Insulin dose determination method and device based on growth hormone stimulation test
A growth hormone and determination method technology, applied in the field of clinical medicine, can solve the problems of test failure, affect the psychological state of patients, increase the workload of medical staff, etc., and achieve the effect of accurate insulin dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
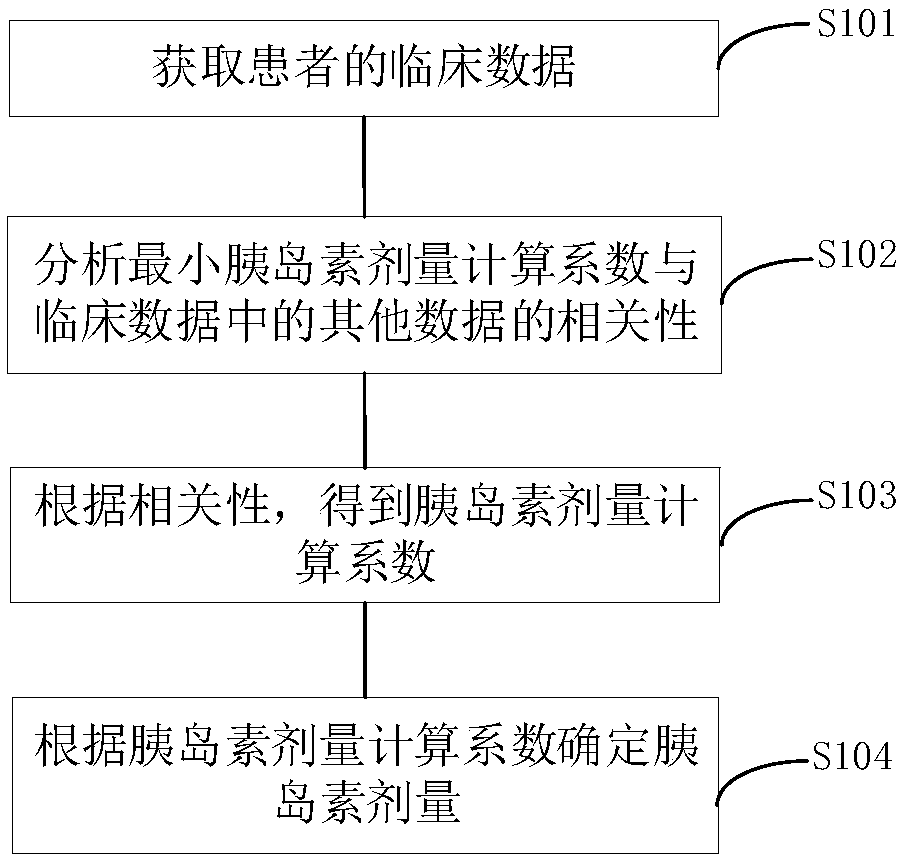
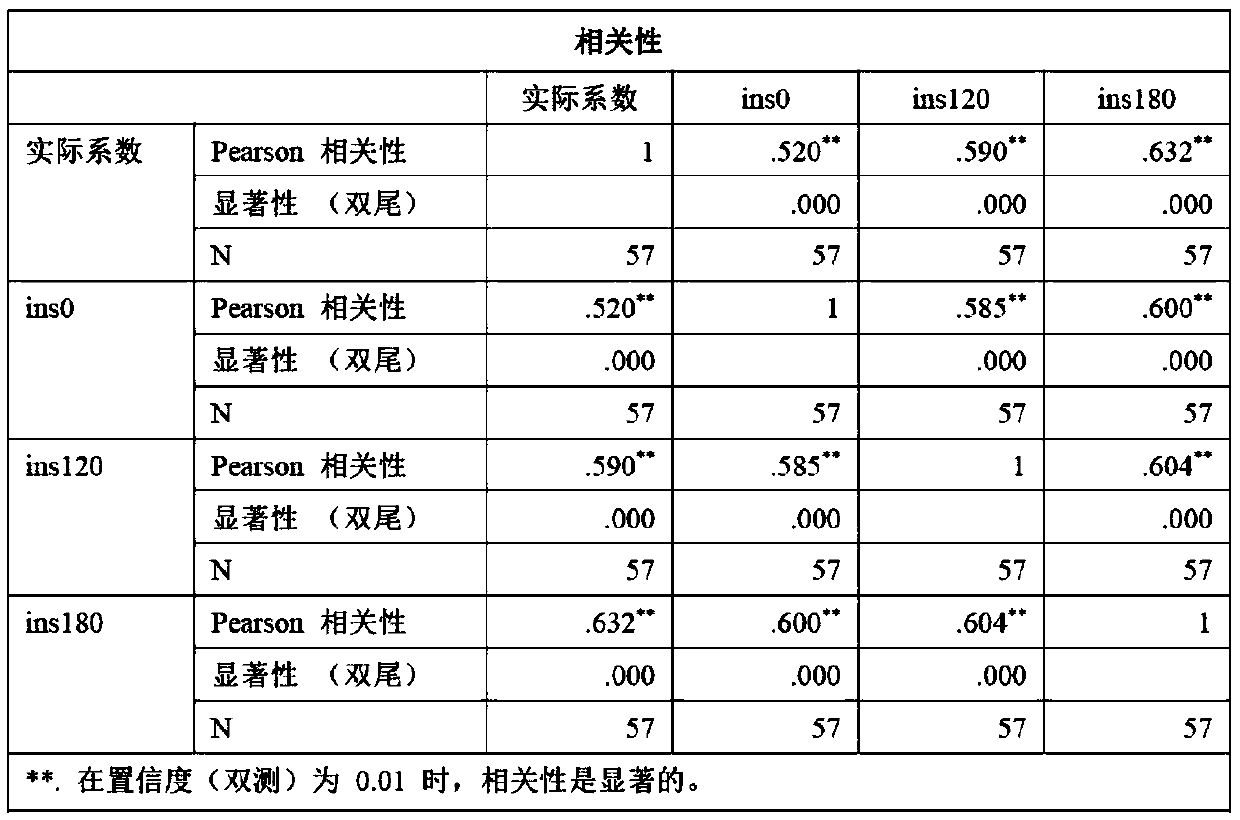
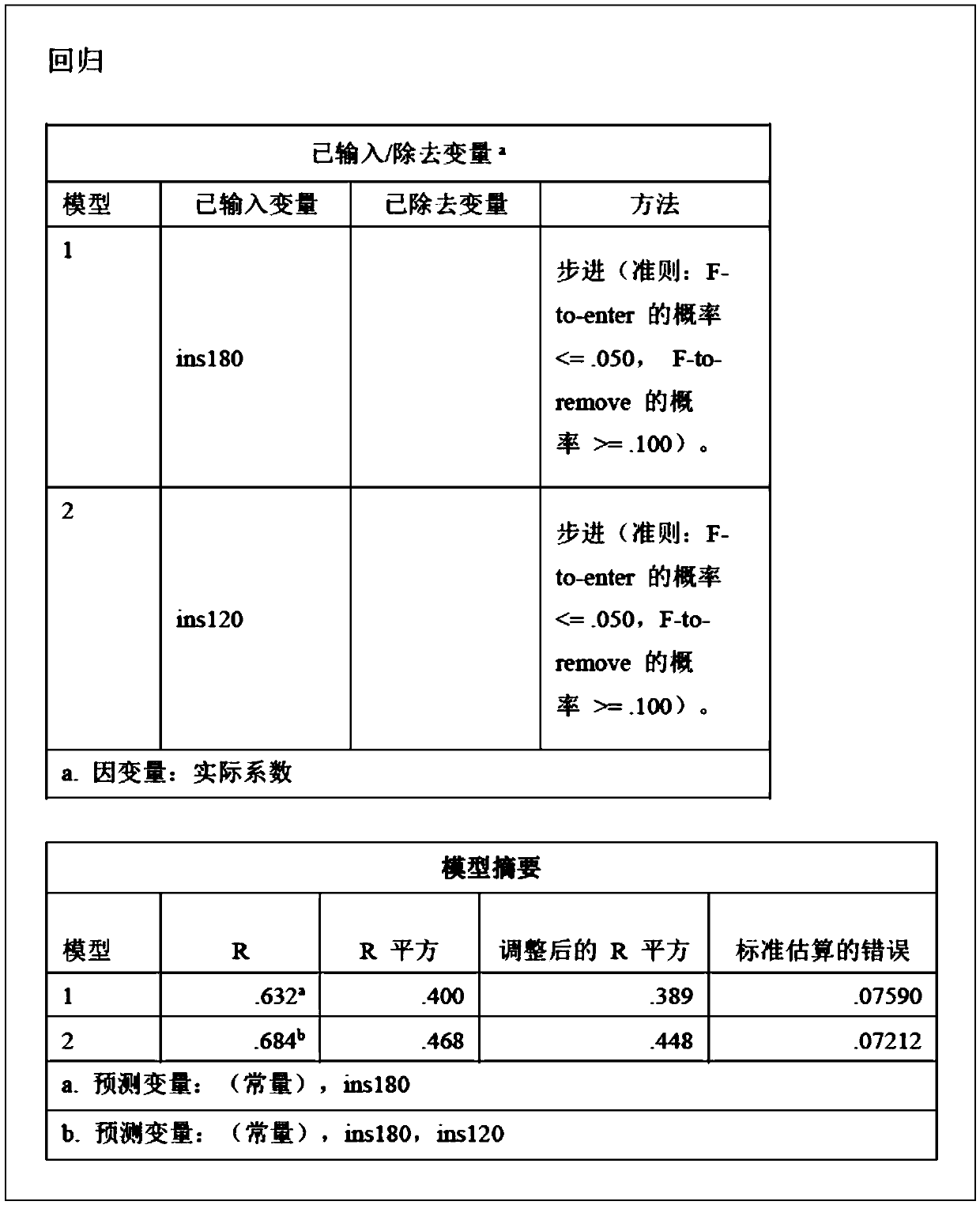
[0016] In order to better understand the embodiments of the present invention, the ITT test is first described below:
[0017] In the early morning, the patient is fasting, quiet, and awake on the bed, and the vital signs are monitored by the ECG monitor. From 8:00 to 9:00 in the morning, a subcutaneous venous channel is established in the elbow vein of the forearm and a three-way device is placed in the vein, and the intravenous injection (for example, Novo Nordisk China Pharmaceutical Co., Ltd.) biosynthetic human insulin 0.10-0.15U / kg, 5ml of venous blood was collected 30 minutes before injection, immediately before injection, and 30, 45, 60, 90, and 120 minutes after injection, and blood glucose , GH and cortisol detection, while monitoring glycogen (Note: Generally, blood glucose, GH, cortisol and glucose monitoring should also be taken when symptoms of hypoglycemia begin to appear). Closely monitor changes in blood sugar and record heart rate and blood pressure at each t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com