Yeast recombinant human type I collagen alpha 1 chain protein, synthesis method and application thereof
A collagen and catenin technology, applied in the field of bioengineering, can solve the problems of difficult purification process, complex operation of purification process, difficulty in improving purity, etc., and achieve the effect of improving cutting efficiency, simple fermentation process and short fermentation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] In Example 1, the preparation of recombinant human type I collagen α1 chain protein was carried out, and the experimental steps were as follows:
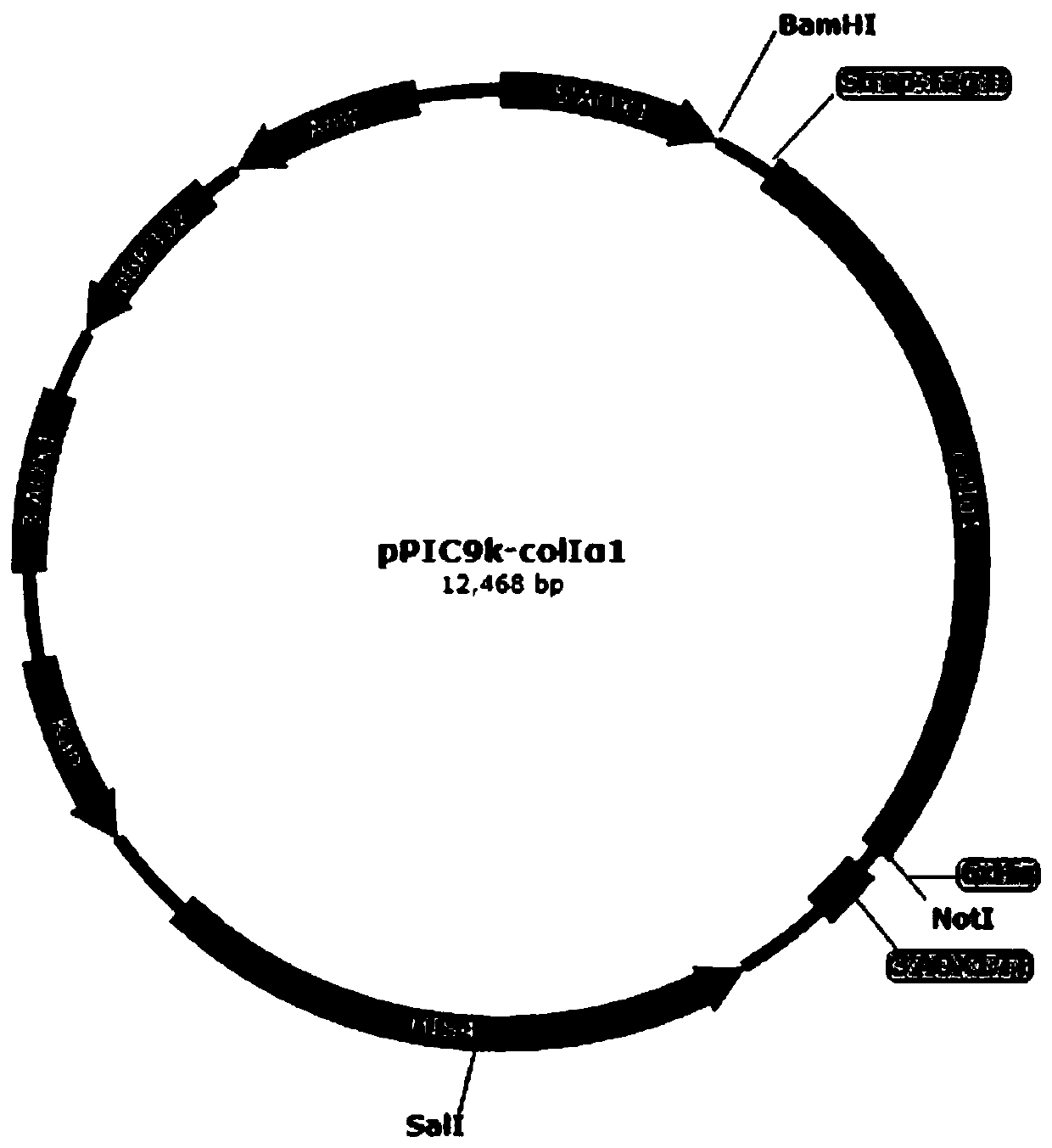
[0062] 1. Synthesize the gene sequence of human type I collagen α1 chain mature peptide: under the condition of not changing the amino acid sequence of human type I collagen α1 chain mature peptide, the corresponding gene sequence corresponding to Pichia pastoris is not commonly used The codons were optimized to the preferred codons of Pichia pastoris, and DNASTAR software was used to optimize the gene sequence, and the restriction endonuclease sites such as ApaI, Bgl II, BamHI, EcoR I, Not I, Sac I, Sal I were removed, The gene sequence of human type I collagen α1 chain mature peptide was synthesized, see SEQ ID NO.3;
[0063]2. Prepare the modified plasmid pPIC9ks containing the Strep-Tag II coding sequence: first use the pPIC9k plasmid as a template, and use primers P1 and P2 to perform PCR amplification, and the product l...
Embodiment 2
[0141] An example of the use of yeast recombinant human type I collagen α1 chain protein in cosmetic and skin care products for external use is now given:
[0142] According to the following mass ratio, use purified water as a solvent to dissolve all raw materials, and stir well to form a colorless, odorless and transparent moisturizing skin care essence;
[0143] Among them, glycerin 2%, butanediol 2%, sodium hyaluronate 0.5%, recombinant human type I collagen α1 chain protein 0.5%, vitamin C ethyl ether 0.5%, vitamin C sodium phosphate 0.5%, dipotassium glycyrrhizinate 0.3%, EDTA·Na 2 0.5%, silk peptide 0.5%, β-glucan 0.3%, Luba oil 0.3%, water-soluble azone 0.5%, and the rest is purified water.
[0144] How to use: After cleansing in the morning and evening, apply directly to the face, gently pat until fully absorbed.
Embodiment 3
[0146] Now provide the embodiment that the recombinant human source type I collagen α1 chain protein is used in cosmetic and skin care products for external use:
[0147] According to the following mass ratio, use purified water as a solvent to dissolve all raw materials, and stir well to form a colorless, odorless and transparent moisturizing skin care essence;
[0148] Glycerin 1%, Butylene Glycol 1%, Sodium Hyaluronate 0.01%, Recombinant Human Type I Collagen α1 Chain Protein 0.01%, Vitamin C Ethyl Ether 0.01%, Vitamin C Sodium Phosphate 0.01%, Dipotassium Glycyrrhizinate 0.1%, EDTA·Na 2 0.01%, silk peptide 0.01%, β-glucan 0.1%, Luba oil 0.1%, water-soluble azone 0.1%, and the rest is purified water.
[0149] How to use: After cleansing in the morning and evening, apply directly to the face, gently pat until fully absorbed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| capacitance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com