyl hexapeptide-8, 0.5 to 2 parts of acetyl octapeptide-3 and 0.5 to 2 parts of nonapeptide-1. Experiments prove that the anti-aging composition disclosed by the invention has an extremely obvious removing effect on quiescent wrinkles, dynamic wrinkles and posture extrusion wrinkles, meanwhile can obviously promote multiplication of human skin fibroblast, extremely obviously promotes synthesis of I and III type collagen and extremely obviously inhibits expression of MMP1 and MMP3.
Owner:佐登妮丝(广州)美容化妆品有限公司
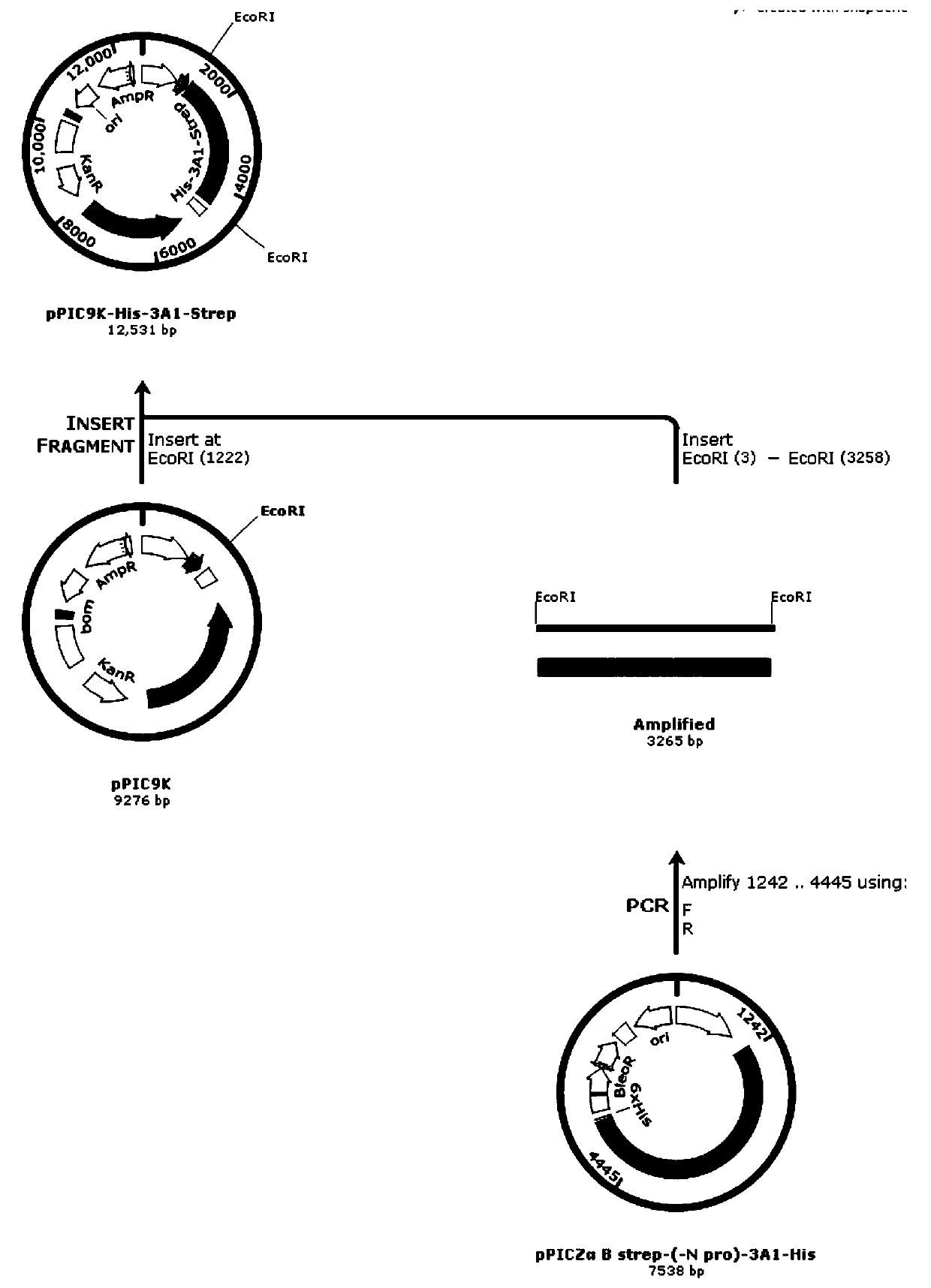
Recombinant human source collagen type-III alpha 1 chain and application thereof
InactiveCN109988243AEasy to purifyHigh expressionCosmetic preparationsFungiNucleotideType III collagen
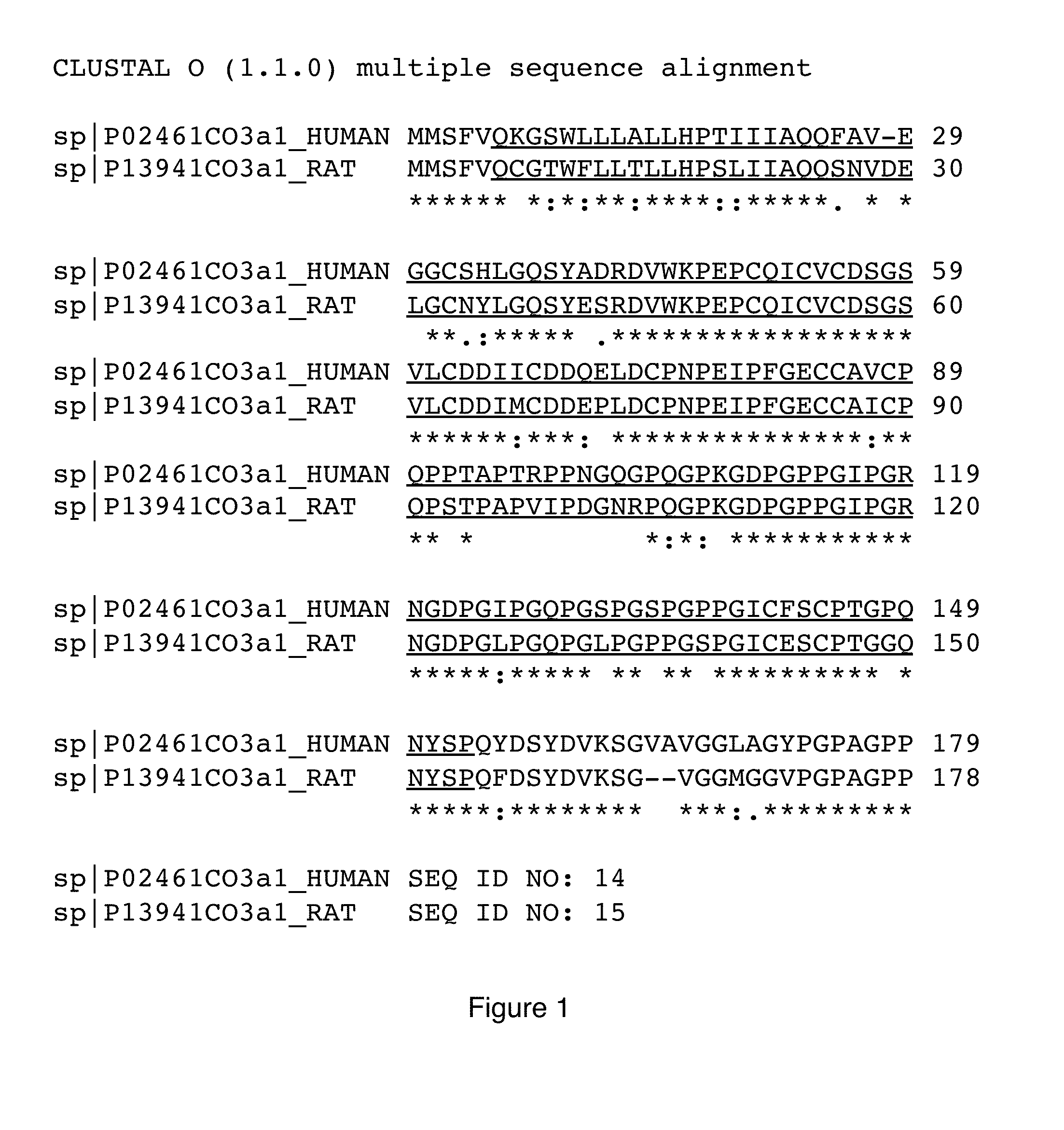
The invention discloses a recombinant human source collagen type-III alpha 1 chain and application thereof. The nucleotide sequence of the recombinant human source collagen type-III alpha 1 chain is encoded as shown in SEQ ID NO: 1, and the recombinant human source collagen type-III alpha 1 chain comprises in sequence from an amino terminal: an amino terminal affinity purification tag, a human source collagen type III mature peptide chain and a carboxyl terminal affinity purification tag. By designing specific affinity purification tags at two terminals of the human source collagen type-III mature peptide chain, recombinant human source collagen type-III in the embodiment is conducive to the purification of full-length protein and can further be used for the identification of collagen. Propeptide at two terminals is removed according to the mature peptide chain of the collagen type-III adopted by the recombinant human source collagen type-III in the embodiment, and the expression quantity is far higher than those of genes containing the propeptide.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
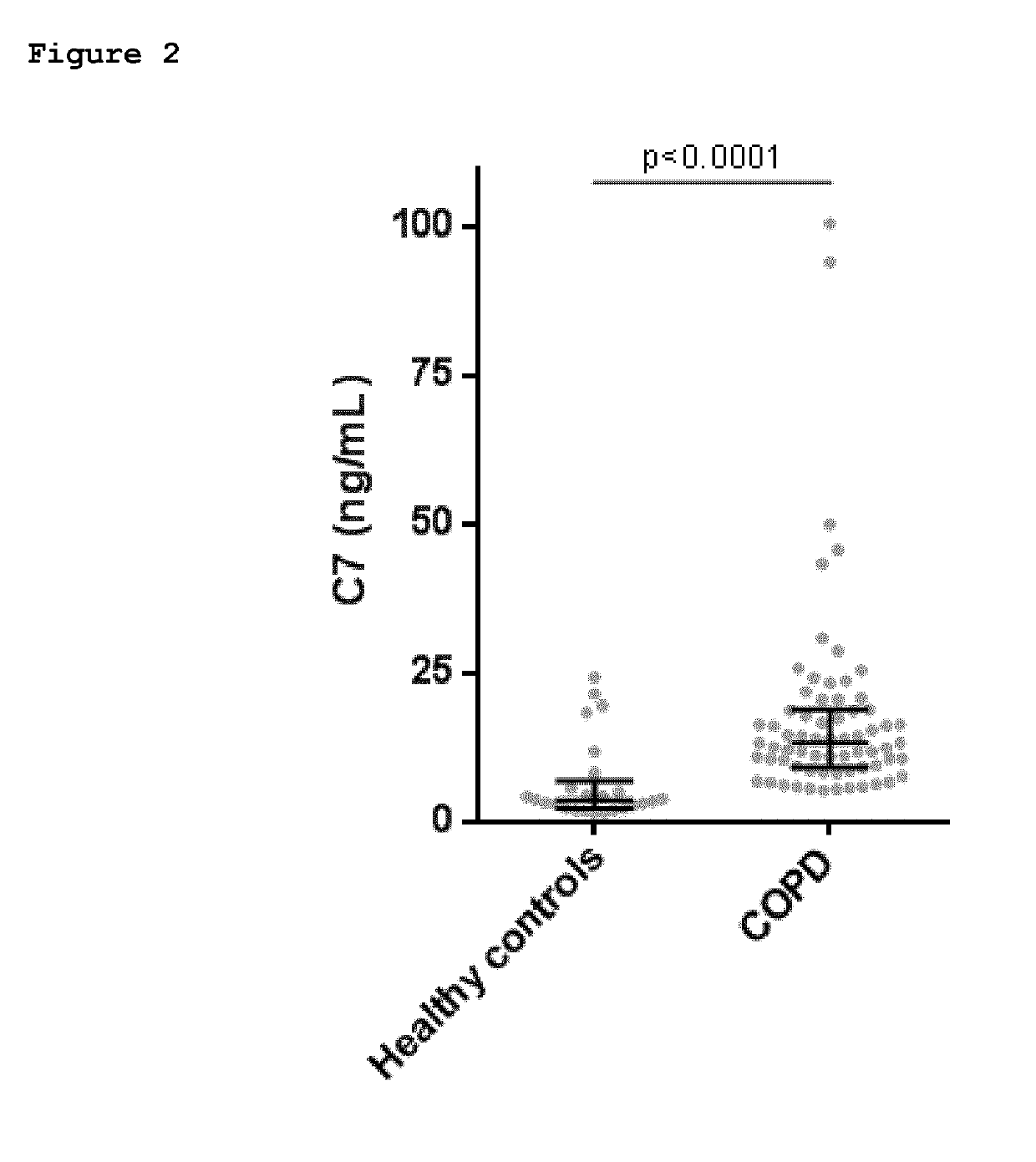
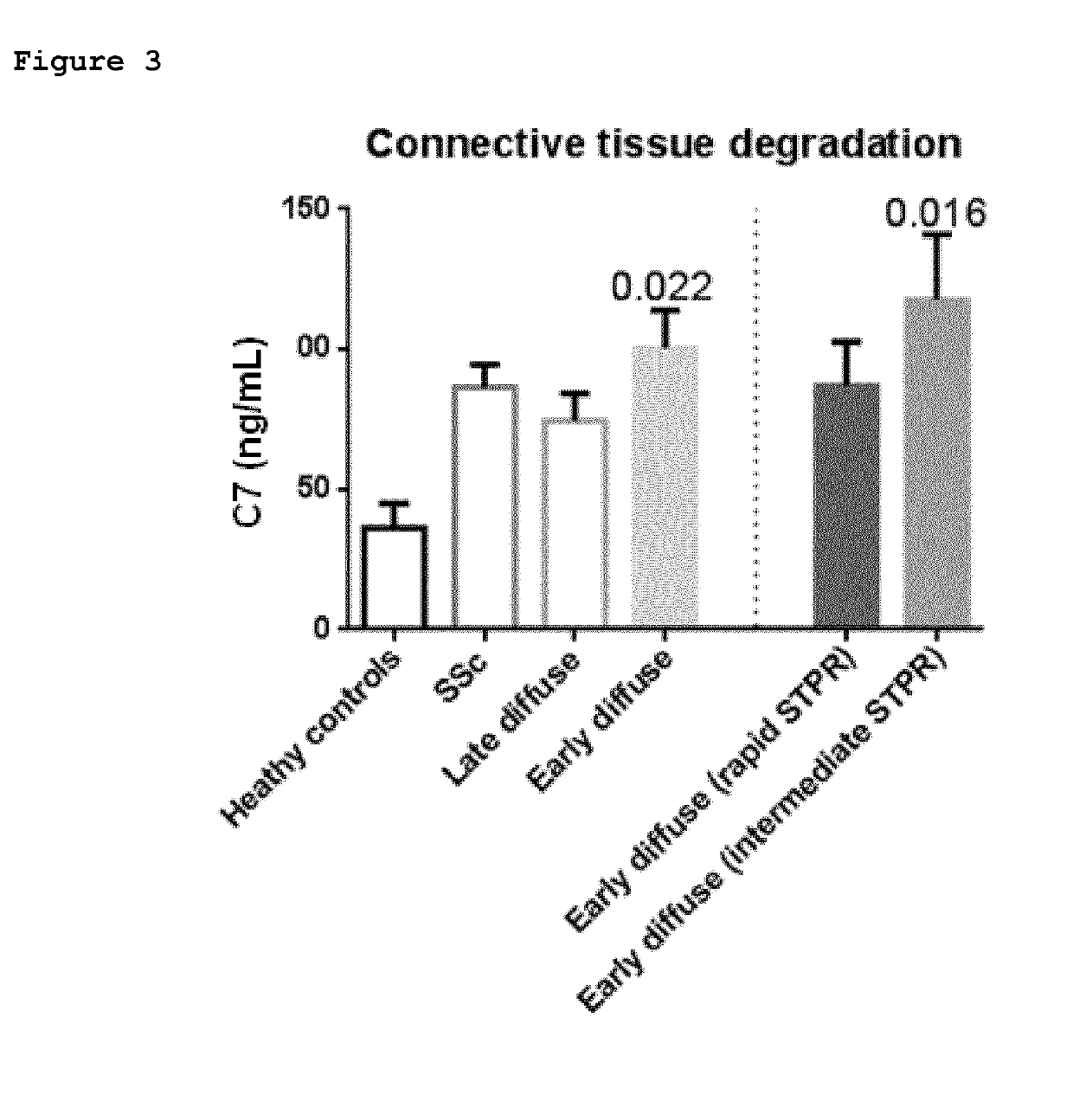
Fibrosis biomarker assay
Provided herein are methods of diagnosis or of quantitation of fibrosis. An immunoassay is conducted to measure neo-epitope containing protein fragments of collagen type III, collagen type I, collagen type IV, collagen type V, or collagen type VI, elastin, biglycan, decorin, lumican, versican, perlecan, neurocan, brevican, fibromodulin, serglycin, syndecan, betaglycan, vimentin, or C-reactive protein naturally present in a biofluid sample obtained from a patient. An above normal elevation of the measured protein fragments in the patient is associated with the presence or extent of fibrosis.
Owner:NORDIC BIOSCI
Multimicrolamellar Collagen Membranes
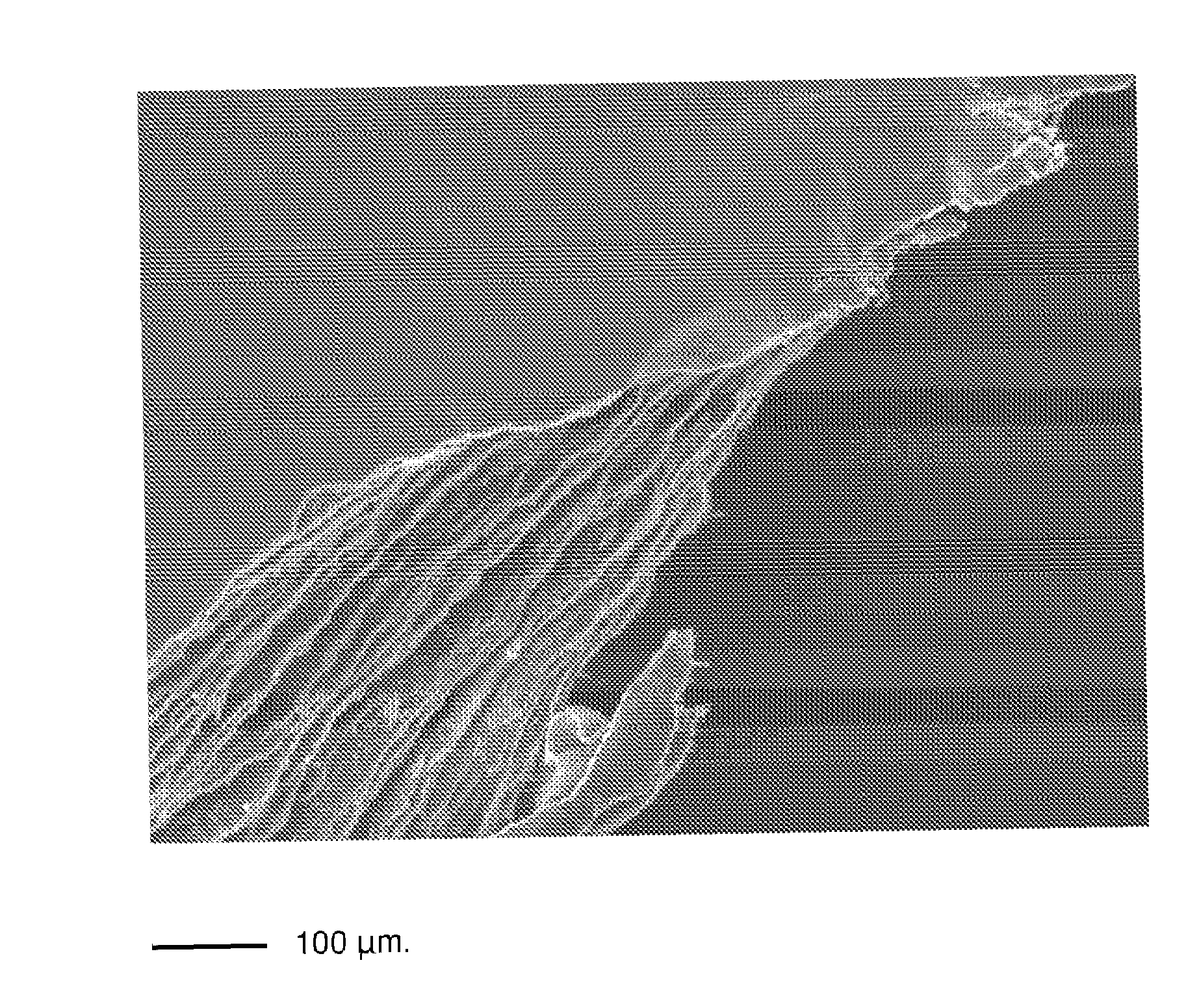
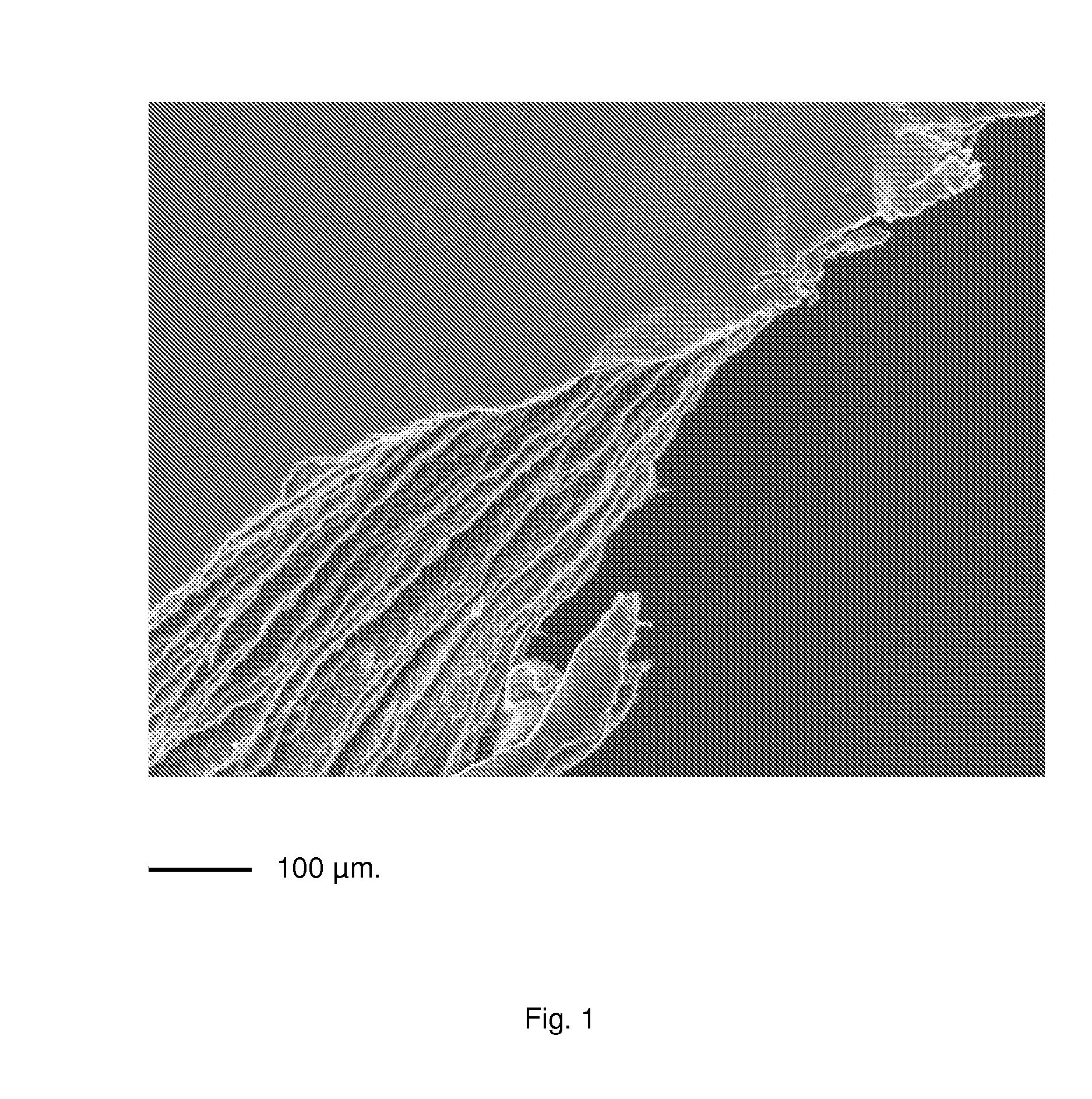
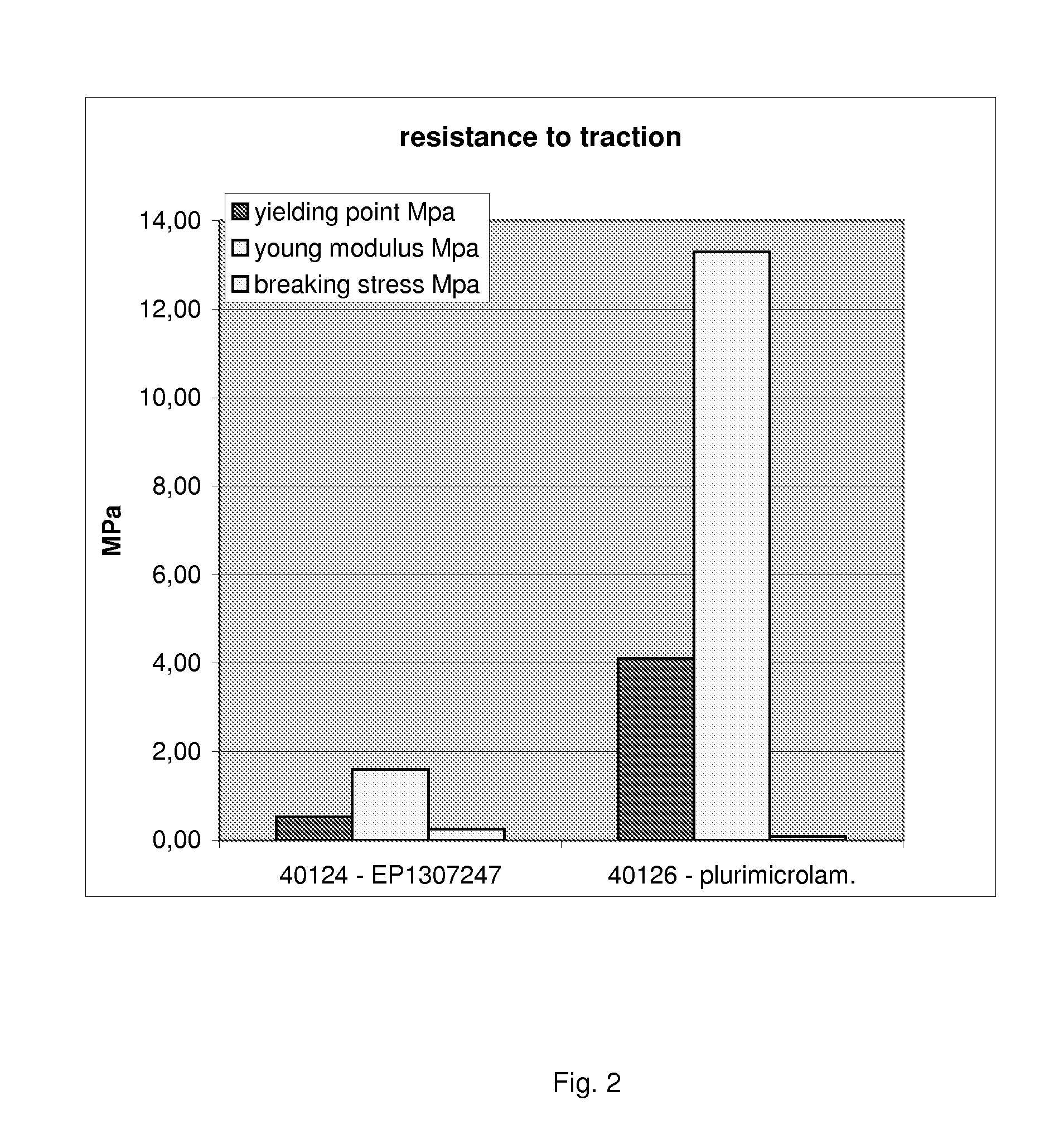
InactiveUS20090269586A1Semi-permeable membranesLayered productsRough surfaceThree dimensional scaffolds
The present invention relates to multimicrolamellar membranes of collagen of several types and structures (e.g. collagen type I, type II, type III, etc.), of only one type or two types in association, but more particularly of type I and type II collagen and / or of their mixture. The multimolecular arrangement is obtained by preparing the membrane in several steps involving the sequential addition of collagen gel layers. Membranes look thin, with a variable rough surface depending on the type of collagen used. Such membranes show a parallel horizontal lamellar microstructure and they can be soaked with different fluids, act as three-dimensional scaffold for cultured cells and are apt to be infiltrated and colonized by cells in vivo, therefore working as support suitable for direct, guided tissue regeneration. Moreover, the present invention relates to the preparation of said membranes from collagen gels and to the optimization of the preparation of collagen gel from tissues.
Owner:OPOCRIN
Composite collagen dressing for burnt injury wound surface restoration and preparation method thereof
ActiveCN109675085AReflect different rolesSimple preparation processAbsorbent padsBandagesAdditive ingredientBiocompatibility Testing
The invention discloses a composite collagen dressing for burnt injury wound surface restoration and a preparation method thereof. 99.9 to 85 parts of type I collagen, 0.01 to 10 parts of type III collagen and 0.01 to 5 parts of type V collagen are proportionally dispersed into a solvent; after homogenizing, shaping is performed; a finished product is obtained. Related ingredients are selected from different kinds of multi-ingredient collagen materials of different contents; no foreign materials are introduced; low immunogenicity and good biocompatibility are realized; the characteristics of all collagen ingredients are sufficiently achieved; the wound restoration is accelerated; the wound healing quality is improved.
Owner:BEIJING QINGYUAN WEIYE BIO TISSUE ENG
Primary culture method of rheumatoid arthritis synovial fibroblasts
InactiveCN110117570AHigh yieldFull shapeCell dissociation methodsArtificial cell constructsPurification methodsSynovial Cell
The invention provides a primary culture method of rheumatoid arthritis synovial fibroblasts. The method comprises the steps of sufficiently crushing pretreated synovial tissue; adding type-III collagenase and type-II collagenase into the synovial tissue and conducting blowing and beating; after culture digestion, scattering the synovial tissue into single cells; after filtering, centrifuging a filtrate, taking a substratum precipitate, and inoculating the substratum precipitate into a DMEM high-glucose culture medium for adherent culture; adopting a natural purification method and a repeatedadherent method for purifying the cells to obtain the rheumatoid arthritis synovial fibroblasts. By means of the culture method, about 1,010 RASFs can be obtained within 30 days, the cell yield is greatly increased, multiple experiment demands can be met, and therefore, the primary culture method is a quick and effective primary RASFS isolation culture method. In the meanwhile, compared with synovial cell lines of in-vitro construction lines, the RASFs obtained through primary culture have higher study value, and have a good practical application prospect.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Specific binding sites in collagen for integrins and use thereof
The present invention identified a high affinity binding sequence in collagen type III for the collagen-binding integrin I domains. Provided herein are the methods used to characterize the sequence, the peptides comprising this novel sequence and the use of the peptides in enabling cell adhesion. Also provided herein are methods to identify specific integrin inhibitors, sequences of these inhibitors and their use in inhibiting pathophysiological conditions that may arise due to integrin-collagen interaction.
Owner:TEXAS A&M UNIVERSITY
Construction method of swine-origin collagen membrane-autologous chondrocyte composite scaffold
InactiveCN106924814AStrong persistenceTear resistantSkeletal/connective tissue cellsCell culture supports/coatingRough surfaceTherapeutic effect
The invention discloses a method for constructing a composite scaffold of pig-derived collagen membrane-autologous chondrocytes. The specific steps are as follows: take the patient's articular cartilage, shake and clean it; cut the cartilage tissue into pieces; obtain cell suspension; precipitate as chondrocytes; Dried the porcine type Ⅰ / Ⅲ collagen membrane, placed it in a sterile plastic plate, slowly dripped the cell suspension on the rough surface of the prepared porcine type Ⅰ / Ⅲ collagen membrane with a plastic sleeve until the collagen membrane reached Wet and saturated state; wait for 10-15 minutes after the collagen membrane adsorbs the cells. The invention has the advantages of good therapeutic effect and the like.
Owner:安徽安龙基因科技有限公司
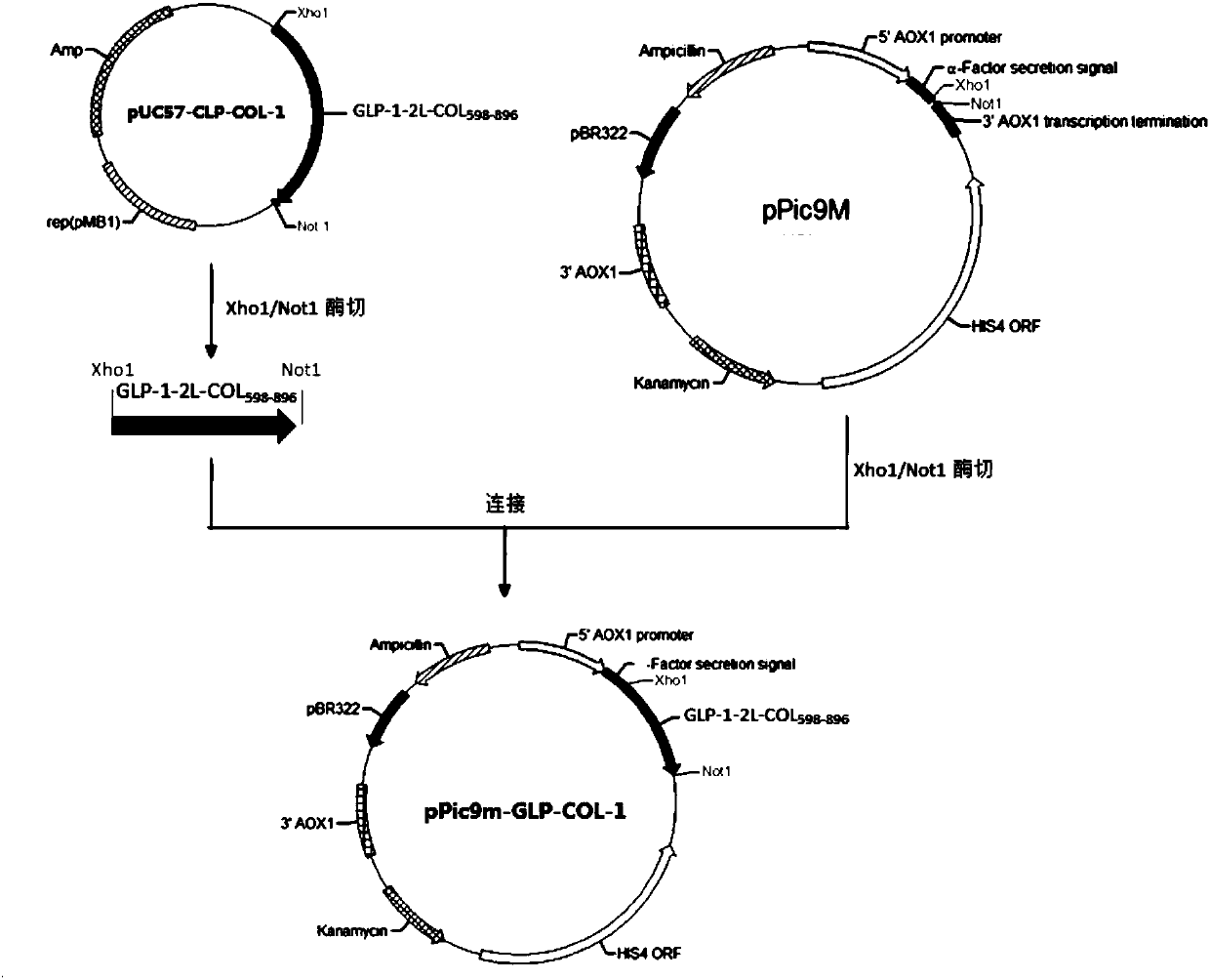
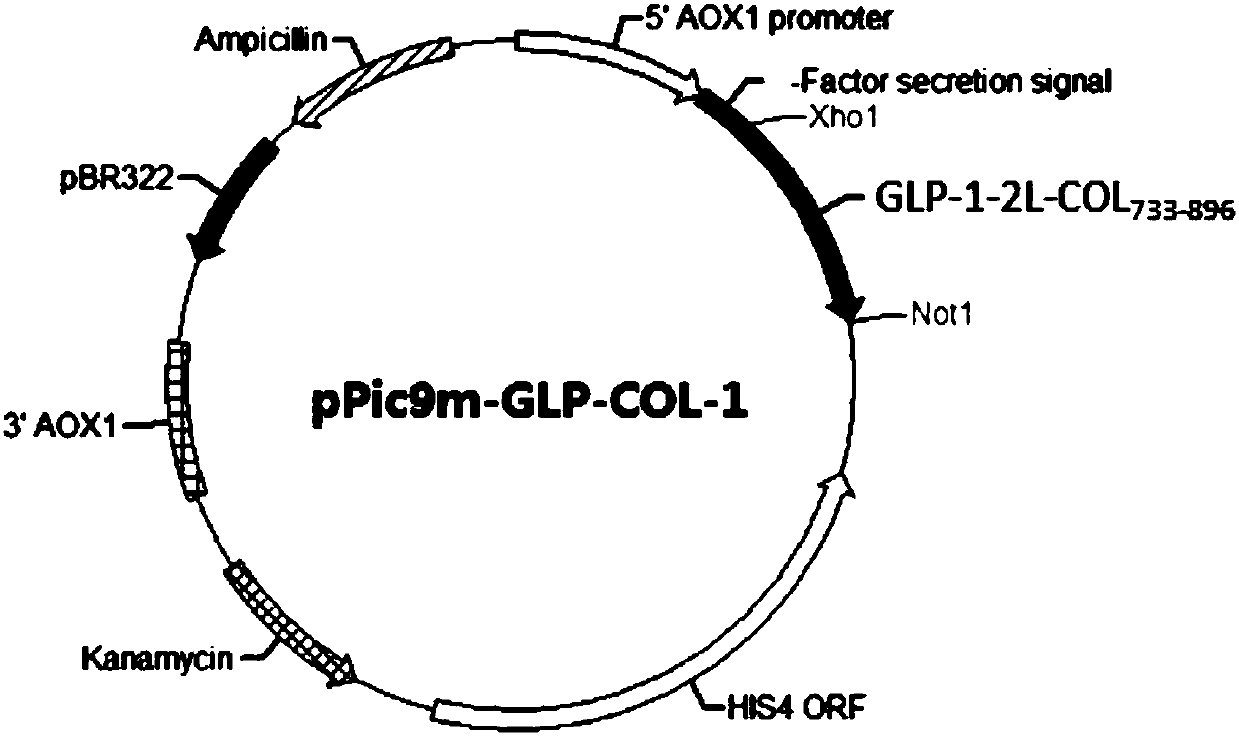
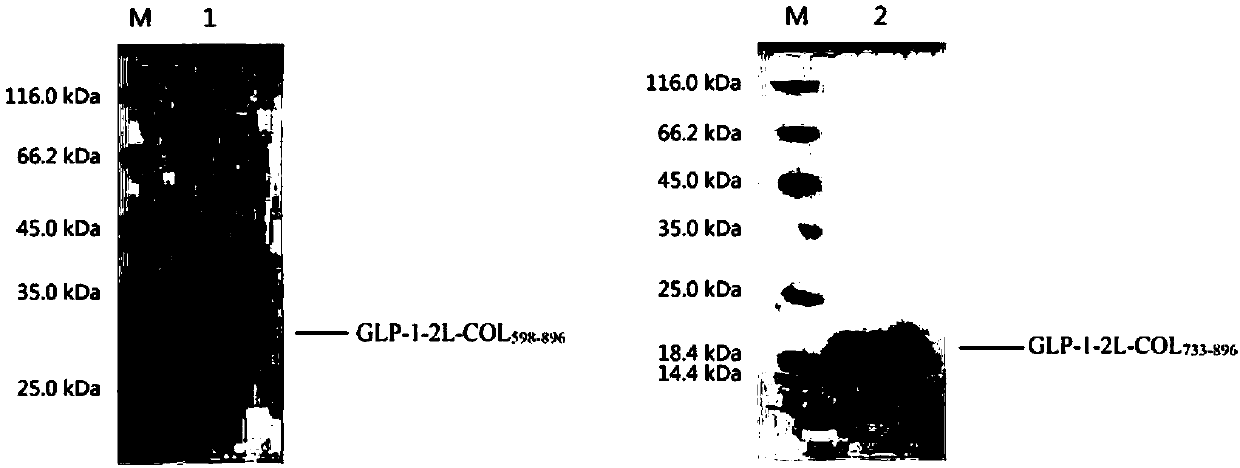
GLP-1 analogue-COL3A1 fusion protein
InactiveCN110092835AActivating activityPreserve hypoglycemic activityConnective tissue peptidesPeptide/protein ingredientsHuman typeHalf-life
The invention relates to a GLP-1 analogue-COL3A1 fusion protein. Specifically, the fusion protein comprises the glucagon-like peptide 1 analog and a human type III collagen alpha1 chain fusion protein, and the fusion protein has the hypoglycemic effect of the glucagon-like peptide 1 and has an extended half-life in vivo.
Owner:SHANGHAI HUIMMUTECH BIOTECHNOLOGY CO LTD
A kind of anti-aging composition and its preparation and application
ActiveCN107441017BSignificant anti-aging effectEasy to removeCosmetic preparationsToilet preparationsWrinkle skinAdditive ingredient
The invention belongs to the field of cosmetics and particularly relates to an anti-aging composition and preparation and application thereof. The anti-aging composition is prepared from the following ingredients in parts by weight: 1 to 3 parts of hydrolyzed pearl powder, 1 to 3 parts of dioscorea villosa root extract, 0.5 to 2 parts of acetyl hexapeptide-8, 0.5 to 2 parts of acetyl octapeptide-3 and 0.5 to 2 parts of nonapeptide-1. Experiments prove that the anti-aging composition disclosed by the invention has an extremely obvious removing effect on quiescent wrinkles, dynamic wrinkles and posture extrusion wrinkles, meanwhile can obviously promote multiplication of human skin fibroblast, extremely obviously promotes synthesis of I and III type collagen and extremely obviously inhibits expression of MMP1 and MMP3.
Owner:佐登妮丝(广州)美容化妆品有限公司
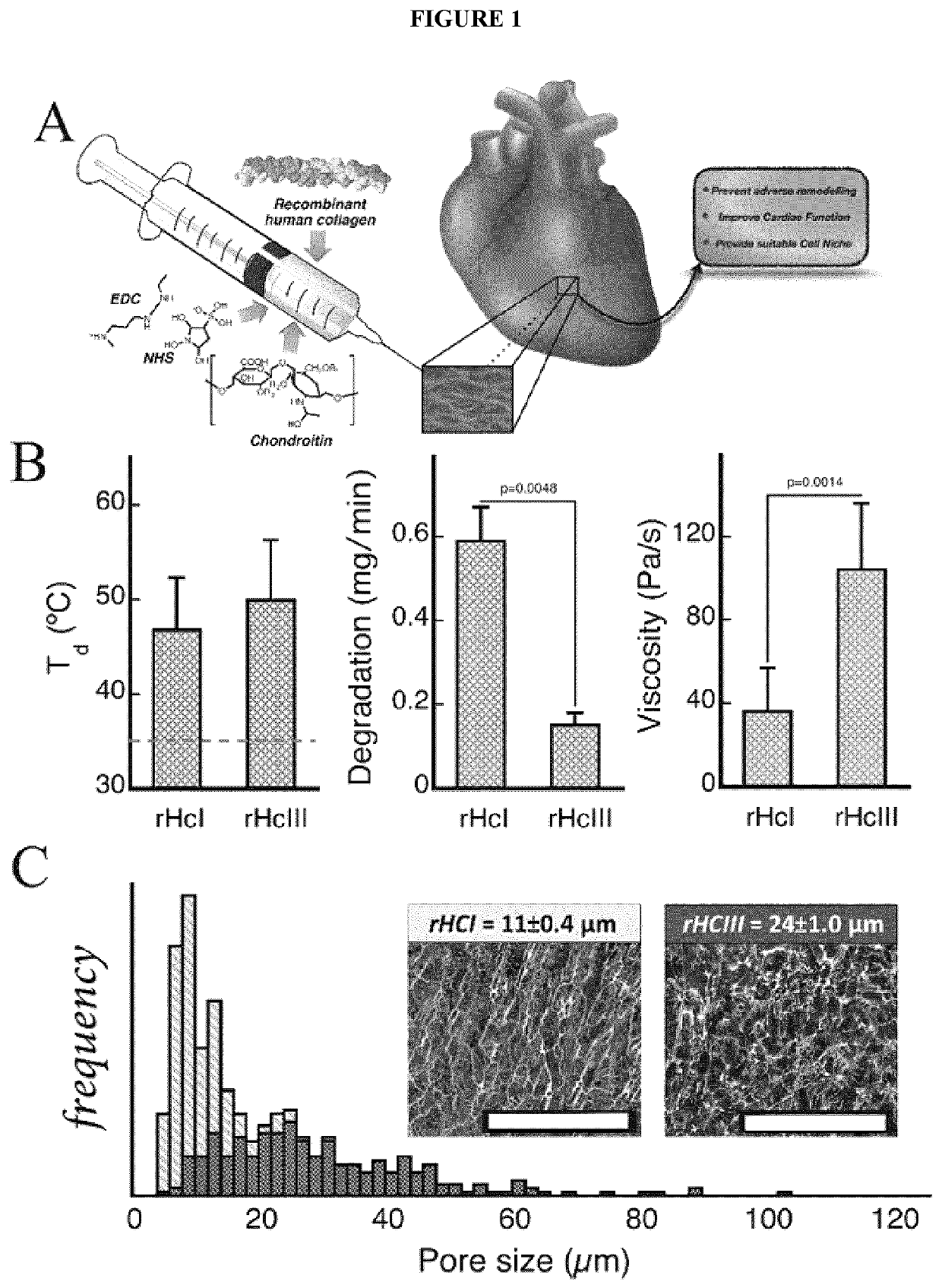
Biocompatible hydrogel compositions and uses thereof
ActiveUS20200345897A1Avoid tissue damageFunction increaseOrganic active ingredientsConnective tissue peptidesRepair tissueCollagen Type III
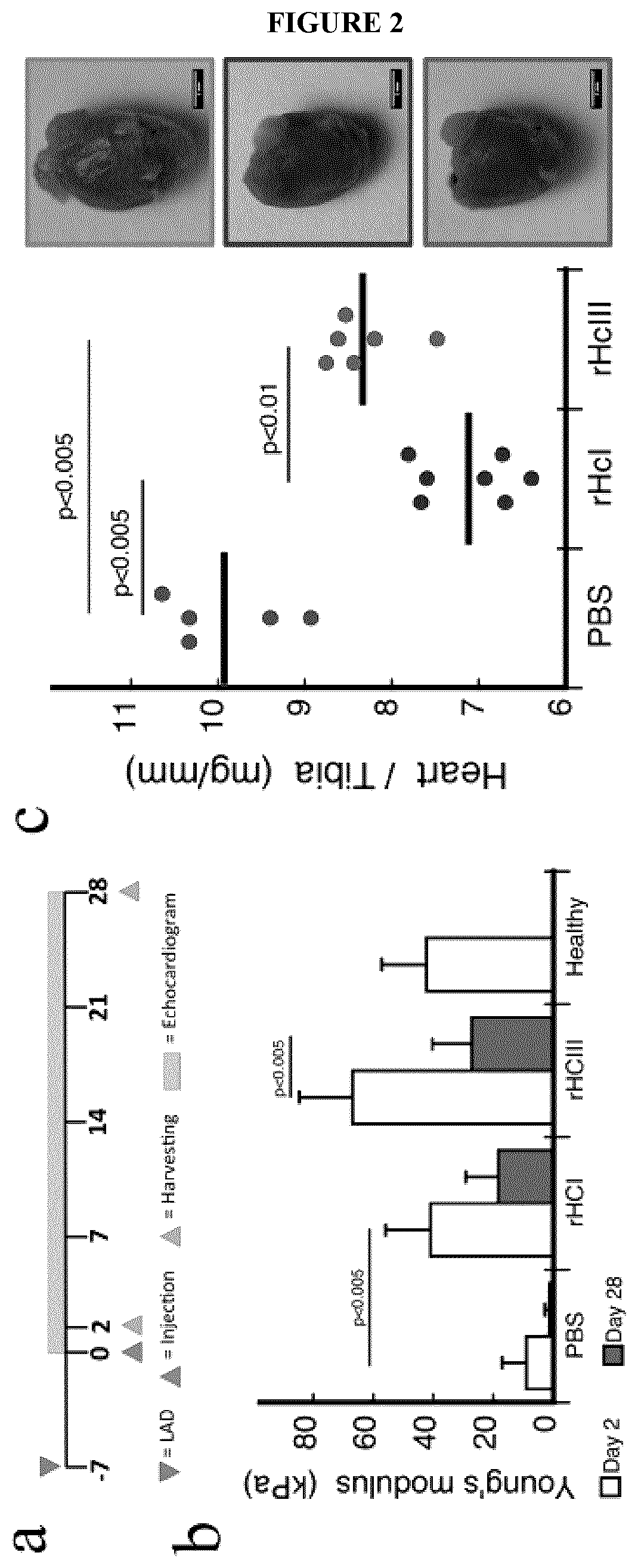
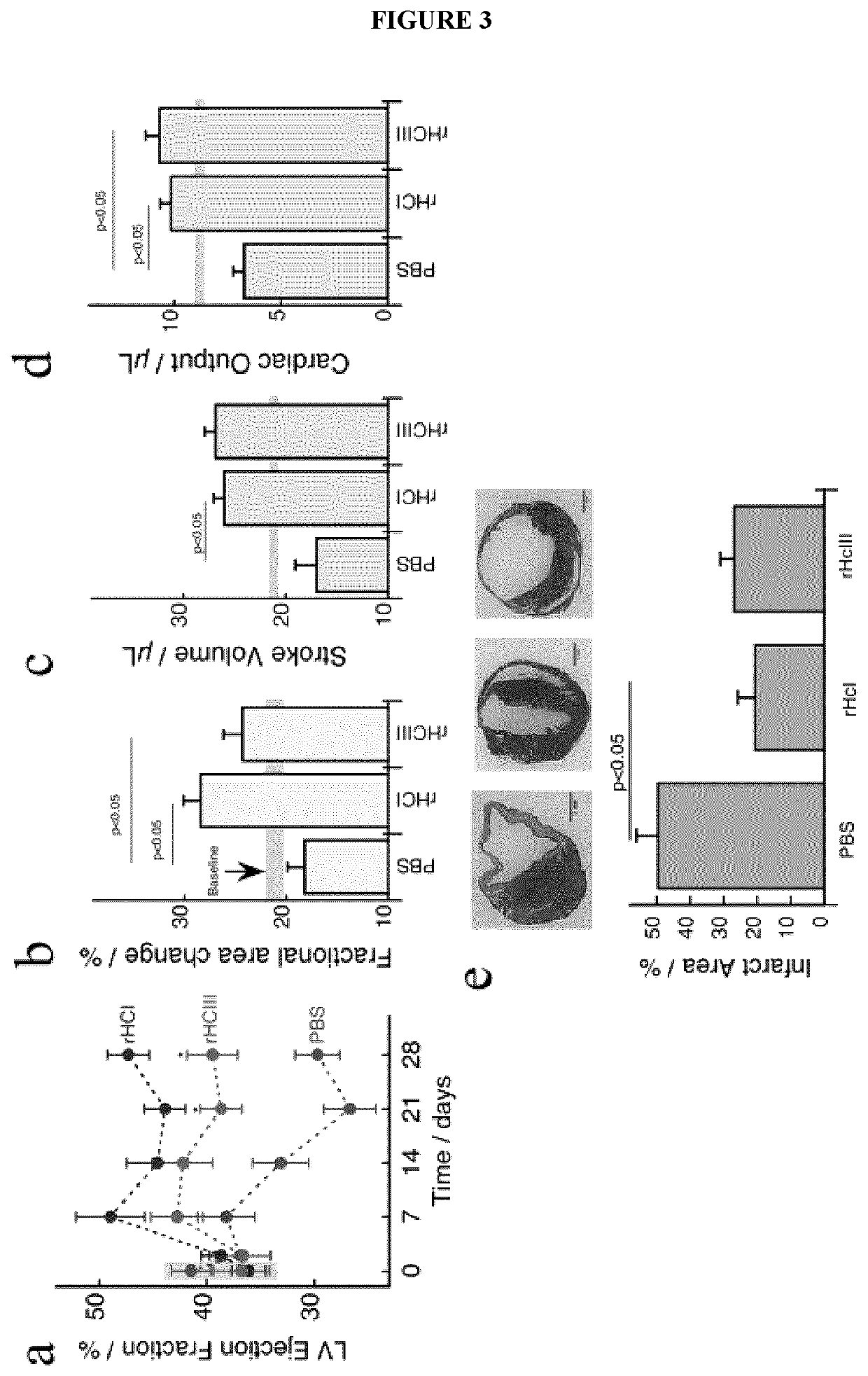
Provided herein are biocompatible and / or biodegradable hydrogel compositions comprising native collagen and chondroitin sulfate, the collagen and chondroitin sulfate being chemically cross-linked thereby forming a matrix. The native collagen may comprise recombinant human collagen type I (rHCI), recombinant human collagen type III (rHCIII), or a combination thereof, for example. Methods and uses thereof for regeneration or repair of tissue, improvement of tissue function, mechanical stabilization of tissue, prevention of tissue damage, or prevention of tissue loss of function are described, particularly with respect to cardiac tissue and myocardial infarction events.
Owner:OTTAWA HEART INST RES
PIIINP neo-epitope assay
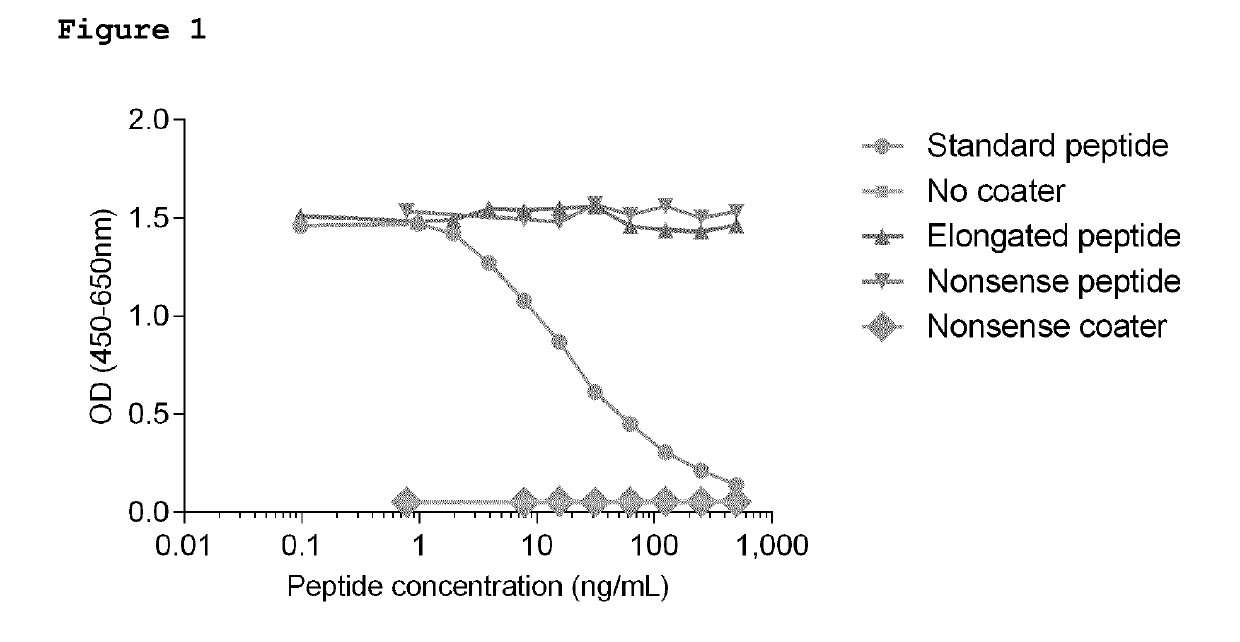
ActiveUS9726674B2Immunoglobulins against animals/humansDisease diagnosisMonoclonal antibodyCollagen Type III
Provided is a monoclonal antibody specifically reactive with a C-terminal neo-epitope of PIIINP comprised in a C-terminal amino acid sequence CPTGXQNYSP-COOH (SEQ ID NO: 4) in which X is Gly or Pro, and where the monoclonal antibody does not recognize or bind an elongated version of the C-terminal amino acid sequence CPTGXQNYSPQZ-COOH (SEQ ID NO: 5), in which Z is absent or is one or more amino acids of the sequence of collagen type III. Also provided is a method of immunoassay for detecting in a biological sample the C-terminal neo-epitope of PIIINP generated by N-protease cleavage of intact type III procollagen, by contacting the sample with the monoclonal antibody, and determining the amount of binding of the antibody.
Owner:NORDIC BIOSCI
Biochemical marker for CVD risk evaluation
The invention relates to a biochemical marker for CVD risk evaluation and a method of bioassay for the quantification of peptide fragments comprising a neo-epitope formed by cleavage of a protein of an atherosclerotic plaque such as lumican, versican, perlecan, decorin, biglycan, collagen type III, CRP, ApoE, or elastin, by a proteinase, said comprises contacting a sample such as urine or serum with an antibody reactive with the neo-epitope and determining the level of binding of said immunological binding partner to peptide fragments in said sample. The assay is predictive of risk of cardiovascular disease events.
Owner:NORDIC BIOSCI
Method for preparing medical III type collagen
InactiveCN101717804BRich sourcesLow priceConnective tissue peptidesPeptide/protein ingredientsWater bathsSalting out
Owner:SICHUAN UNIV
Preparation method for collagen membrane inoculated with autologous mesenchymal stem cells
InactiveCN106967678AStop the spreadGood adhesionSkeletal/connective tissue cellsProsthesisTherapeutic effectCollagen Type III
The invention discloses a preparation method for a collagen membrane inoculated with autologous mesenchymal stem cells. The preparation method comprises the following concrete steps: carrying out uniform mixing so as to obtain a marrow suspension; preparing autologous serum; adding the marrow suspension into a centrifuge tube containing a cell separating medium and carrying out gradient centrifugation to obtain a sediment which is target cells; adding the target cells into 15 ml of a DMEM high-glucose medium containing FGF-2, the autologous serum and gentamycin, carrying out culturing in an incubator and replacing the DMEM high-glucose medium once every three days; after cell culture for 3 to 4 weeks, carrying out passage three times; subjecting cells to resuspension with normal saline; slowly adding the cell suspension onto the rough surface of a prepared pig-sourced I / III type collagen membrane with a plastic sleeve drop by drop; and allowing the collagen membrane to adsorb cells for 10 to 15 min so as to obtain the collagen membrane inoculated with the autologous mesenchymal stem cells. The collagen membrane inoculated with autologous mesenchymal stem cells prepared in the invention has the advantages of good treatment effect, convenience in treatment, low cost, etc.
Owner:安徽安龙基因科技有限公司
Composite bone repairing material and preparation method thereof
ActiveCN109675117APromote proliferationPromote formationTissue regenerationProsthesisForeign matterSynthesis methods
The invention discloses a composite bone repairing material and a preparation method thereof. The composite bone repairing material is compounded by taking hydroxyapatite and two different types of collagen as matrices through an in-situ synthesis method; the ratio of the total collagen to the hydroxyapatite in parts by weight ranges from 1:9 to 1:1; the ratio of type-I collagen to type-III collagen in parts by weight is (85 to 99.99):(0.01 to 15). The composite bone repairing material has the advantages that the different types of collagen are involved in the composite bone repairing material, no foreign matter is introduced, and accordingly, low immunogenicity and high biocompatibility of the collagen can be maintained; different effects of the different types of collagen can be embodiedat the same time, and accordingly, the composite bone repairing material is multifunctional.
Owner:河北科硕生物技术有限公司
Kadsurae Caulis-loading I/III type collagen composite scaffold
The invention discloses a Kadsurae Caulis-loading I / III type collagen composite scaffold, which is prepared by using bovine source I type collagen, recombinant human source III type collagen, KadsuraeCaulis and PLGA as raw materials. The preparation process comprises: preparation of Kadsurae Caulis-loading PLGA microspheres, preparation of I / III type collagen composite scaffold, loading of Kadsurae Caulis-loading PLGA microspheres, and other steps. According to the present invention, the prepared composite scaffold integrates the characteristics of the I type collagen ribbon fiber structure and the III type collagen reticular fiber structure, further has characteristics of suitable three-dimensional network structure, good biocompatibility, no toxicity, degradability and low immunogenicity, integrates the osteoarthritis treatment effect of Kadsurae Caulis, and is the good medical material for osteoarthritis cartilage defect repair and treatment.
Owner:FUZHOU UNIV
Collagen type vii alpha 1 assay
InactiveUS20190195875A1Connective tissue peptidesImmunoglobulins against animals/humansCollagen Type IIICollagen VI
A method of immunoassay for detecting in a biological sample a fragment of collagen type VII alpha 1 comprising an N- or C-terminal neo-epitope, the method comprising contacting the biological sample comprising the fragment of collagen type VII alpha 1 comprising the N- or C-terminal neo-epitope with an antibody of the invention, and determining the amount of binding of the antibody
Owner:NORDIC BIOSCI
Cardiac fibroblast-derived extracellular matrix and injectable formulations thereof for treatment of ischemic disease or injury
PendingUS20190111113A1Effective treatmentReduce injuriesAntibacterial agentsPowder deliveryDiseaseExtremity injury
Owner:WISCONSIN ALUMNI RES FOUND
New use of sugar -based decorative polyphenol compounds
ActiveCN107951871BImprove antioxidant capacityFunction increaseHydroxy compound active ingredientsAntinoxious agentsLung protectionOxygen
The invention discloses an application of glycosyl modified polyphenol compounds as drugs for relieving paraquat poisoning and treating pulmonary fibrosis. The glycosyl modified polyphenol compounds have the structure shown in the description. The glycosyl modified polyphenol compounds have a good oxidation resistance function and a lung protection function and have a notable protection function on lung injury caused by paraquat, and a mechanism is related with reduction of formation of oxygen radicals, relieving of oxidative stress and inflammatory reaction, increase of content of GSH in blood serum and SOD in lung tissue and reduction of content of MDA and HYP in lung tissue. The glycosyl modified polyphenol compounds can effectively inhibit bleomycin-induced pulmonary fibrosis of mice,and the mechanism is related with reduction of expression of TGF-beta 1, IL-6, alpha-SMA, P-smad2, type I collagen and type III collagen in lung tissue, increase of content of GSH and SOD in blood serum and reduction of the content of HYP in lung tissue.
Owner:天津海润家和创新医药研究有限责任公司
Biochemical markers for CVD risk assessment
The present invention relates to biochemical markers for CVD risk assessment and bioassays for the quantification of peptide fragments containing neo-epitopes that are cleaved by proteases from proteins of atherosclerotic plaques For example, leucin, versican, perlecan, decorin, biglycan, collagen type III, CRP, ApoE or elastin, the method comprises the sample For example urine or serum is contacted with antibodies reactive to the neo-epitope and the level of binding of the immunological binding partner to the peptide fragment in the sample is determined. The assay predicts the risk of a cardiovascular disease event.
Owner:NORDIC BIOSCI
PIIINP Neo-epitope Assay
ActiveUS20160061844A1Immunoglobulins against animals/humansDisease diagnosisProteinase activityMonoclonal antibody
Provided is a monoclonal antibody specifically reactive with a C-terminal neo-epitope of PIIINP comprised in a C-terminal amino acid sequence CPTGXQNYSP-COOH (SEQ ID NO: 4) in which X is Gly or Pro, and where the monoclonal antibody does not recognise or bind an elongated version of the C-terminal amino acid sequence CPTGXQNYSPQZ-COOH (SEQ ID NO: 5), in which Z is absent or is one or more amino acids of the sequence of collagen type III. Also provided is a method of immunoassay for detecting in a biological sample the C-terminal neo-epitope of PIIINP generated by N-protease cleavage of intact type III procollagen, by contacting the sample with the monoclonal antibody, and determining the amount of binding of the antibody.
Owner:NORDIC BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com