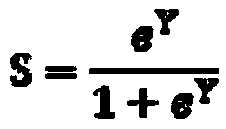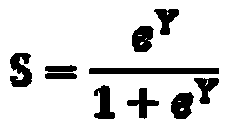Methods for diagnosing and evaluating non-alcoholic steatohepatitis
A steatohepatitis, non-alcoholic technology, used in biochemical equipment and methods, disease diagnosis, pharmaceutical formulations, etc., can solve problems such as unreasonable advice and trouble for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
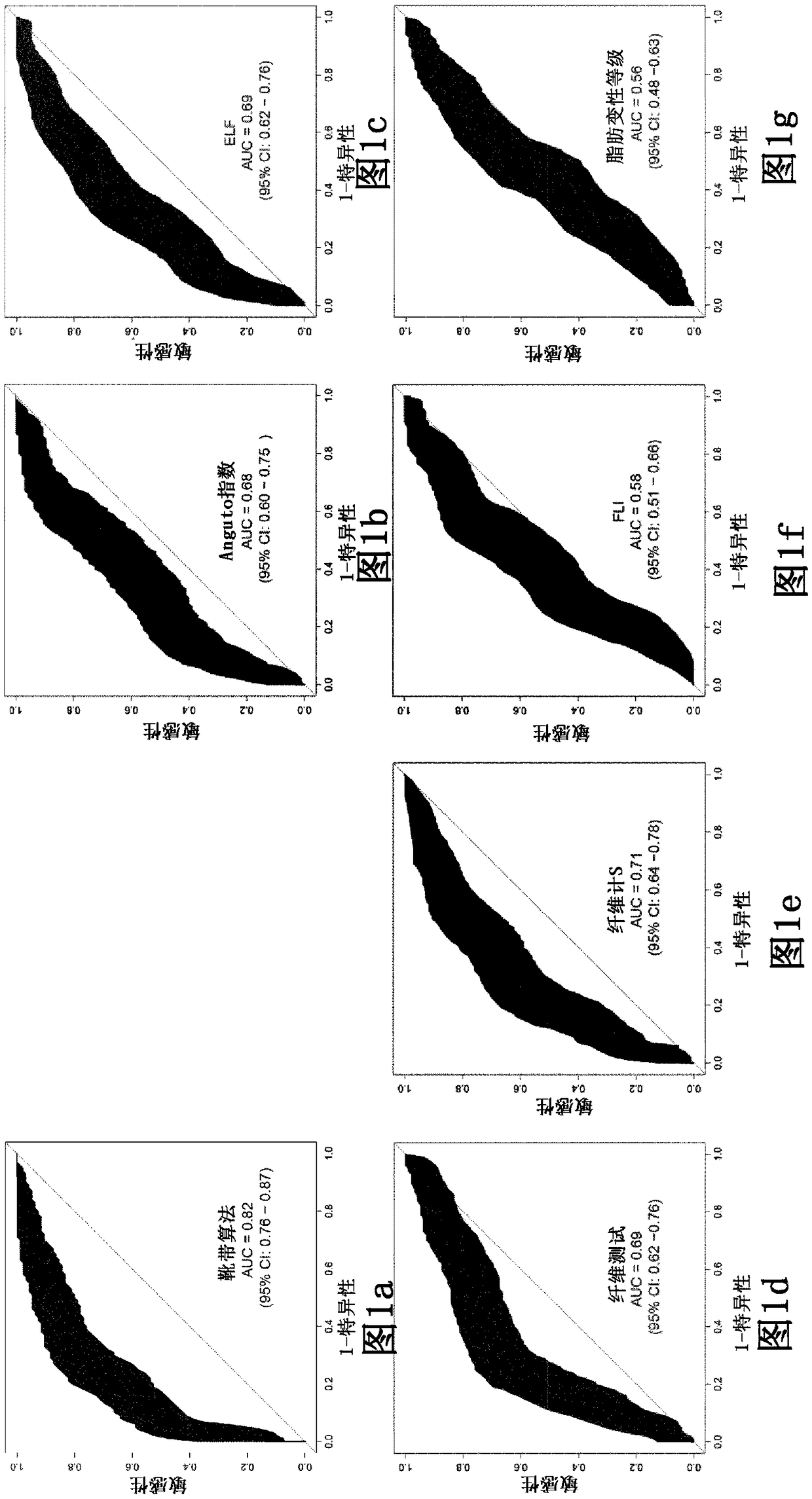
[0318] Sample Collection and Analysis
[0319] clinical research
[0320]The clinical trial (NASH phase 2 GOLDEN-505 trial (GFT505-212-7-NCT01694849) was a multicenter, randomized, double-blind, placebo-controlled study evaluating once-daily Elafibranor (1-[ 4-methylthiophenyl]-3-[3,5-dimethyl-4-carboxydimethylmethoxyphenyl]prop-2-en-1-one) on nonalcoholic steatohepatitis Efficacy and safety of steatohepatitis in (NASH) patients.
[0321] After appropriate exclusion of other etiologies of liver disease, a liver biopsy was performed to confirm the diagnosis of NASH. NASH was diagnosed as steatohepatitis as assessed by liver biopsy within 6 months before randomization. Confirmation of steatohepatitis was based on central reading of liver biopsy. Patients with NASH were defined as NAS ≥ 3, including steatosis grade ≥ 1, hepatocellular ballooning ≥ 1, and lobular inflammation ≥ 1.
[0322] The study was approved by the appropriate regulatory agency at each participating cen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com