Degradable medicine slow release function composite enteric stent and making method thereof
A production method and functional technology, applied in the field of medical devices, can solve the problems of stent slippage, intestinal perforation, bleeding, inability to achieve the effect of treatment, endangering the life safety of patients, etc., and achieve a stable and efficient production method. The effect of promotion, excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
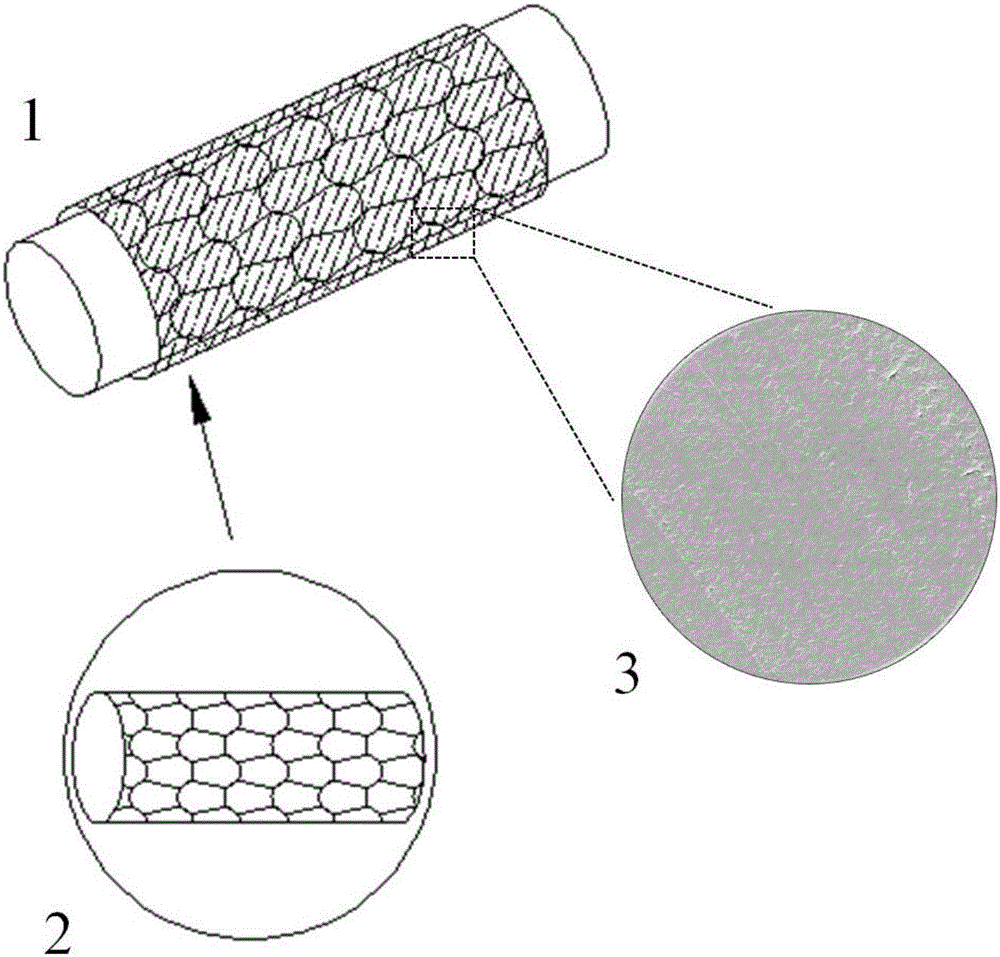
[0035] Inner tubular stent: The radial force is 0.3-3cN / mm prepared by the weft knitting method, the tube wall is smooth, and the tubular stent has good resilience. The selected scaffold material is polydioxanone (PDO), which has high strength (tensile strength ≥ 400N / mm2, breaking strength ≥ 600MPa), high elastic modulus, and a single filament diameter of about 0.2mm. The model of the selected knitting machine is No. 27 small-diameter circular weft loom, and the process parameters: the diameter of the needle cylinder is 30mm, 27 needles, the yarn tension is 2.1Kgf, the fabric tension is 150cN, the bending triangle is 3mm, and the knitting speed is 3m / minute. The parameters of the prepared inner tubular stent: transverse density 20 longitudinal rows / 5cm, longitudinal density 21 rows / 5cm, coil length 6mm, total density 1200 coils / 25cm 2, fiber volume content 15%, underfill coefficient 15. Thereafter, the inner tubular stent is decontaminated, cleaned, and heat-set.
[0036]...
Embodiment 2
[0039] Inner tubular stent: The radial force is 0.3-3cN / mm prepared by braiding, the tube wall is smooth, and the tubular stent has good resilience. The selected scaffold material is PLGA monofilament, which has a large elastic modulus and a diameter of about 0.3mm. The model of the selected knitting machine is No. 27 small-diameter circular weft loom, and the process parameters: the diameter of the needle cylinder is 30mm, 27 needles, the yarn tension is 2.1Kgf, the fabric tension is 150cN, the bending triangle is 3mm, and the knitting speed is 3m / minute. Prepared bare stent, parameters: horizontal density 20 longitudinal rows / 5cm, vertical density 21 rows / 5cm, coil length 6mm, total density 1200 coils / 25cm 2 , fiber volume content 15%, underfill coefficient 15. Thereafter, the inner tubular stent is decontaminated, cleaned and shaped.
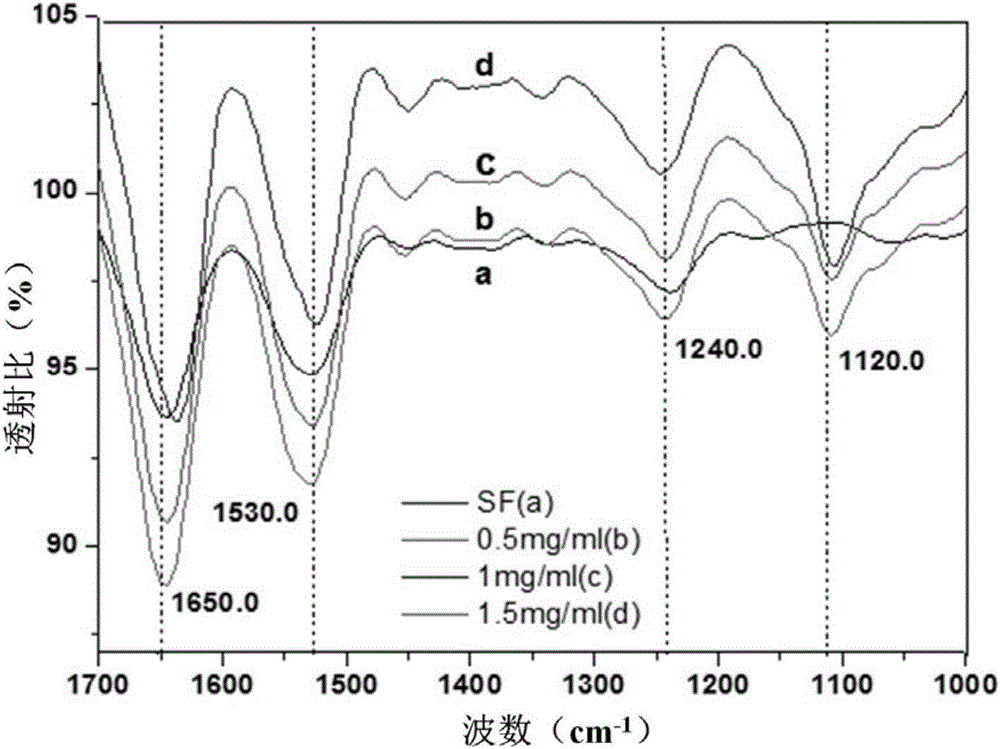
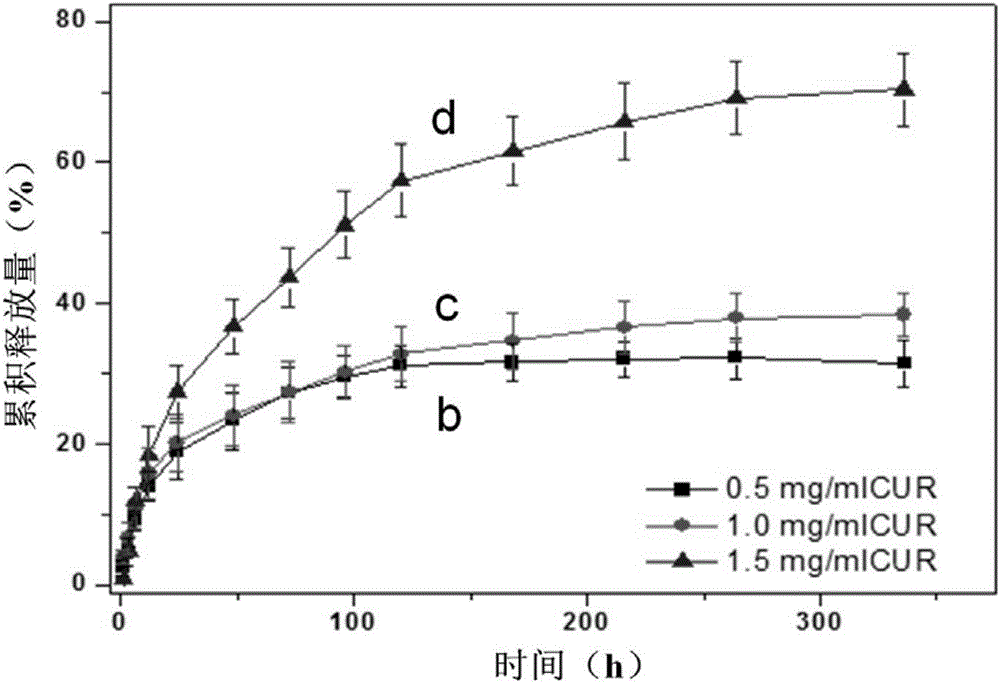
[0040] Outer drug film layer: pure silk fibroin solution (mass fraction is 15%), 5-fluorouracil (concentration is 15mg / ml), curcumin (co...
Embodiment 3
[0043] Inner tubular stent: The tubular stent with a radial force of 0.3-3cN / mm, smooth tube wall and good resilience is prepared by knitting. The selected scaffold material is PGA, which has a large elastic modulus and a single filament diameter of about 0.4mm. The model of the selected knitting machine is No. 27 small-diameter circular weft loom, and the process parameters: the diameter of the needle cylinder is 30mm, 27 needles, the yarn tension is 2.1Kgf, the fabric tension is 150cN, the bending triangle is 3mm, and the knitting speed is 3m / minute. The parameters of the prepared inner tubular stent: transverse density 20 longitudinal rows / 5cm, longitudinal density 21 rows / 5cm, coil length 6mm, total density 1200 coils / 25cm 2 , fiber volume content 15%, underfill coefficient 15. Thereafter, the inner tubular stent is decontaminated, cleaned, and heat-set.
[0044] Outer drug film layer: adopt silk fibroin solution drug loading and drying film forming process to obtain 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com