Methods for promoting cell reprogramming
a cell reprogramming and cell technology, applied in the field of induced pluripotent stem cells, can solve the problems of low reprogramming efficiency, low reprogramming efficiency, and extreme low efficiency of the reprogramming process, so as to avoid immune rejection, increase the number of cells, and prevent or improve the effect of ips
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mouse Embryonic Fibroblast Reprogramming
[0135]293FT cell culture and lentiviral infection: 293FT cells cultured in a growth medium (DMEM, 10% FBS, 500 μg / ml G418) are seeded in 96-well (primary screen) or 24-well plates (secondary and tertiary screens) at 2.2×104 cells or 1.32×105 cells per well, respectively, and incubated at 37° C., 5% CO2 for subsequent lentiviral infection. The RNAi Consortium (TRC) mouse kinase activity lentiviral shRNA library is purchased from ThermoScientific (RMM4957). 293FT cells are transfected with pLKO.1 plasmid DNA-expressing shRNAs targeting the mouse kinase gene family using Lipofectamine™ 2000 according to the manufacture's instructions. On the following day (Day 2), the medium is replaced with fresh 293FT medium containing 1.1% BSA, and the cells are incubated at 37° C., 5% CO2 for an additional two days for lentivirus production. On Day 4, 40 μl (96-well) or 200 μl (24-well) of lentivirus-containing medium is transferred to the retrovirus-infected...
example 2
Differentiation from iPS Cells
[0144]In Vitro Differentiation: To induce spontaneous differentiation of iPSCs, iPS clones that show ES-like proliferation and morphology undergo embryoid body formation using the hang-drop method. CCE ES cells. A mouse embryonic stem cell line (StemCell Technologies), is used as a control. On Day 3, embryoid bodies are transferred to gelatin-coated 6-well plates and cultured with EB medium (DMEM, 15% FBS, MEM-NEAA, L-Glutamine, MTG) for another 11 days. On Day 14, cells are fixed with 4% paraformaldehyde for immunostaining with the following antibodies: anti-AFP (Abcam, ab7751), anti-Beta III tubulin (R&D systems, MAB1368), and anti-alpha actinin (Sigma, A7811).
[0145]Immunostaining: Established iPSC clones are fixed in 4% paraformaldehyde and permeablized by 0.1% Triton-X-100 in PBS. Cells are then blocked in 5% BSA in PBS containing 0.1% Triton X-100 for 1 hour at room temperature. Primary antibody is diluted from 1:100 to 1:400 in 2.5% BSA PBS contai...
example 3
Function of Kinases for Somatic Cell Reprogramming
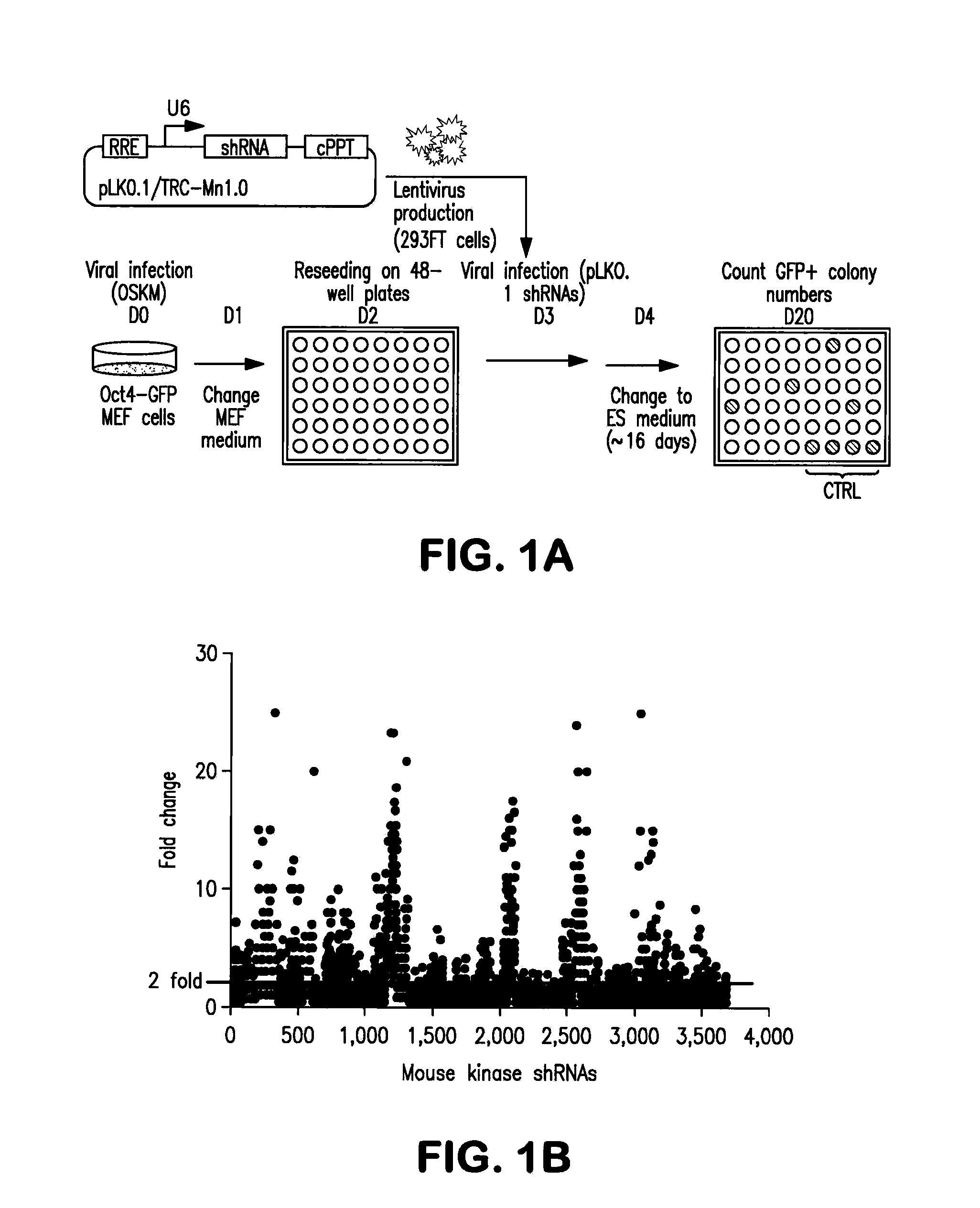
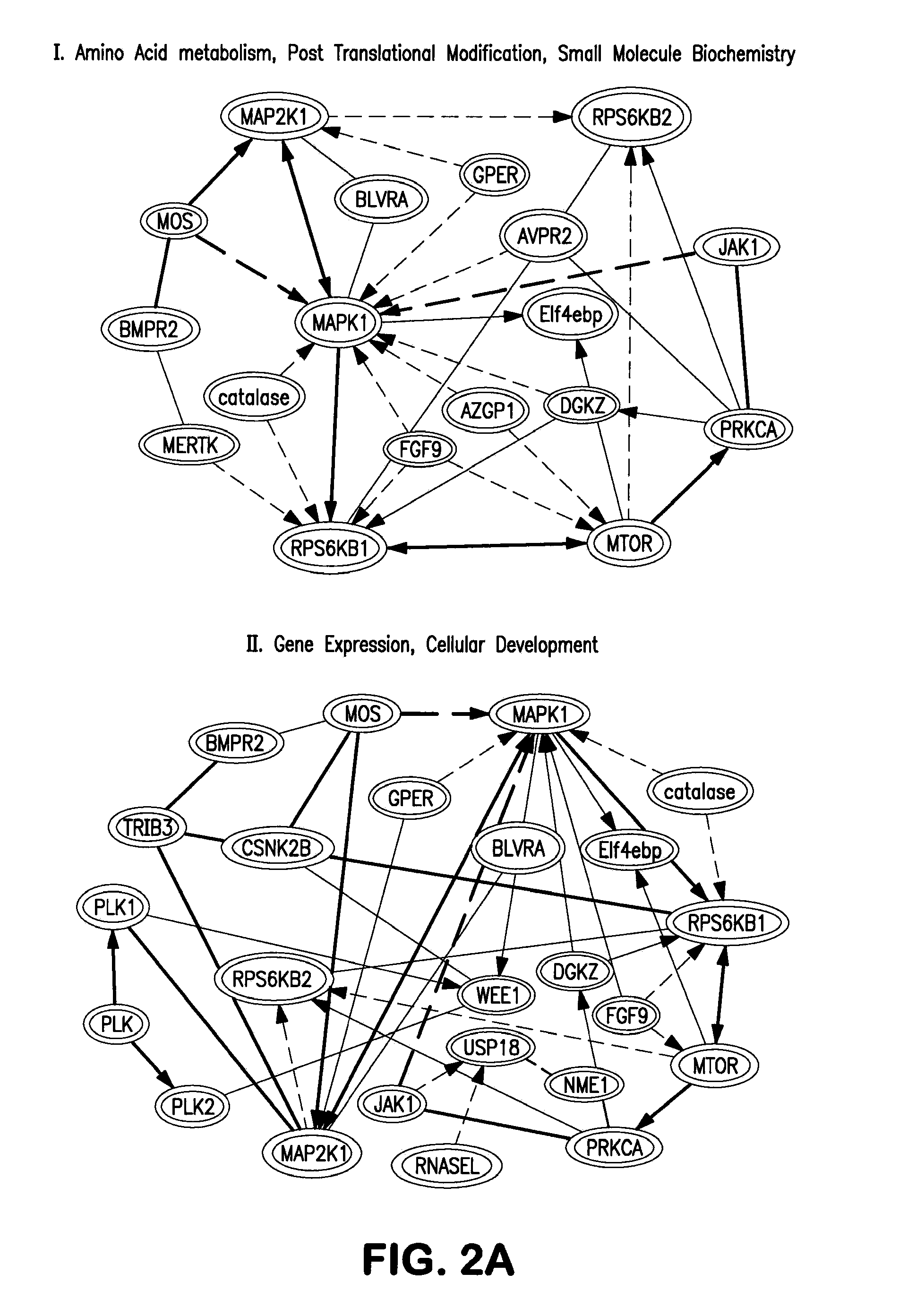
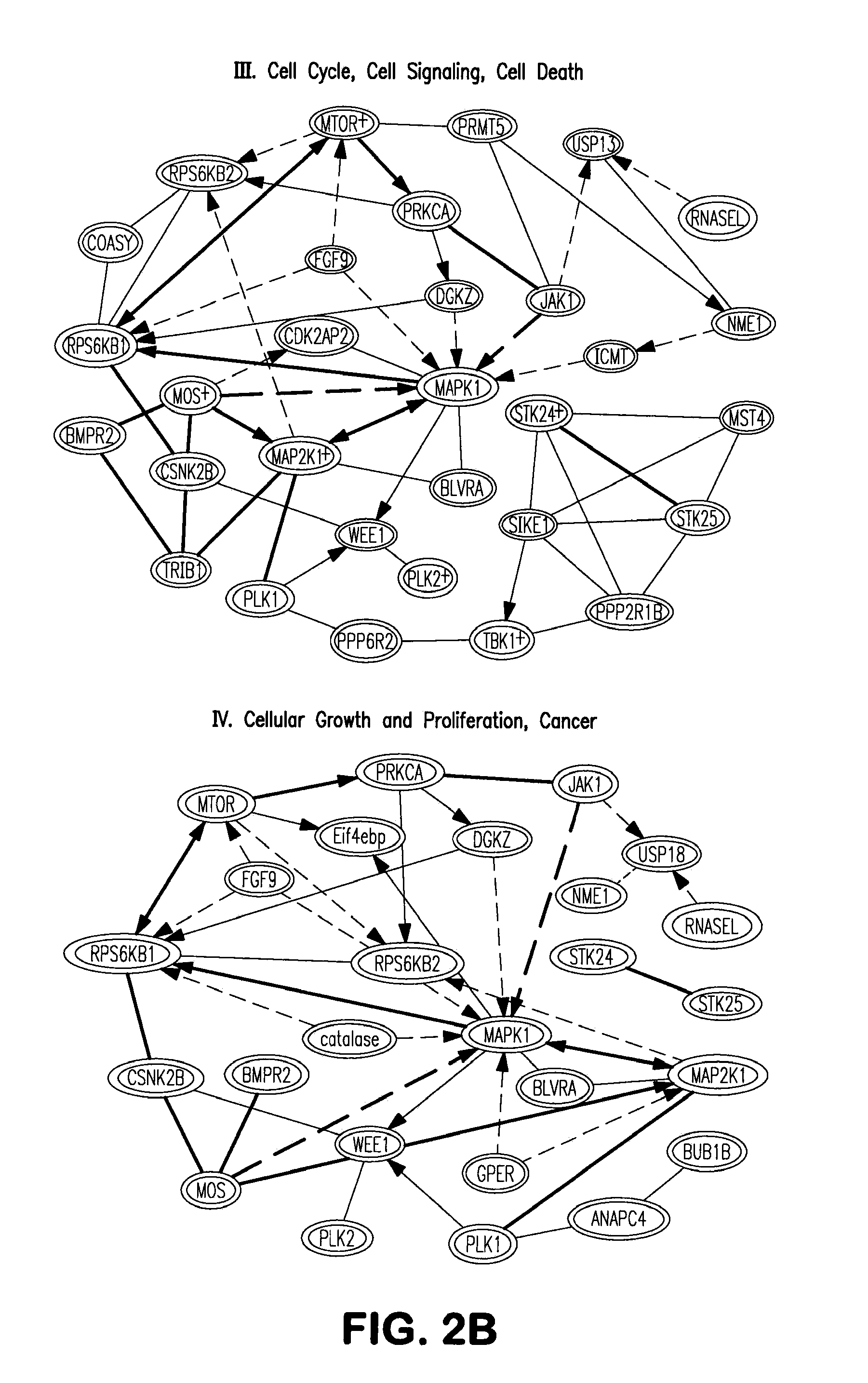
[0149]To determine the function of kinases that regulate somatic cell reprogramming to iPSCs, the present invention provides a whole kinome RNAi screen (FIG. 1). Mouse embryonic fibroblast (MEF) stably integrated POU5F1 (OCT4) -driven GFP construct is used as a reporter cell line to quantitatively monitor iPSC generation. Oct4-GFP MEFs are transduced with a retrovirus of four pluripotency factors, Oct4, Sox2, Klf4, and c-Myc (OSKM), and GFP colonies are quantified. Using OCT4-GFP MEFs, a lentiviral shRNA library targeting 734 kinase genes covering the entire mouse kinome is screened. 3,686 shRNA lentiviruses are prepared in 293FT cells and individually screened in iPSC generation assays (FIG. 1). Dot-plot represents the results of the primary screen, where GFP+ colony counts are shown as fold changes after normalization with that of control pLKO lentiviral infected cells.
[0150]A 2-fold enhancement in iPSC generation by a kinase knock...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com