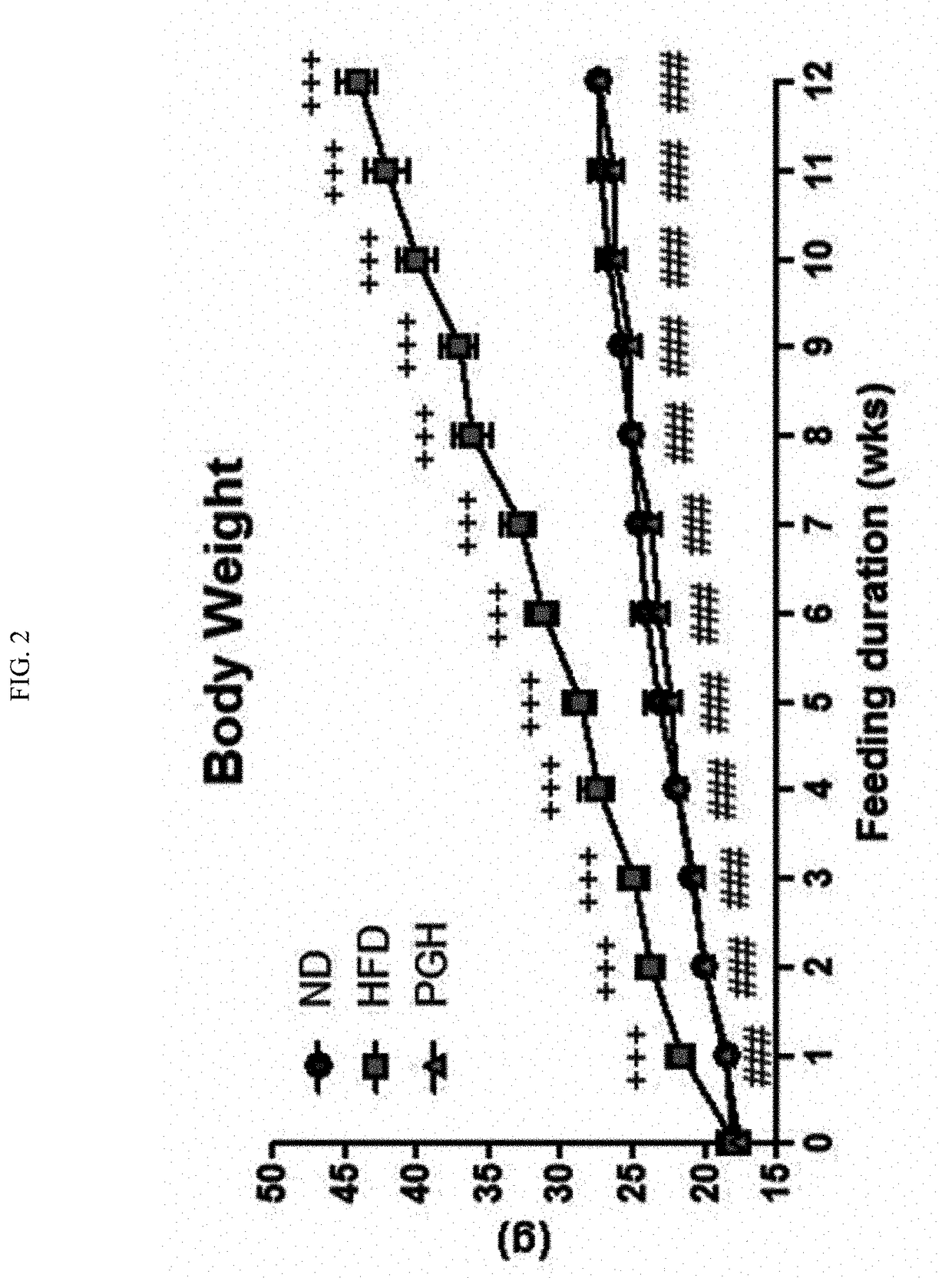
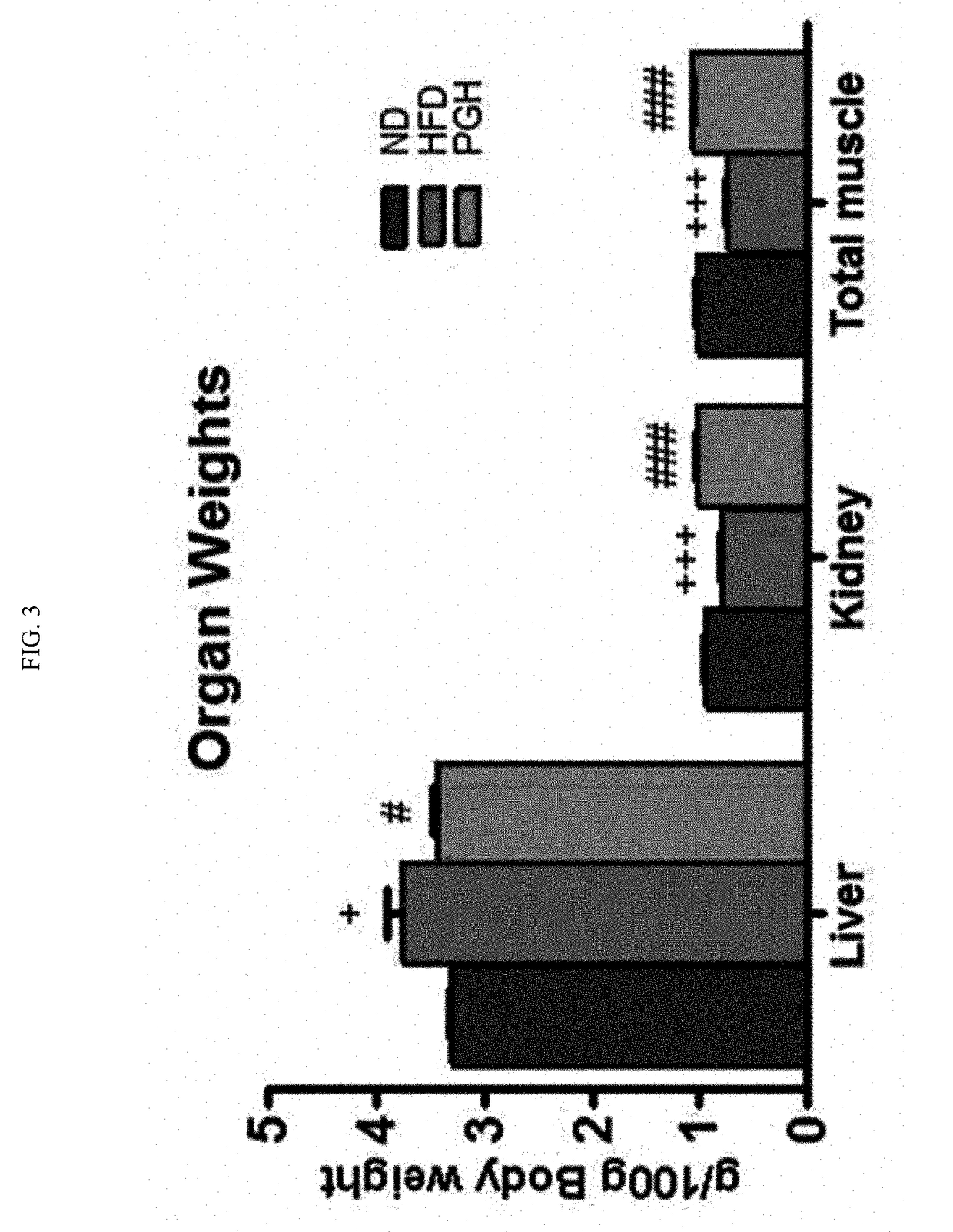
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "FBG - Fasting blood glucose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fasting blood glucose (FBG) is a blood test done to measure the amount of glucose present in the blood after an eight-hour fast. It is thus not affected by recent food intake.
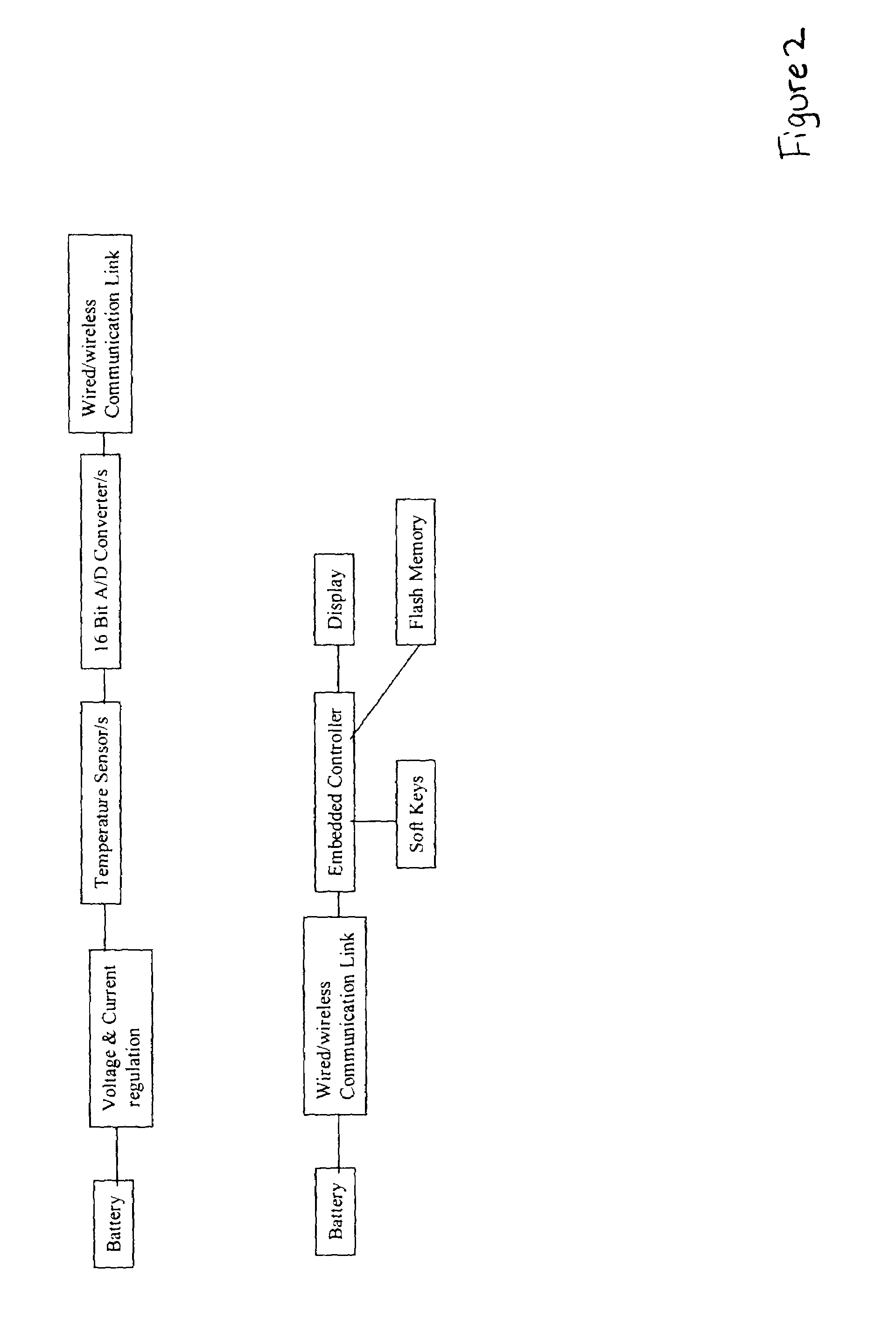
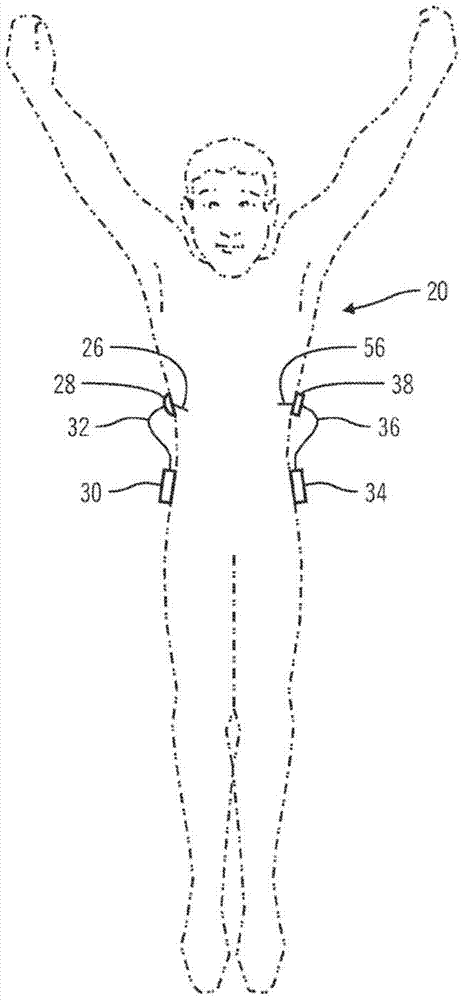
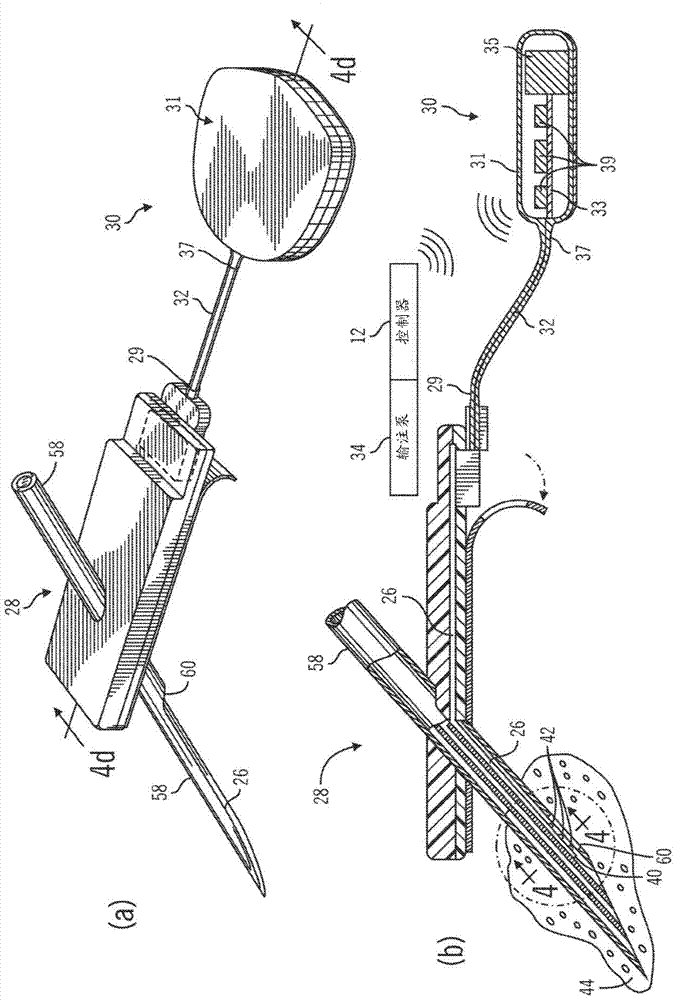
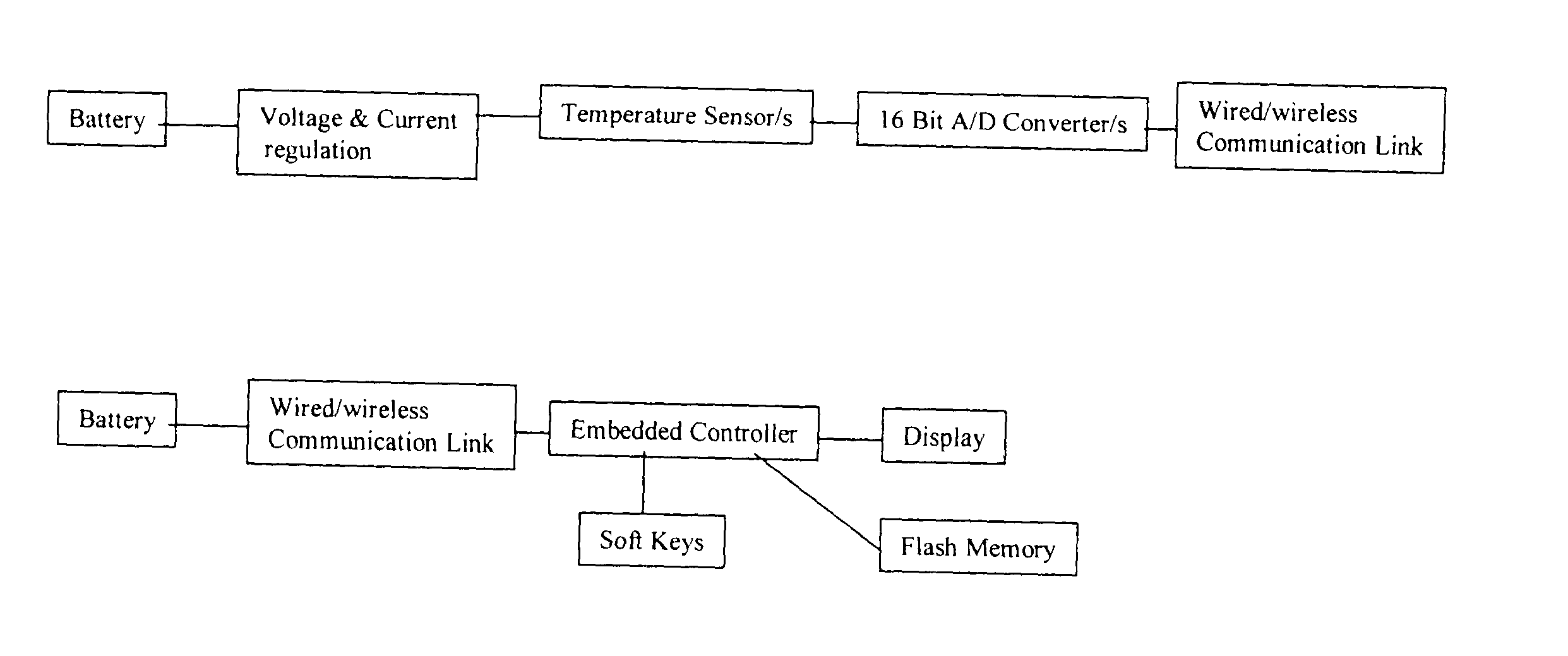
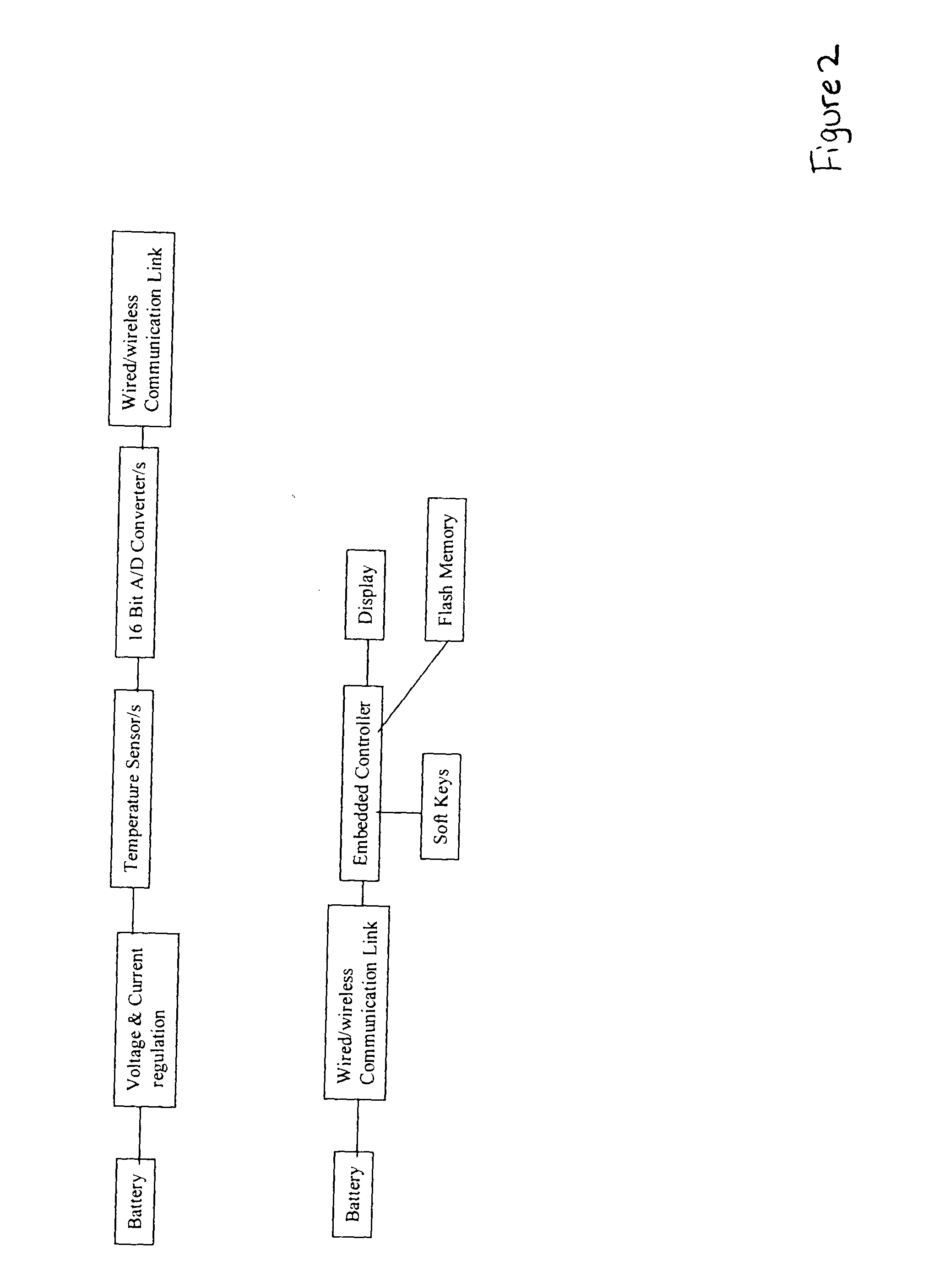
Non-invasive blood glucose monitoring system
ActiveUS6949070B2Low costReduce the numberSensorsTelemetric patient monitoringEmergency medicineGlucose polymers
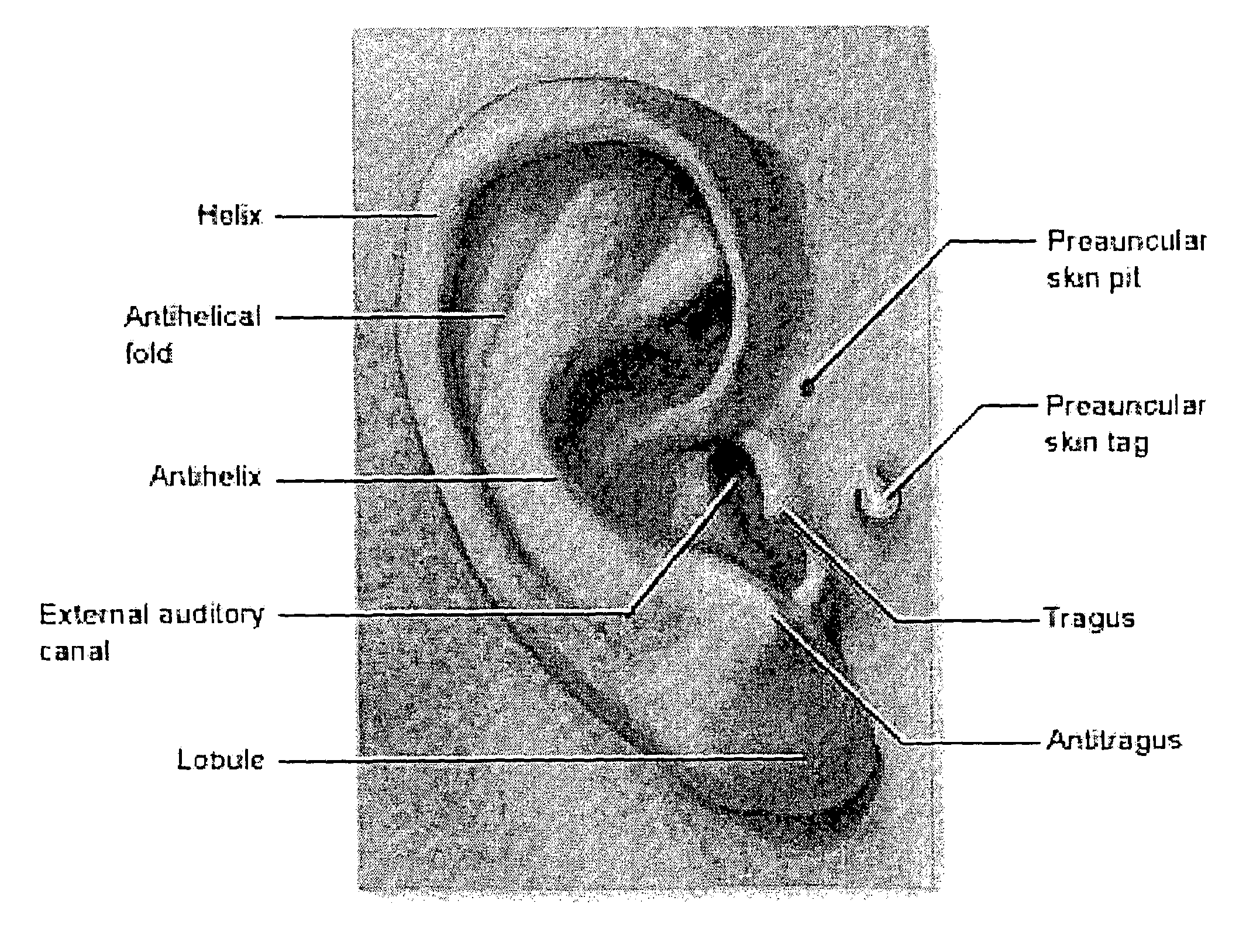
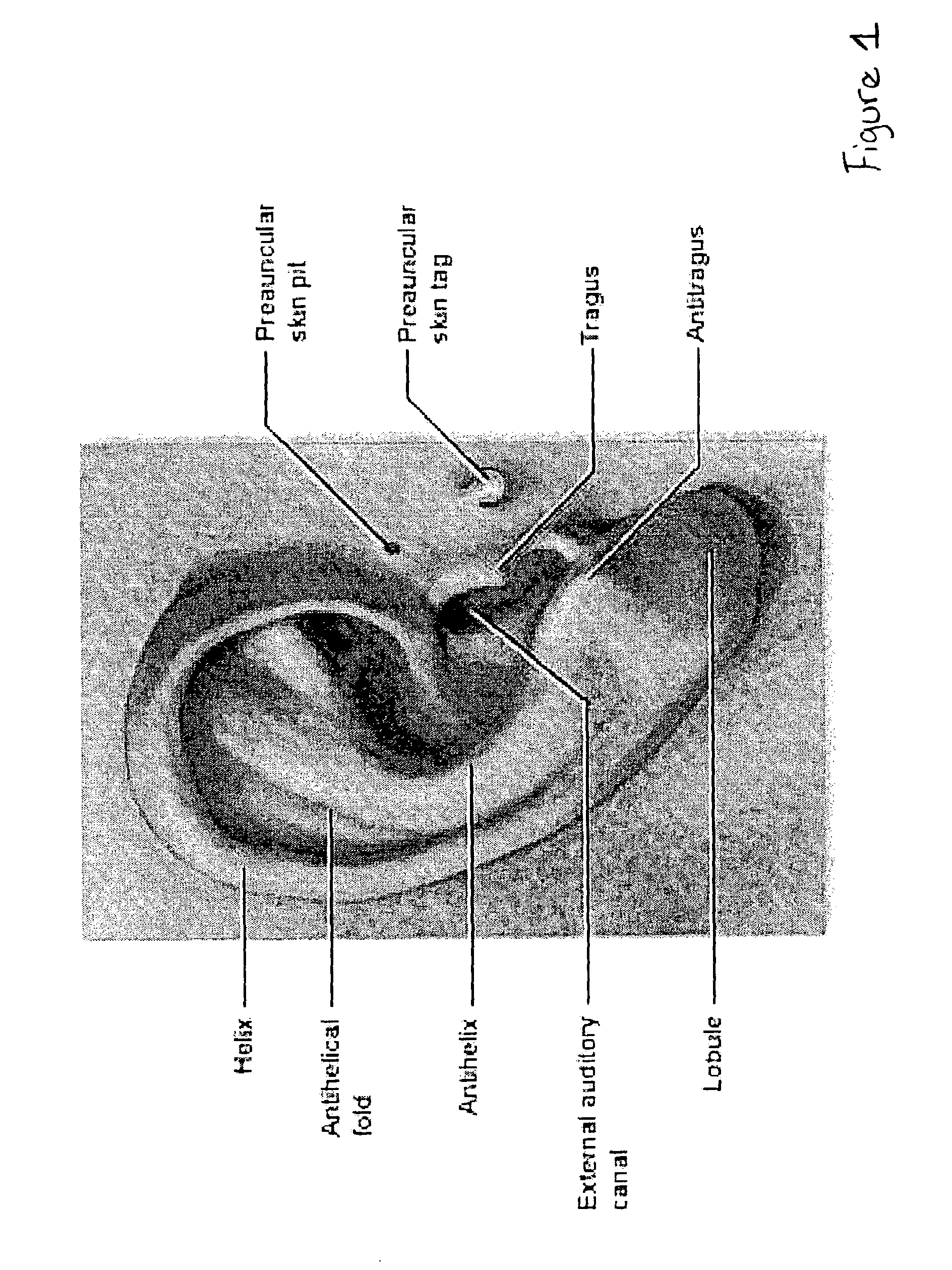
A non-invasive blood glucose monitoring system wherein sensors in contact with separate locations on the ear and calibrated to be accurate to at least ±0.035 degrees Centigrade take the ear temperatures at these locations up to four times per minute continuously to calculate the temperature differential, and using this temperature differential in conjunction with a value determined by taking the square root of the product of the fasting blood glucose and HbA1c that becomes the base line glucose reference level, it can be determined that if the temperature differential decreases, then the blood glucose has increased 1 mg / dl per approximately 0.024 C, while if the temperature differential increases, the blood glucose has decreased 1 mg / dl per approximately 0.024 C.
Owner:L W I ASSOC
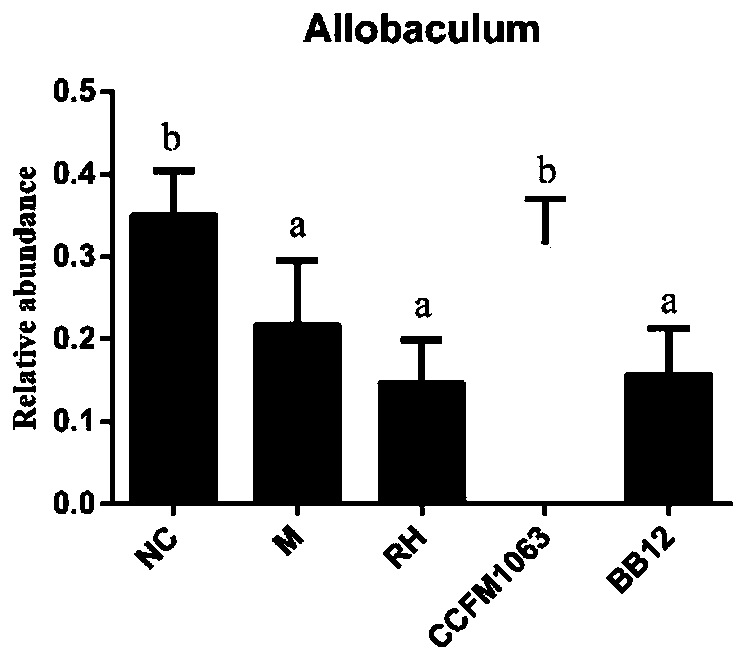
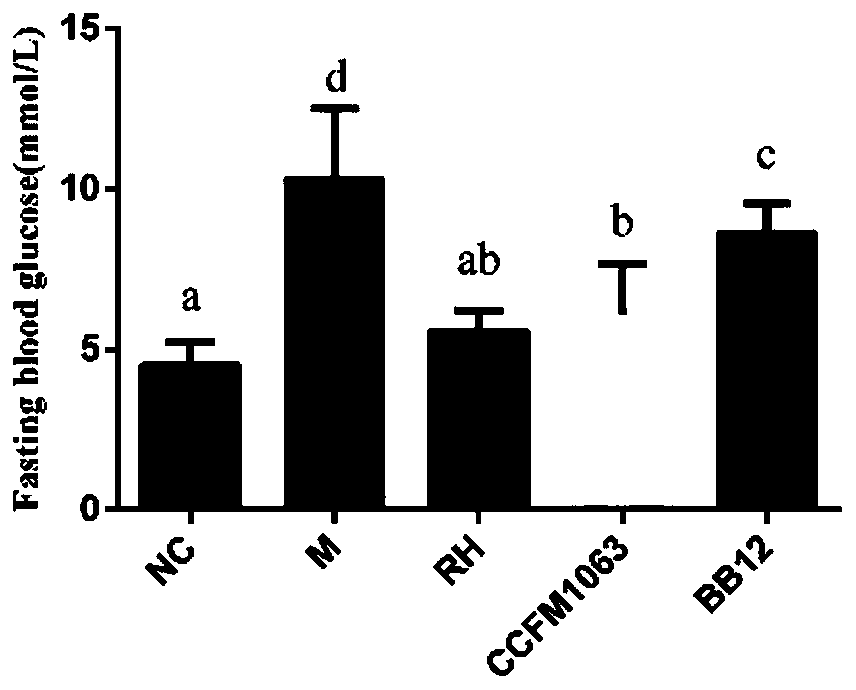
Bifidobacterium bifidum CCFM1063 and application thereof
ActiveCN110331119AImprove the immunityImprove pathological damageNervous disorderBacteriaPerfluorooctanoic acidAllobaculum
The invention discloses bifidobacterium bifidum CCFM1063 and application thereof. The bifidobacterium bifidum CCFM1063 can be rapidly planted in an intestinal tract, fasting blood-glucose and oral glucose tolerance caused by type II diabetes is significantly improved, and the area under the curve for glucose tolerance is reduced; total cholesterol in a serum increased and high-density lipoproteincholesterol reduced caused by the type II diabetes are significantly improved; the condition of insulin resistance caused by the significantly improved is significantly improved; the level of inflammation in type II diabetes liver tissue is significantly improved; pathological injuries of tissue such as pancreas and liver caused by the type II diabetes are significantly improved; the bifidobacterium bifidum CCFM1063 has higher adsorption capacity on perfluoro caprylic acid, and has the capacity of relieving toxicity of the perfluoro caprylic acid; and the constipation condition caused by the type II diabetes is significantly improved and the level of Allobaculum genus in the intestinal tract is improved, and functions of relieving anxiety and depression and colonitis are achieved.
Owner:JIANGNAN UNIV
Method for preparing 2 type diabetes rat model
InactiveCN101095958AVerify authenticityVerify reliabilityFood processingAnimal feeding stuffHigh fatRat model
The invention relates to a method for preparing type-2 diabetes mouse model, which comprises following steps: using streptozocin to make the reaction of islets of pancreas beta cell of new-born mouse to sugar be reduced, injecting treptozocin to weaning mouse to injure islets of pancreas lightly, feeding mouse with high-sugar high-fat food to enable it resist to insulin, and getting type-2 diabetes mouse model. The type-2 diabetes mouse model is characterized by typical symptom of high fasting blood-glucose concentration, high insulinemia, reduced sugar-resistant property and insulin-resistant property. The time for preparing model is shortened and the success rate is high.
Owner:FIRST HOSPITAL OF SHANXI MEDICAL UNIV
Method for establishing type 2 diabetes animal model and application of type 2 diabetes animal model in screening of blood sugar reducing medicaments
The invention provides a method for establishing a type 2 diabetes animal model. An animal is fed with a high-fat and high-sugar feed for a period of time, and the animal is drenched with alcohol with certain concentration at the same time. The attack process of human type 2 diabetes is simulated in the method; and the prepared animal model has typical characteristics of the type 2 diabetes such as high fasting blood glucose, high insulinemia, hyperlipidemia and impaired glucose tolerance, and is suitable for mechanism research of the type 2 diabetes and screening of a large quantity of medicaments.
Owner:NORTHEAST NORMAL UNIVERSITY
Moringa leaf extract with blood sugar lowering activity and preparation method thereof
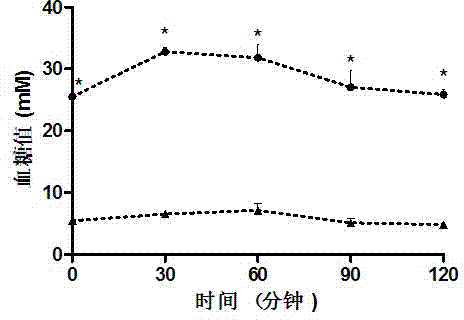
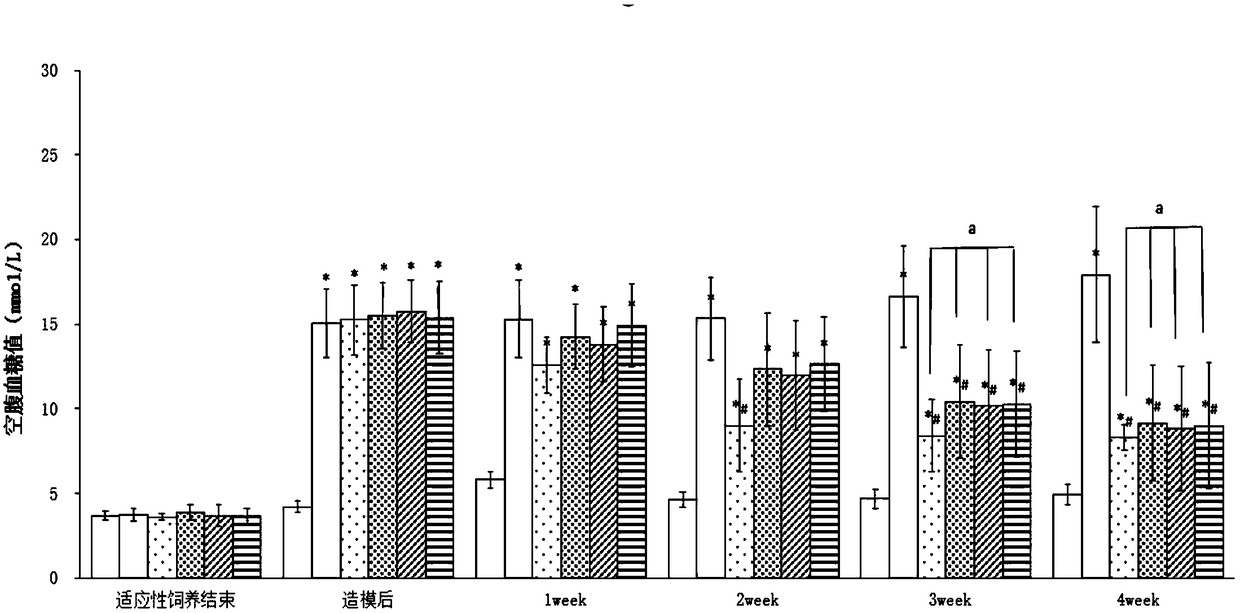
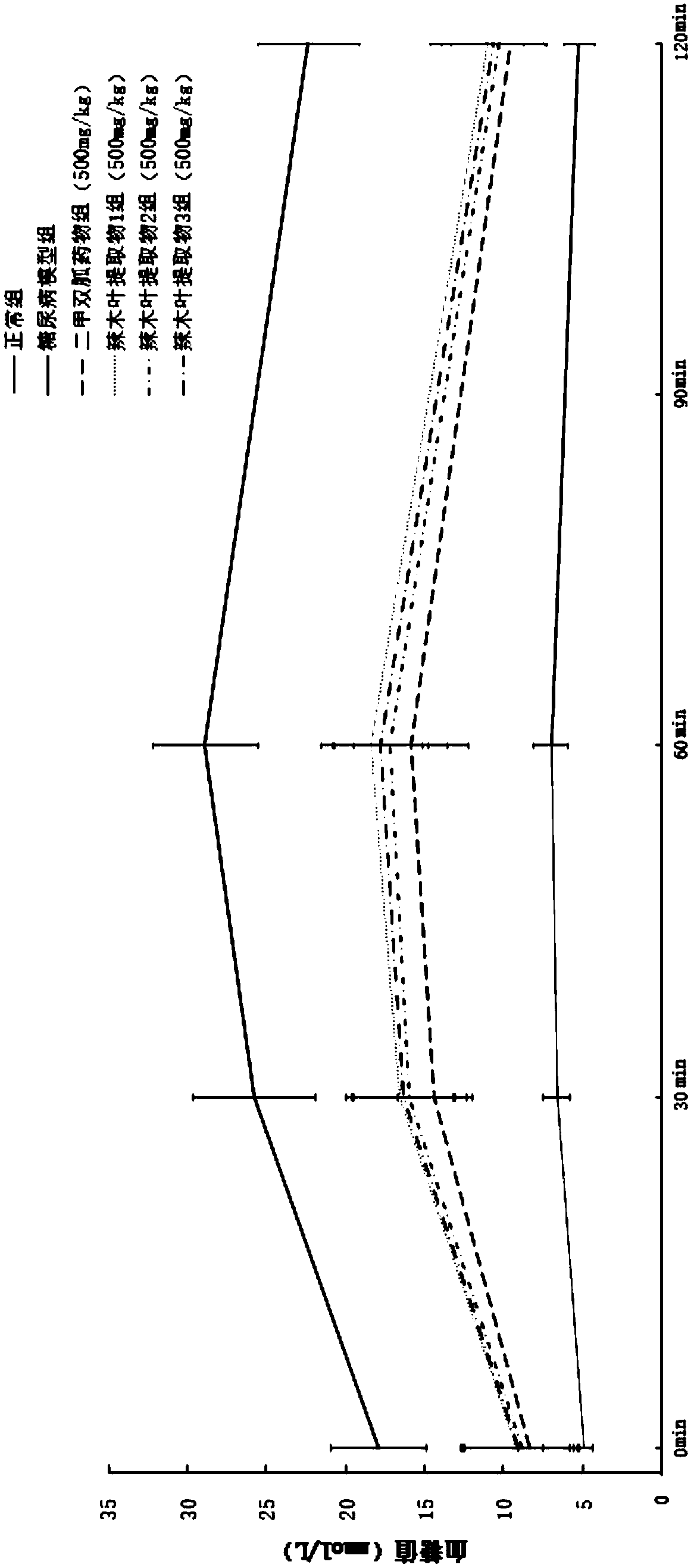
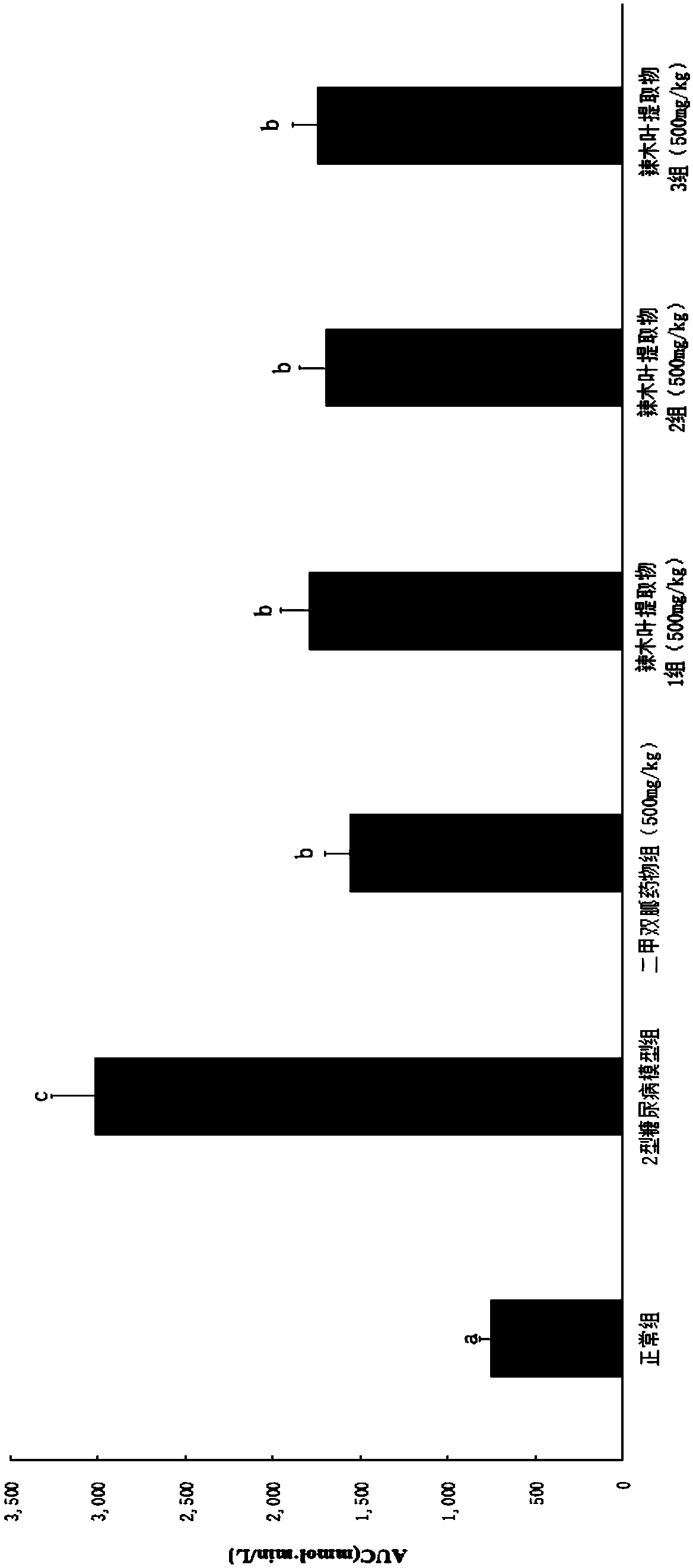
ActiveCN108785342AGood hypoglycemic effectLower fasting blood sugarMetabolism disorderFood ingredient functionsOral glucoseUltrafiltration
The invention discloses a moringa leaf extract with blood sugar lowering activity and a preparation method thereof. The preparation method comprises the following steps: crushing and sieving, performing alkali treatment, performing protease enzymolysis, performing cellulase enzymolysis, performing high-pressure homogenization, performing high-temperature extraction, performing low-temperature high-speed centrifugation, performing ultrafiltration separation and concentration, performing protease enzymolysis, performing low-temperature high-speed centrifugation, performing secondary ultrafiltration separation and concentration, performing freeze drying and the like to obtain the moringa leaf extract with the blood sugar lowering activity. The extract has the polysaccharide content of 30% orabove; an animal experimental model of a type-2 diabetic rat verifies that the extract has good blood sugar lowering activity and can effectively reduce the fasting blood glucose of the type-2 diabetic rat and enhance the oral glucose tolerance dosage thereof; the acting effect of the extract is similar to that of metformin with the same dosage. An extracting technology is simple, the whole technological process can meet a food-grade requirement, and the moringa leaf extract can be applied to the field of medicines, health-care products, food and the like.
Owner:SOUTH CHINA UNIV OF TECH
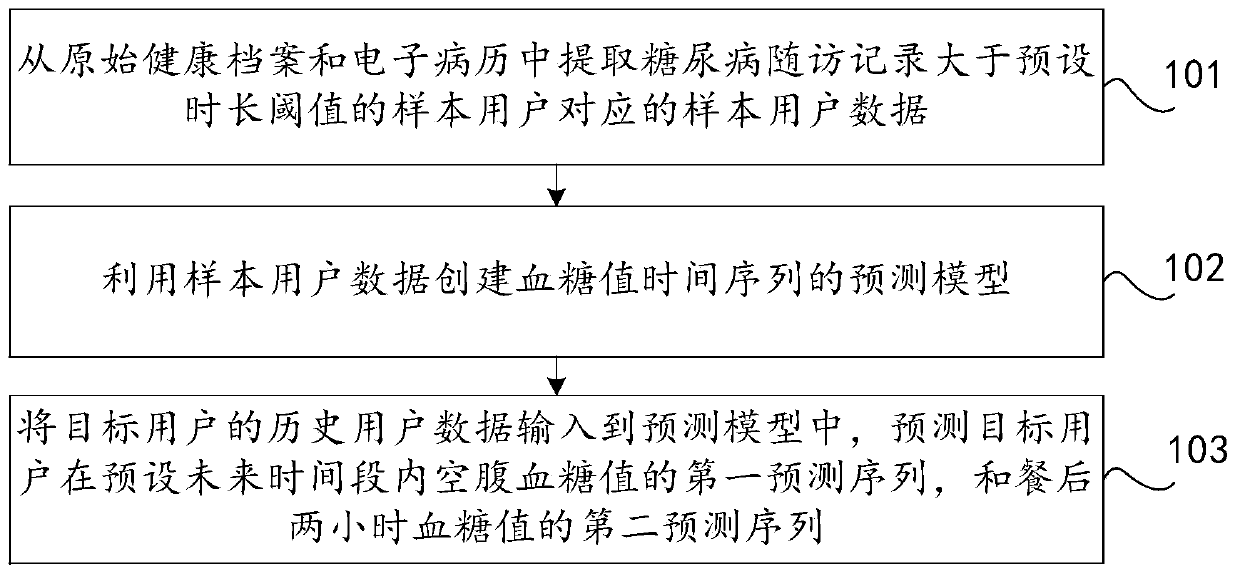
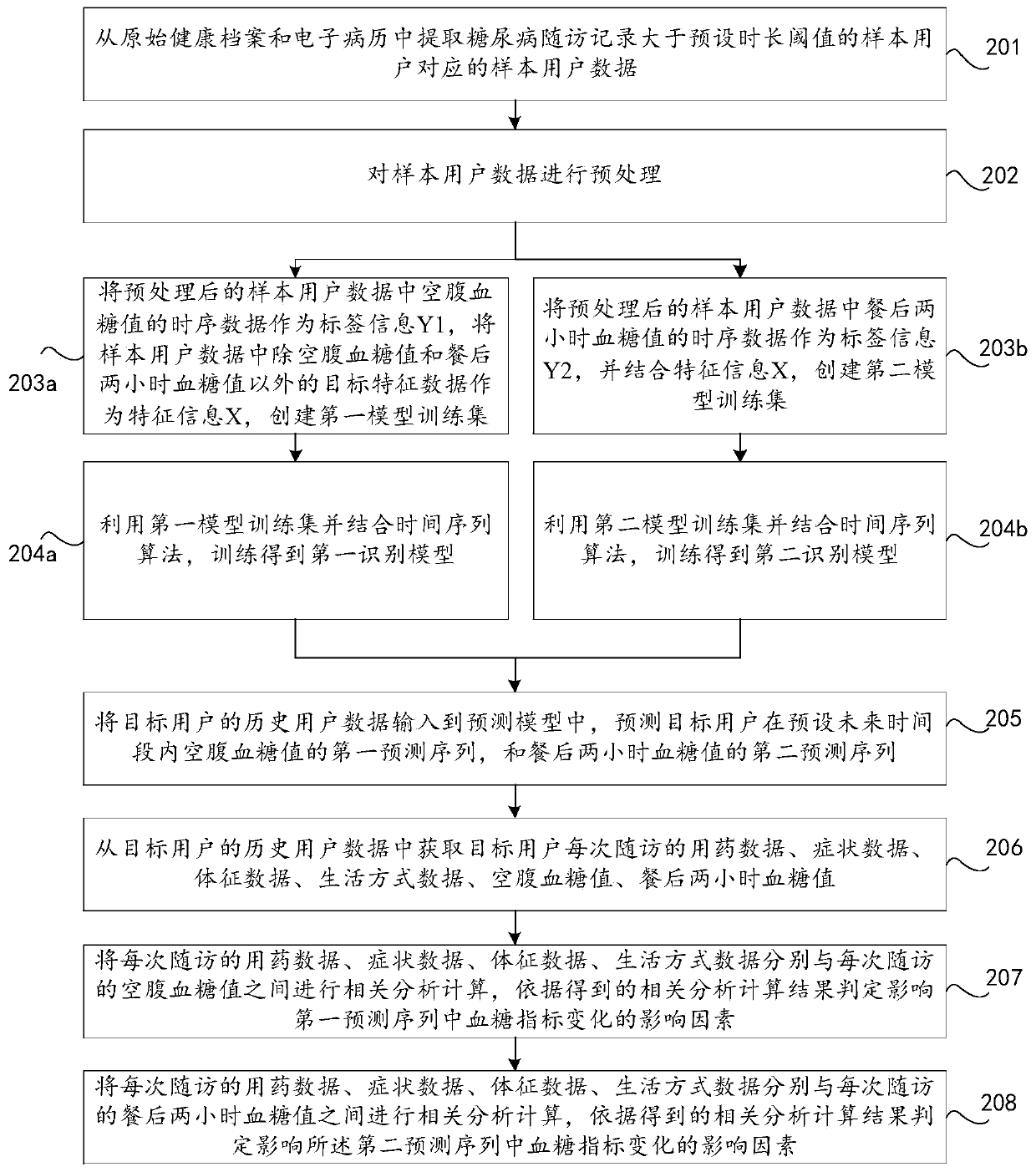
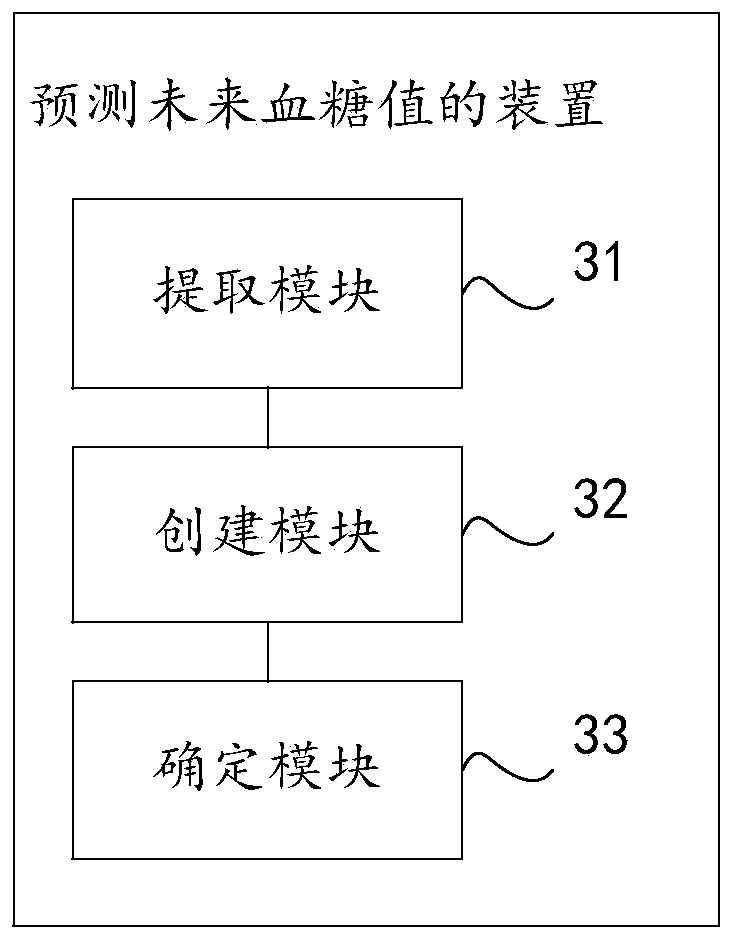
Method and device for predicting future blood glucose value and computer equipment
PendingCN110085318AMonitor developments in real timeMedical data miningHealth-index calculationMedical recordDiabetic patient
The invention discloses a method and device for predicting a future blood glucose value and computer equipment, and relates to the technical field of computers, capable of effectively solving the problem that in the prior art, only the current blood glucose value of a user can be judged, but the future blood glucose value of the user cannot be predicted. The method comprises the following steps: extracting sample user data corresponding to a sample user whose diabetes follow-up record is greater than a preset duration threshold from an original health record and an electronic medical record; utilizing the sample user data to create a prediction model of a blood glucose value time sequence; and inputting historical user data of a target user into the prediction model, and predicting a firstprediction sequence of fasting blood glucose values of the target user in a preset future time period and a second prediction sequence of blood glucose values of two hours after meal, wherein the historical duration corresponding to the historical user data is smaller than the preset duration threshold, and both the target user and the sample user are diabetic patients. The method and device forpredicting a future blood glucose value are suitable for predicting the future blood glucose value of the target user.
Owner:PING AN TECH (SHENZHEN) CO LTD
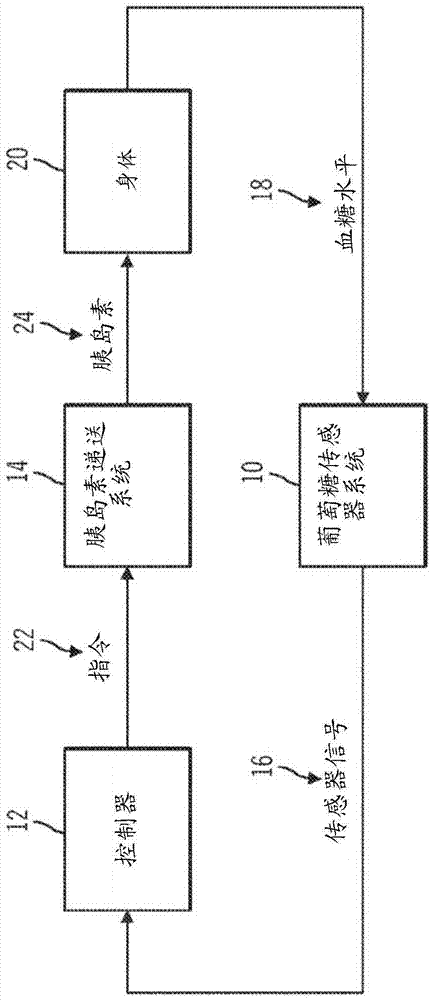
Safeguarding techniques for a closed-loop insulin infusion system
A method of controlling an insulin infusion device comprises operating a processor architecture comprising at least one processor device to calculate a maximum insulin infusion rate for the user based on a fasting blood glucose value associated with the user, a total daily insulin value associated with the user, and fasting insulin delivery data that is indicative of insulin delivered to the user during a fasting period, wherein the maximum insulin infusion rate is applicable during a period of closed-loop operation of the insulin infusion device; obtaining a first closed-loop insulin infusion rate for the user, wherein the first closed- loop insulin infusion rate is obtained for a current sampling point during the period of closed- loop operation; and providing a second closed-loop insulin infusion rate for the user when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate, wherein the second closed-loop insulin infusion rate is less than the first closed-loop insulin infusion rate; the first closed-loop insulin infusion rate is calculated in accordance with a proportional-integral-derivative insulin feedback (PID-IFB) control algorithm; and inhibiting windup of an integral component of the PID-IFB control algorithm when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate.
Owner:MEDTRONIC MIMIMED INC
Lactobacillus acidophilus La-SJLH001 with probiotic function of regulating blood sugar and cholesterol level and application thereof
ActiveCN109182207AGood gastrointestinal toleranceImprove survival rateBacteriaConfectioneryIn vivoLactobacillus acidophilus
The invention relates to a self-screened Lactobacillus acidophilus SJLH001 (La SJLH001 for short) with probiotic function of regulating blood sugar and cholesterol level and application thereof in probiotic food. The strain has good tolerance to gastrointestinal environment, and has antioxidant, hydrophobic, ultrasonic resistance, reducing power and other in vitro benefits. In vitro and in vivo functional tests showed that La-SJLH001 has significant amylase inhibitory activity and alpha-Glucose glycerase inhibitory activity, and can significantly reduce fasting blood glucose, postprandial blood glucose level, enhance the tolerance of glucose, alleviate the pancreatic islet enlargement, reduce serum cholesterol level, enhance intestinal mucosal protection and other functions. La- SJLH001 has significant hypoglycemic and cholesterol-lowering activities, which makes it widely used in various forms of probiotic foods (such as probiotic tablet candy, probiotic solid drinks, etc.).
Owner:BEIJING SHOU JIA LI HUA SCI TECH CO LTD +1
Non-invasive blood glucose monitoring system
ActiveUS20050043603A1Low costReduce the numberSensorsTelemetric patient monitoringGlycemicGlucose polymers
A non-invasive blood glucose monitoring system wherein sensors in contact with separate locations on the ear and calibrated to be accurate to at least ±0.035 degrees Centigrade take the ear temperatures at these locations up to four times per minute continuously to calculate the temperature differential, and using this temperature differential in conjunction with a value determined by taking the square root of the product of the fasting blood glucose and HbA1c that becomes the base line glucose reference level, it can be determined that if the temperature differential decreases, then the blood glucose has increased 1 mg / dl per approximately 0.024 C, while if the temperature differential increases, the blood glucose has decreased 1 mg / dl per approximately 0.024 C.
Owner:L W I ASSOC
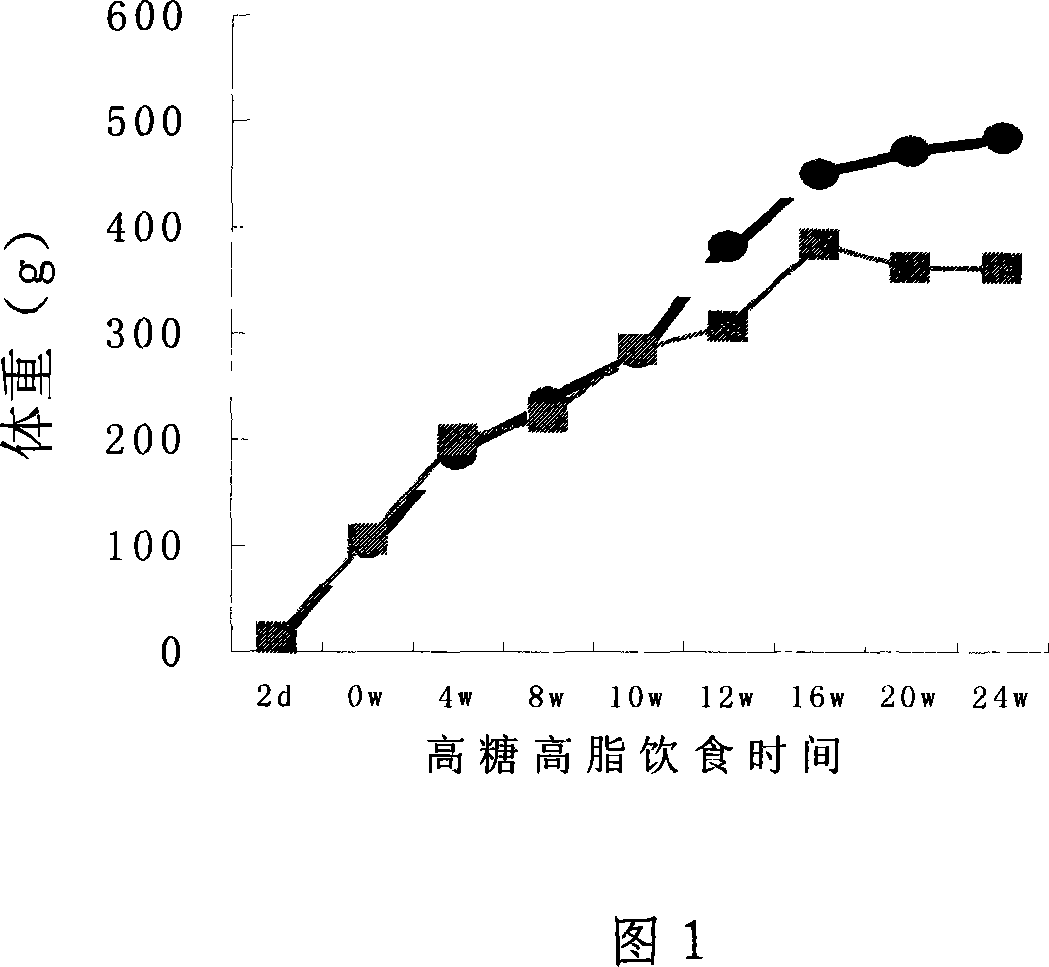
Preparation method of OLETF rat fasting blood-glucose elevation model
The invention discloses a preparation method of an OLETF rat fasting blood-glucose elevation model, belonging to animal zoological medicinal models and feeds specially used for specifically animals. In the invention, an OLETF rat is feed by high-fat feed in 6-8 weeks, and the high-fat feed comprises the following raw materials in percentage by weight: 60-65 percent of standard feed, 10-12 percentof yolk powder, 10-12 percent of lard, 8-12 percent of sugar-free full-cream powder, 2-14 percent of cane sugar and 1-2 percent of water. The OLETF rat fed by the feed can simulate the morbidity process of human type II diabetes more and is good for the research of the type II diabetes; and the invention also shortens the time the OLETF rat develops into the diabetes, accelerates the molding, saves the experimental period and reduces the experimental cost.
Owner:天津市公安医院
Combined food for reducing blood sugar
The invention relates to a combined food for reducing blood sugar. The food is formed by maitake polysaccharide powder and total saponins powder of astragalus which are mixed according to the mass ratio of 1:2 to 2:1; the maitake polysaccharide powder is extracted from maitake fruit bodies, wherein the content of maitake polysaccharide is more than 50%; the total saponins powder of astragalus is extracted from astragalus, wherein the content of total saponins powder of astragalus is more than 50%. The combined food has the functions of reducing fasting blood glucose of a 2-type diabetes KKay mouse model and a nutritional type 2-type diabetes mouse model, enhancing the physiological activity in a synergetic manner, and better improving the respective function of reducing the blood sugar; and the toxicological tests show that the application of the combined food is safe. The combined food can be used for preparing a health-care food and medicaments for reducing the blood sugar in an auxiliary manner, can reduce the fasting blood glucose and improve the clinical symptoms caused by high blood sugar.
Owner:HEFEI UNIV OF TECH
Application of L-arabinose in preparing medicines or health-care products
ActiveCN103156865ALower total cholesterolLower triglyceridesOrganic active ingredientsMetabolism disorderHuman bodyDigestion
The invention discloses application of L-arabinose in preparing medicines or health-care products for preventing or treating metabolic syndrome, and in particular application of L-arabinose in preparing medicines or health-care products for simultaneously reducing total cholesterol, triglyceride, fasting blood-glucose, blood pressure, waistline and weight, blood uric acid and glutamic-pyruvic transaminase of human body. The applicant discovers that for adults, in a taking dosage range of 10-20g / person / day, total cholesterol, triglyceride, fasting blood-glucose, blood pressure, waistline and weight, blood uric acid and glutamic-pyruvic transaminase of human body can be simultaneously reduced clinically. Not only is functions of the body not damaged, but also digestion and absorption of proteins and vitamins are not affected while the indexes are simultaneously reduced, so that the purpose of improving the functions of the body and preventing and treating metabolic syndrome is realized.
Owner:GUANGXI INST OF BOTANY THE CHINESE ACAD OF SCI +3
Method for evaluating insulin resistance
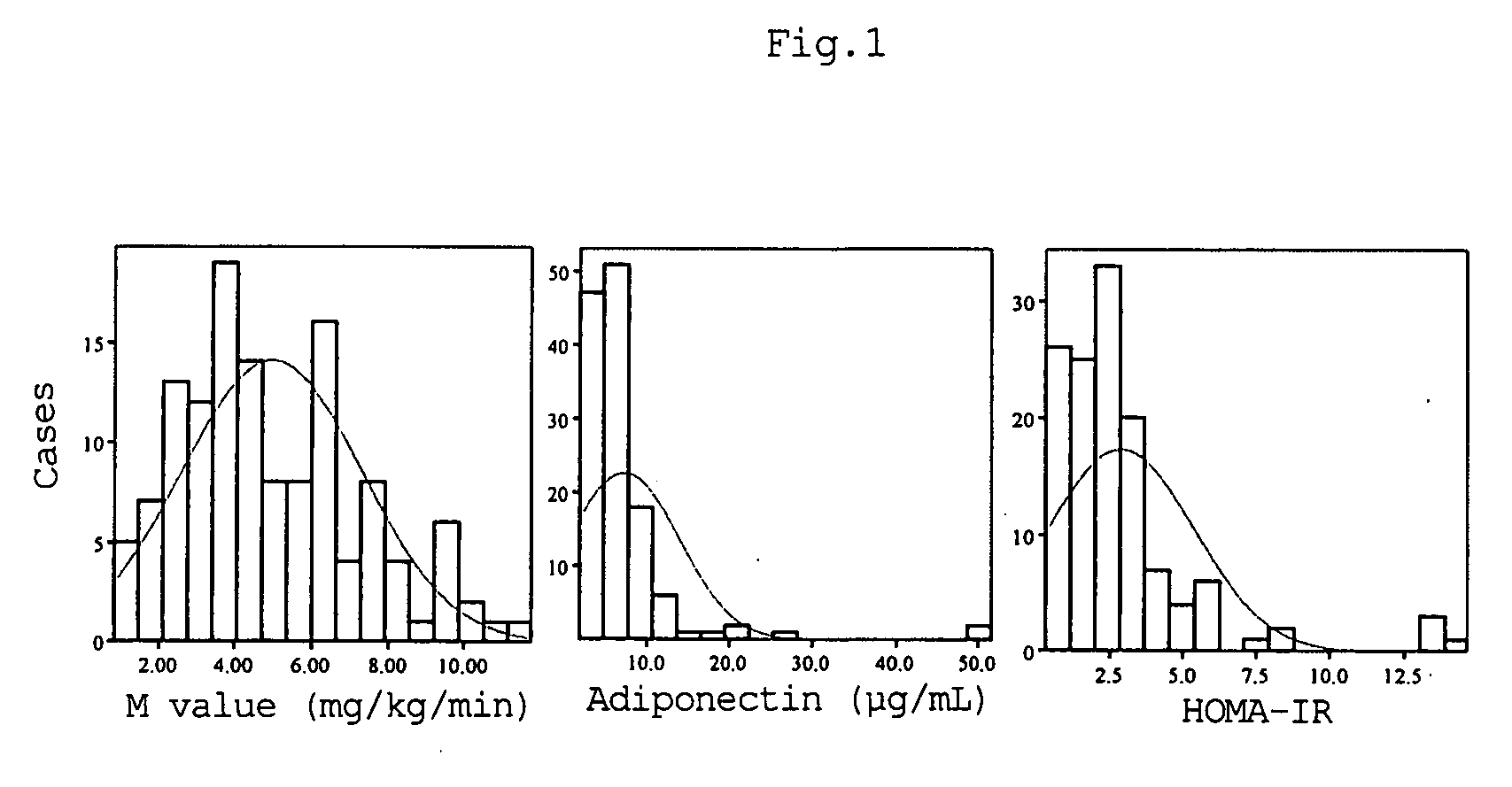
InactiveUS7592180B2Correlativity is excellentInsulin resistance can be uniformly evaluatedPeptide/protein ingredientsDisease diagnosisFast blood sugarFBG - Fasting blood glucose
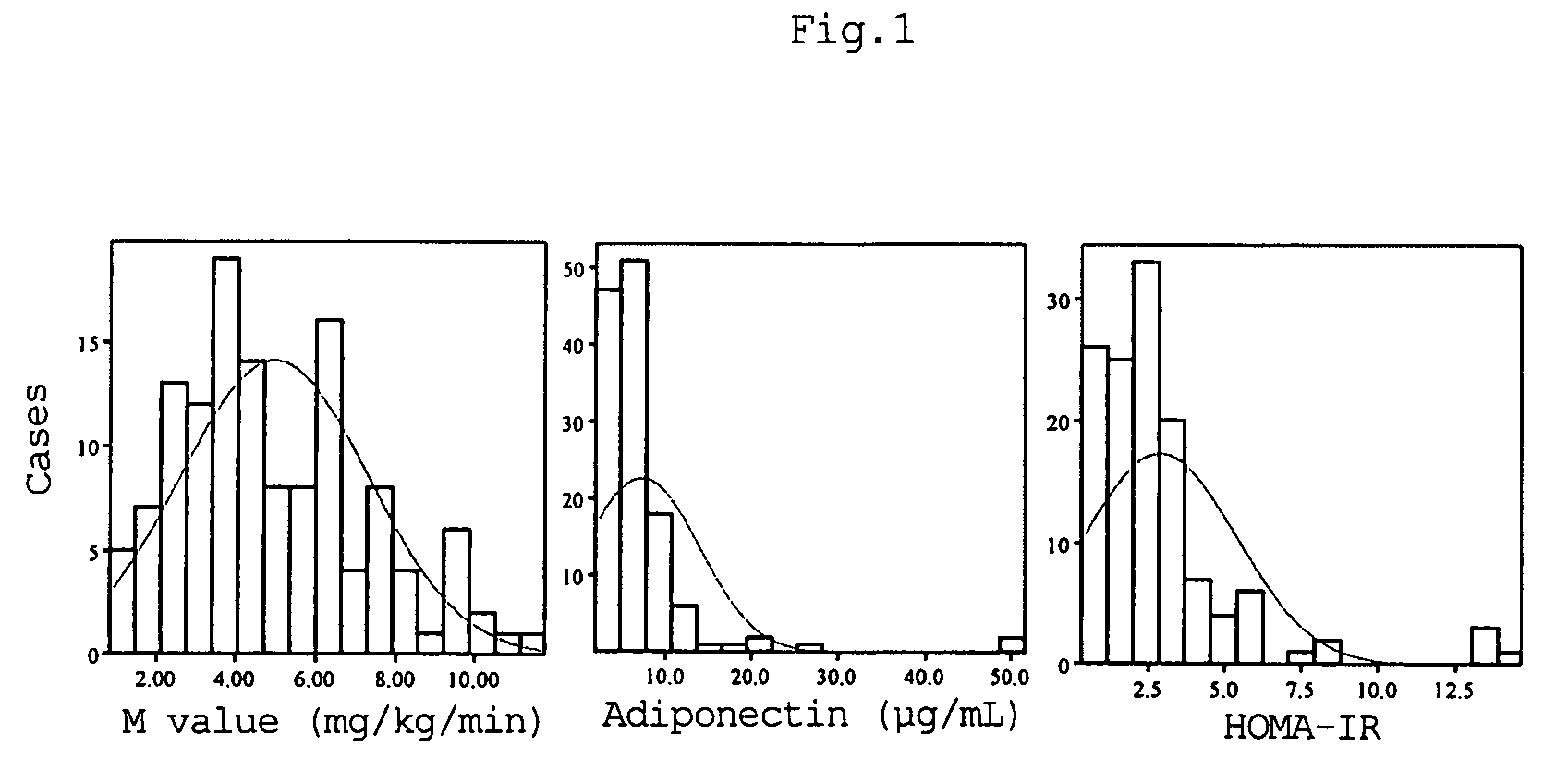
A method for evaluating insulin resistance in a simple and highly reliable manner is provided. This method includes measuring a fasting insulin value in blood, a fasting blood sugar value, and an adiponectin value and evaluating insulin resistance using, as an index, a value obtained by the following calculation formula (I):(Fasting insulin value)×(Fasting blood sugar value) / Adiponectin value (I).
Owner:OTSUKA PHARM CO LTD
Method for Evaluating Insulin Resistance
InactiveUS20080066526A1Correlativity is excellentEasily prevent and treat diabetesPeptide/protein ingredientsFlow propertiesFast blood sugarFast insulin
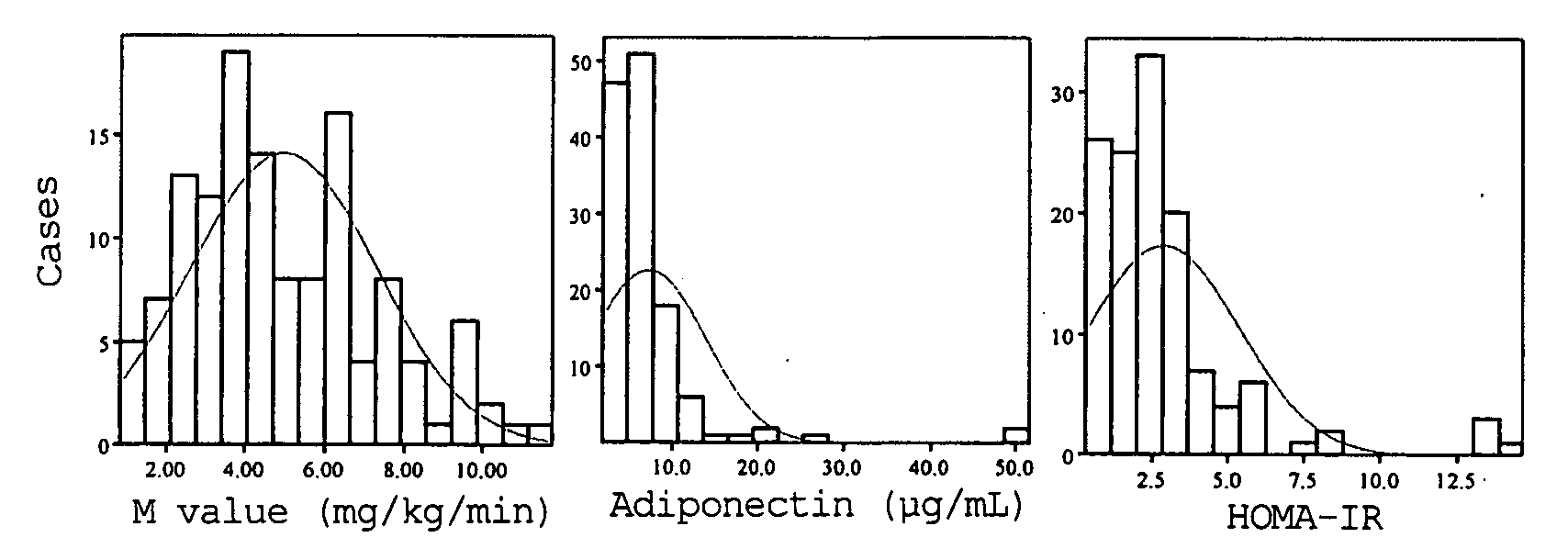
A method for evaluating insulin resistance in a simple and highly reliable manner is provided. This method comprises measuring a fasting insulin parameter in blood, a fasting blood sugar parameter, and an adiponectin parameter and evaluating insulin resistance using, as an index, a value obtained by the following calculation formula (I): (Fasting insulin value)×(Fasting blood sugar value) / Adiponectin value (I)
Owner:OTSUKA PHARM CO LTD
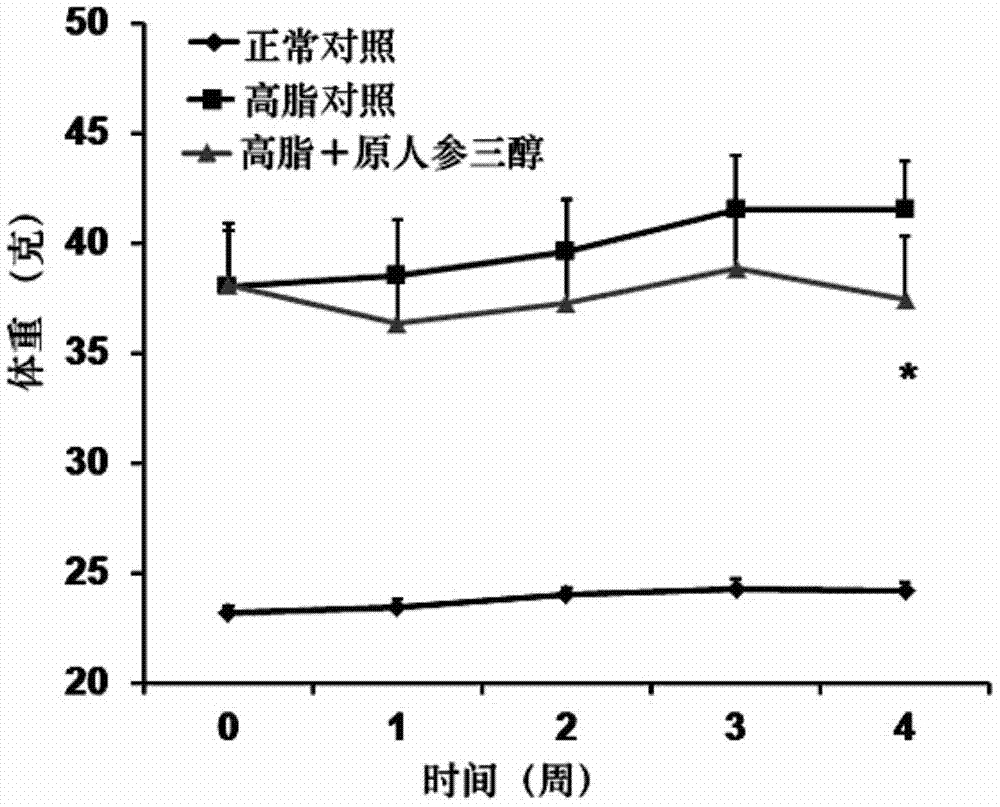
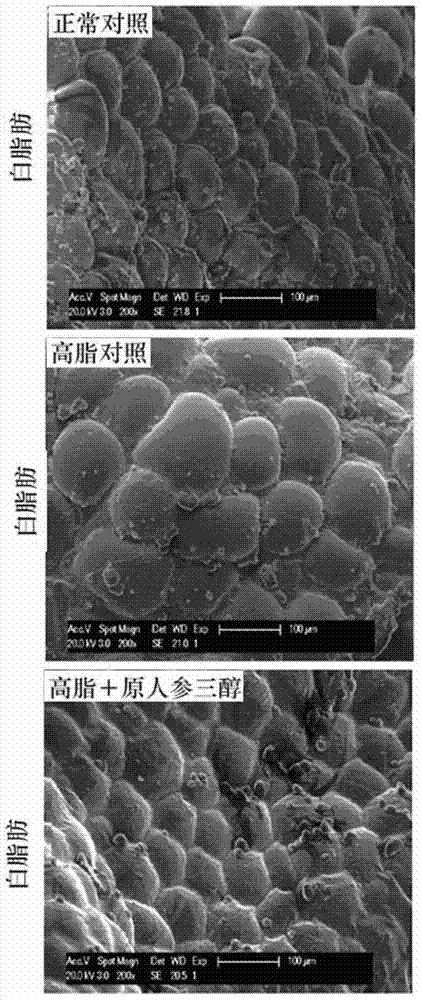
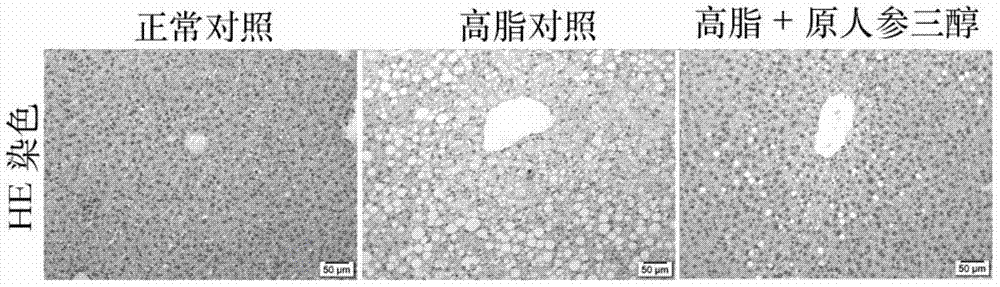
Pharmaceutical use of protopanaxatriol or/and derivatives thereof
InactiveCN104490896AStrong therapeutic activityInhibition of volume increaseOrganic active ingredientsMetabolism disorderDyslipidemiaIn vivo
Owner:SHANGHAI UNIV OF T C M
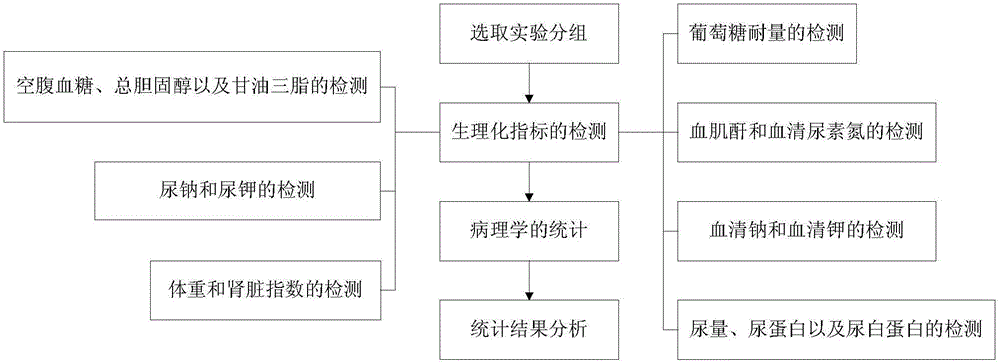
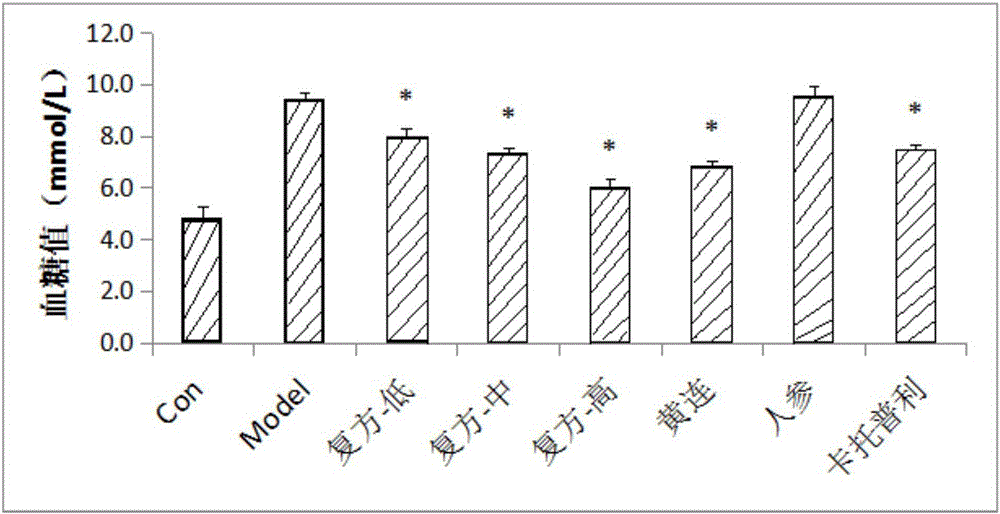
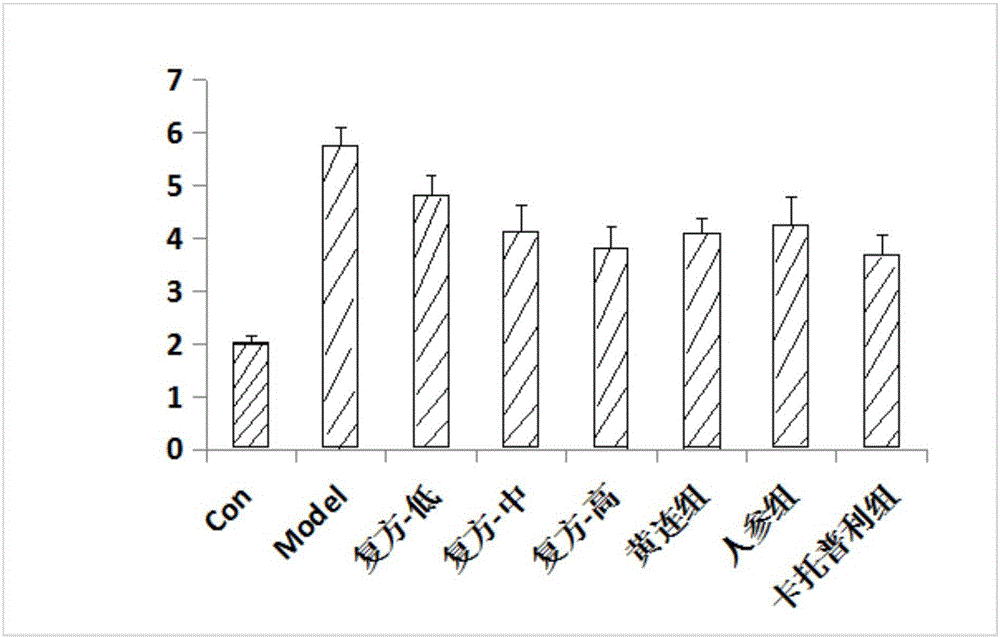
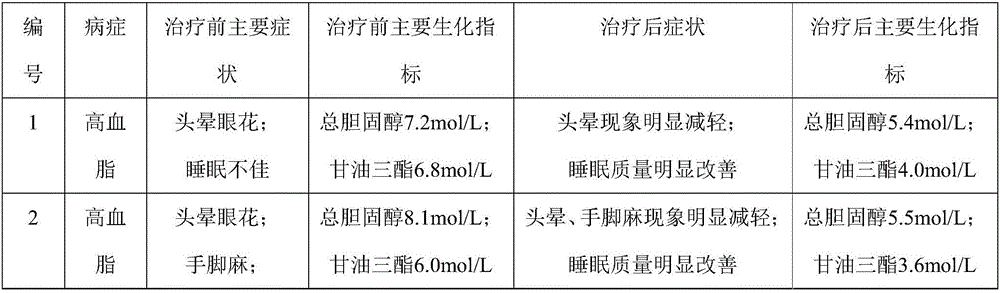
Experiment analysis method for blood glucose reducing efficacy of Shenlian decoction rapidly disintegrating tablets for reducing blood glucose
The invention provides an experiment analysis method for blood glucose reducing efficacy of Shenlian decoction rapidly disintegrating tablets for reducing blood glucose and belongs to the technical field of traditional Chinese medicine research. The method comprises the following steps of step one, selection of experiment grouping; step two, detection of physiological indicators, including detection of mice fasting blood-glucose, total cholesterol and triglyceride, detection of glucose tolerance, detection of urine volume, urine protein and urinary albumin, detection of serum creatinine and serum urea nitrogen, detection of serum sodium and serum potassium, detection of urine sodium and urine potassium and detection of weight and kidney indexes; step three, statistics of pathology; step four, analysis of statistical results; according to the experiment analysis method, an effective constituent ginseng total saponin and coptis total alkaloid compound (RH for short) of the Shenlian decoction rapidly disintegrating tablets for reducing blood glucose can effectively reduce the blood glucose level of a diabetic patient and has a certain therapeutic effects for early diabetic nephropathy.
Owner:CHANGCHUN UNIV OF CHINESE MEDICINE
Low-acid-value Yunnan pine seed oil, preparation method and application thereof
InactiveCN106433949APromote growthAvoid high-quality demandsMetabolism disorderFatty-oils/fats refiningDiseaseThrombus
The invention discloses a low-acid-value Yunnan pine seed oil and a preparation method thereof, the invention further provides an application of the pine seed oil in foods and treatment of cardiovascular and cerebrovascular diseases and other diseases. The capsule disclosed by the invention can promote normal metabolism of cholesterol, so that the plasma cholesterol can be reduced, the function of promoting lipid deposition in the endothelial cells of the artery by blood platelets can be obviously inhibited, the blood platelet aggregation can also be reduced, the thrombosis can be inhibited, and the fasting blood glucose of a patient can be remarkably improved.
Owner:王俊人
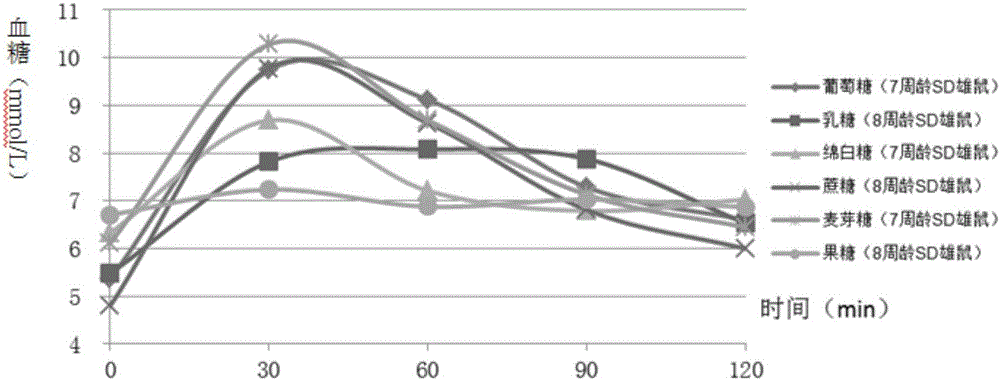
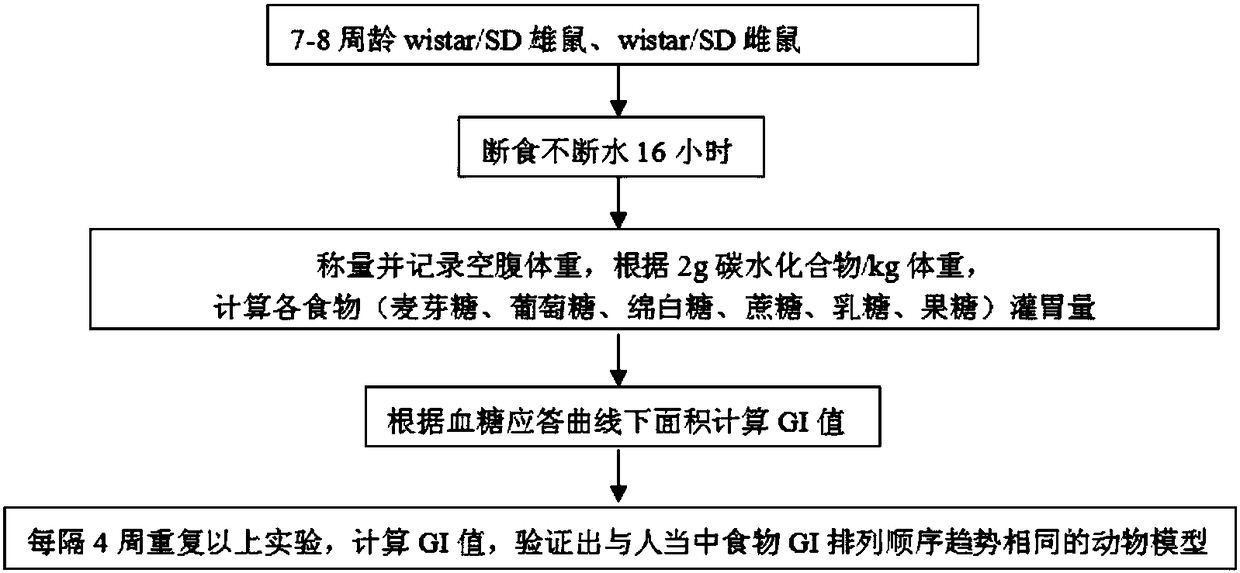
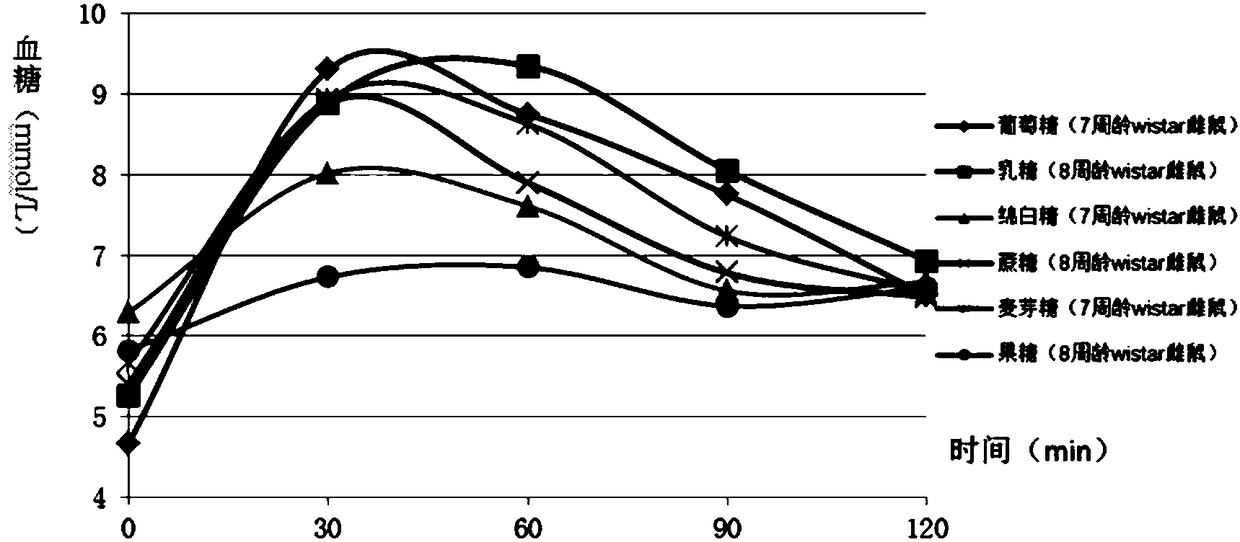
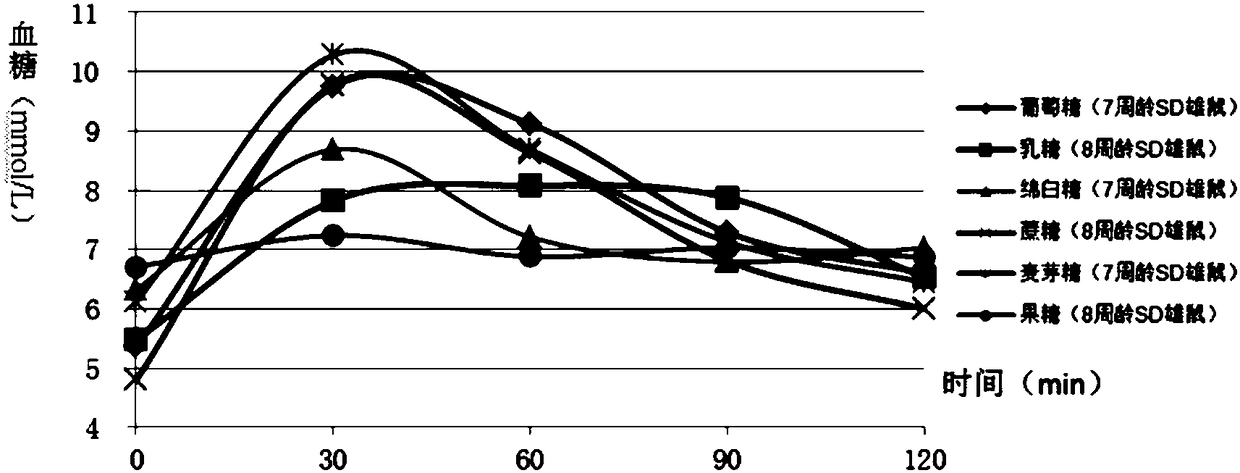
Construction method of animal experimental model for verifying food blood glucose generation index and animal model constructed through method
ActiveCN106135133AEfficient verificationValidate cost-effectiveOrganic active ingredientsAnimal husbandryHuman bodyLactose
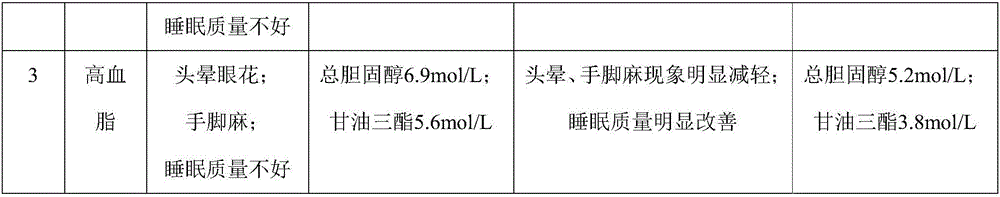
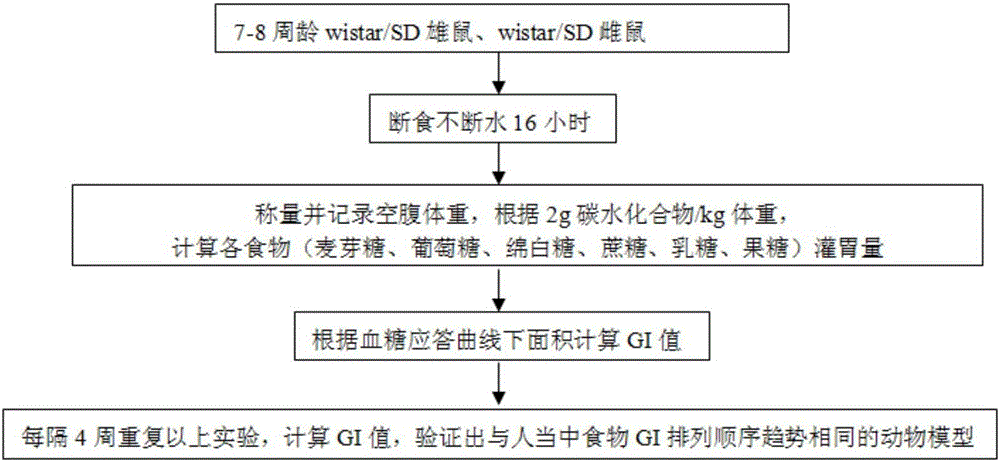
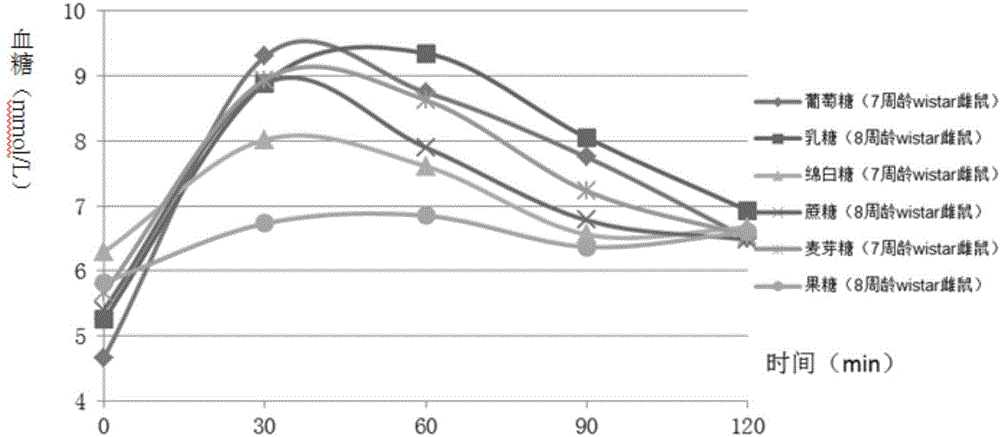
The invention discloses a construction method of an animal experimental model for verifying a food blood glucose generation index and the animal model constructed through the method. The construction method comprises the steps that at least ten female rats and at least ten male rats are selected from wistar rats and SD rats, the rats are not fed on food and fed on water for 10 hours to 16 hours, and the intragastric administration food amount is calculated through weighing; the fasting blood glucose is detected by adopting a Roche glucometer, blood glucose values obtained 30 min, 60 min, 90 min and 120 min after intragastric administration of glucose, maltose, soft sugar, saccharose, lactose and fructose is conducted are detected, and the area under a food blood glucose response curve of all time detection points of each rat in each group is calculated by adopting an integral method; a glucose experiment result is taken as the GI standard 100, and GI values of other food are calculated according to the following formula: GI=100*the area under a blood glucose response curve of the food to be verified / the area of a blood glucose response curve of glucose; trend comparison is conducted on the sequence of the food GI values verified from the rats and the sequence of the GI values verified from a human body, and 7-10-week-age SD male rats with the weight of 210 g to 350 g are taken as the animal experimental model for verifying the food blood glucose generation index.
Owner:SICHUAN UNIV
Use of aucubin in preparing medicine for treating type 2 diabetes
InactiveCN110522758AImprove glucose and lipid metabolismImprove islet morphologyOrganic active ingredientsMetabolism disorderIntraperitoneal routeHigh doses
The invention belongs to the technical field of medicine, and provides the use of aucubin in preparing a medicine for treating type 2 diabetes. A mouse model of type 2 diabetes is established by intraperitoneal injection of streptozotocin after 4 weeks of a high-fat diet. Then, aucubin is injected intraperitoneally at low, medium and high doses for 4 weeks respectively, four items of body weight,fasting blood glucose, insulin and blood lipid of mice are detected, the insulin resistance index and insulin sensitivity index are calculated, and the morphological changes of islets in pancreas areobserved by HE staining. The results show that the levels of insulin, TG, T-CHO and LDL-C in the serum of the mouse model of type 2 diabetes can be reduced, the level of HDL-C can be increased, and the insulin resistance index and insulin sensitivity index can be improved by aucubin in low, medium, and high doses of groups, compared with the model group. The atrophy of islets in the pancreas of the model mouse of type 2 diabetes is also improved by aucubin which has a better protective effect on the pancreas.
Owner:DALIAN UNIV OF TECH
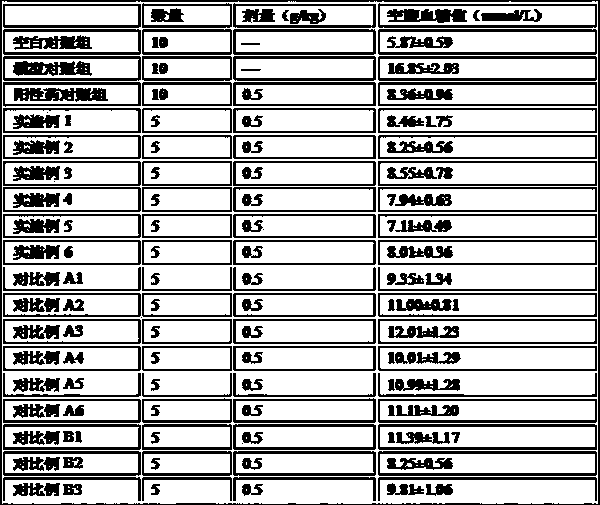
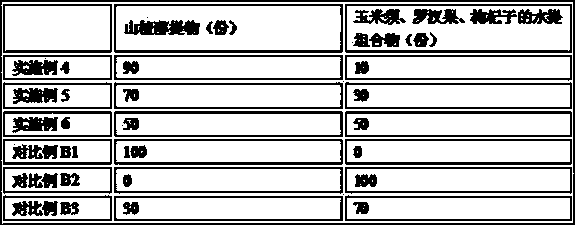
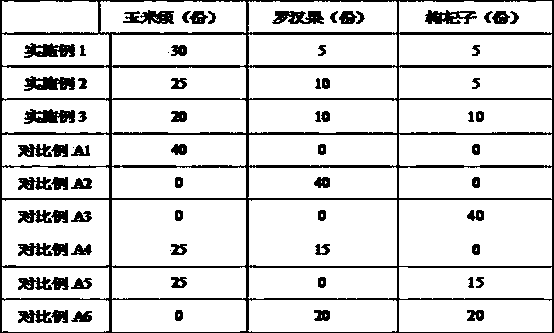
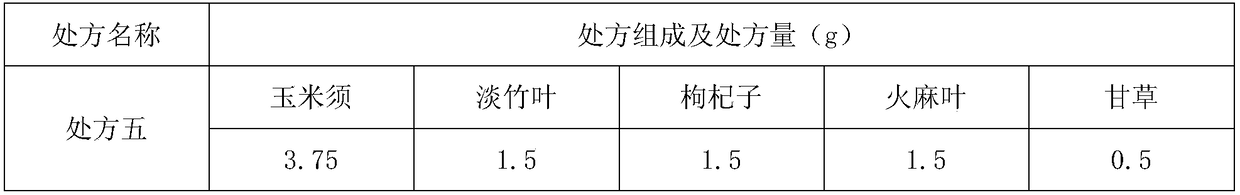
Hawthorn health food assisting regulating blood sugar level
ActiveCN108835636AImprove eating experienceAvoid influenceMetabolism disorderNatural extract food ingredientsAlcoholHigh availability
The invention provides hawthorn health food assisting regulating blood sugar level. The hawthorn health food is formed by performing reasonable compounding on hawthorn alcohol extract and a water extract composition of corn stigma, siraitia grosvenorii and lycium barbarum, and specially comprises the following components in parts by weight: 50 to 100 parts of the hawthorn alcohol extract, and 10 to 50 parts of a water extract composition of the corn stigma, siraitia grosvenorii and lycium barbarum, wherein the water extract composition is prepared from the corn stigma, siraitia grosvenorii andlycium barbarum according to the mass ratio being (20-30): (5-10): (5-10). The hawthorn health food obviously reduce the fasting blood-glucose level, and compared with the prior art, the food has equivalent or better efficacy of assisting regulating blood sugar level, and produces the effect of synergistic interaction. Meanwhile, as all raw materials belongs to medicinal and edible materials, thefood has the characteristics of rice resources, wide distribution, low price and high availability, and the food is simple in production process, low in operation cost, more suitable for common consumer groups, and good in safety.
Owner:广州汝丽多食品科技有限公司
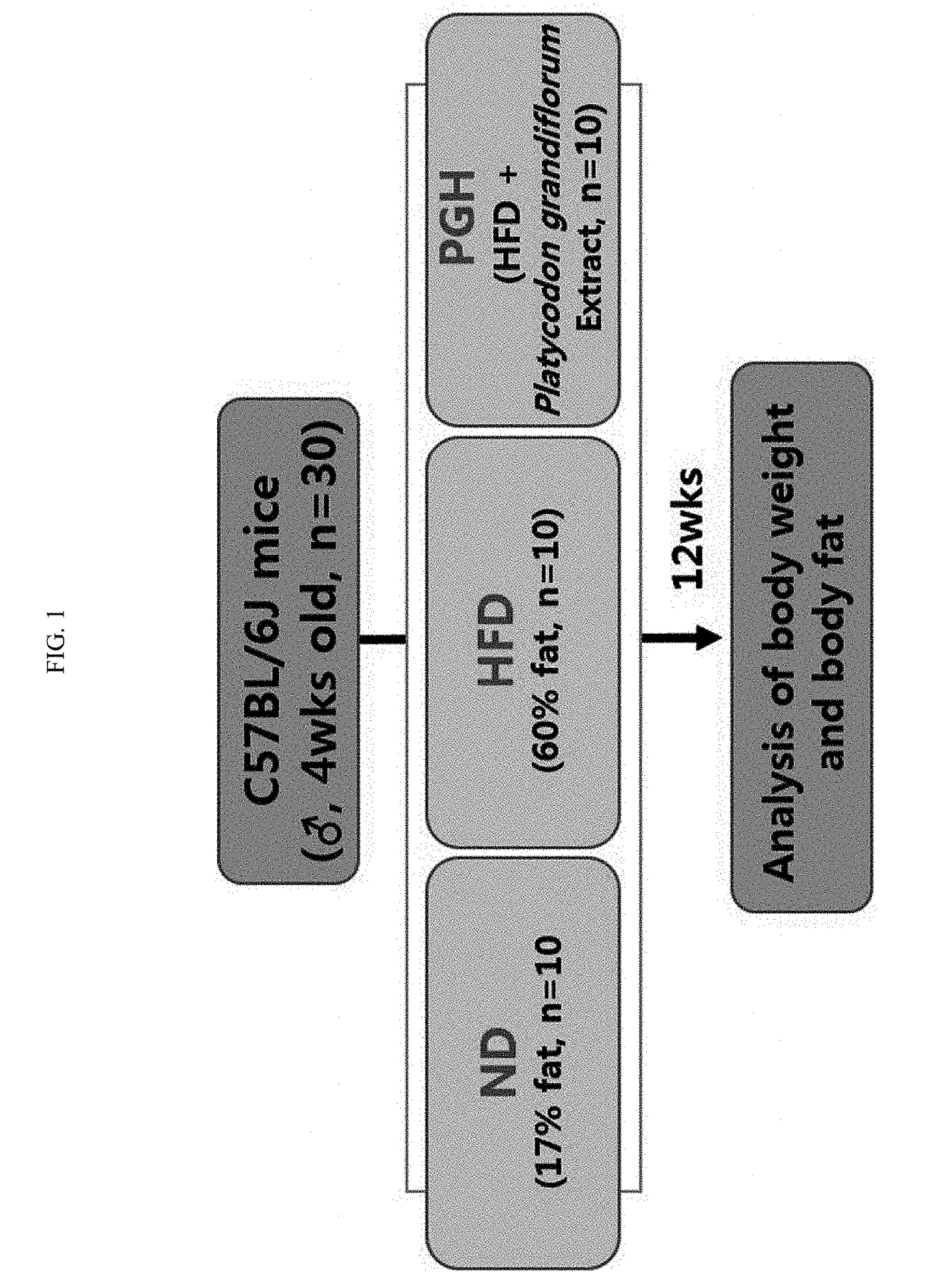
Pharmaceutical composition comprising an extract of platycodon grandiflorum and method for preventing or treating of obesity using the same
ActiveUS20180339007A1Preventing and treating obesitySimilar levelsFood sciencePlant ingredientsEnergy expenditureBULK ACTIVE INGREDIENT
Provided is a composition for preventing, improving or treating obesity, which includes a Platycodon grandiflorum extract as an active ingredient and a method for preparing the extract with an improved anti-obesity effect. The extract shows an effect of inhibiting increases in body weight and fat mass caused by a high-fat diet for normalization, an increase in fasting blood glucose and a decrease in energy expenditure in a diet-induced obesity mouse model. Further, compared to an extract prepared by a conventional extraction method, the Platycodon grandiflorum extract shows a more excellent effect of inhibiting increases in body weight and fat mass, fasting blood glucose, and blood lipid and adipokine contents in experiments, resulting in an excellent anti-obesity effect. Therefore, the Platycodon grandiflorum extract prepared is expected to be useful for preventing obesity and treating obesity-related complications that can be caused by an increase in fat mass as well as obesity.
Owner:KYUNGPOOK NAT UNIV IND ACADEMIC COOP FOUND
Application of a kind of curcumin derivative in the preparation of anti-diabetes and its complication medicine
ActiveCN102293762AReduce fatty degenerationAlleviate atherosclerosisMetabolism disorderDigestive systemDiabetes modelHigh fat
The invention discloses application of a curcumin derivative to preparation of medicines for resisting diabetes and complication thereof and in particular relates to application of (1E,6E)-1,7-di(3,5-ditertbutyl-4-hydroxyl phenyl)heptyl-1,6-diene-3,5-diketone to preparation of medicines, health-care products and food for preventing and treating diabetes and complication thereof. The (1E,6E)-1,7-di(3,5-ditertbutyl-4-hydroxyl phenyl)heptyl-1,6-diene-3,5-diketone can obviously reduce serum, liver triglyceride, total cholesterol and low-density lipoprotein of high fat feed and streptozotocin induced diabetes model mice, can reduce fasting blood glucose and glycated hemoglobin of the model mice, can improve glucose tolerance of the model mice, can obviously improve glycometabolism and lipid metabolism of the diabetes model mice, and can obviously reduce fatty degeneration of liver and aortic arch and lesion of atherosclerosis.
Owner:FUJIAN MEDICAL UNIV
Composition with blood glucose regulating function
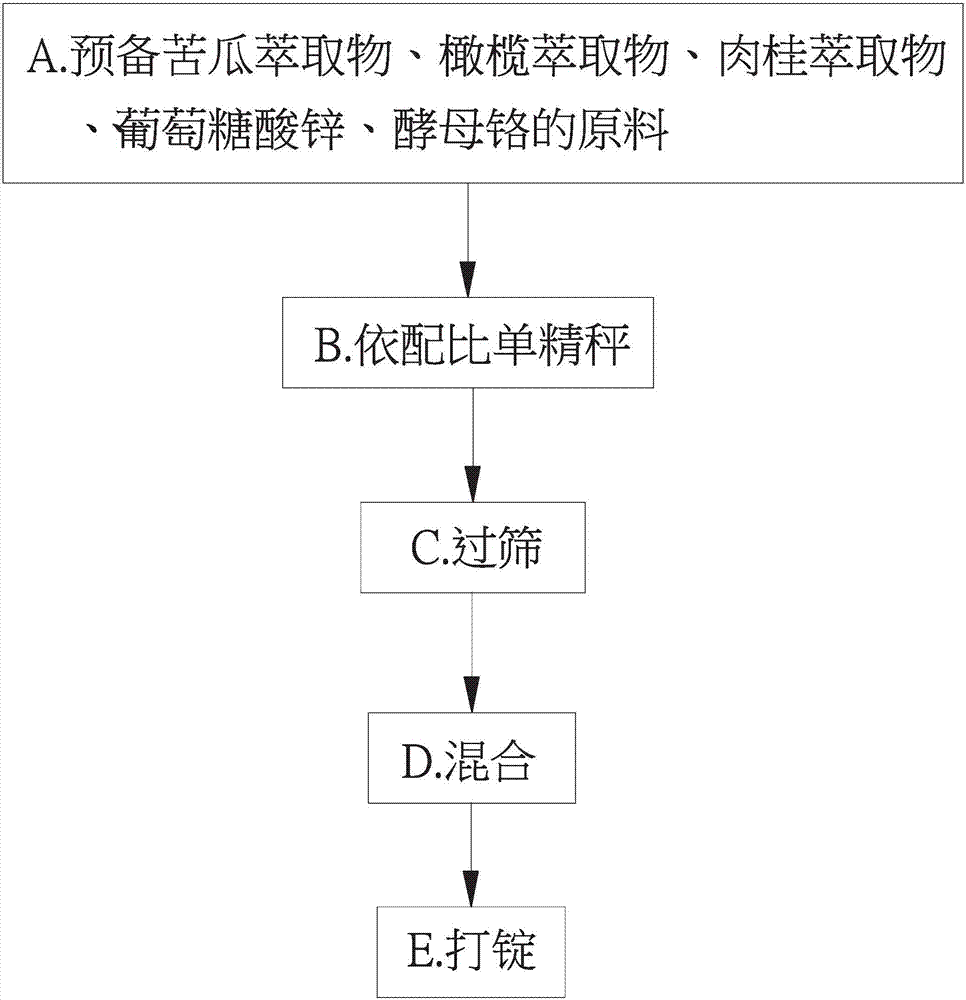
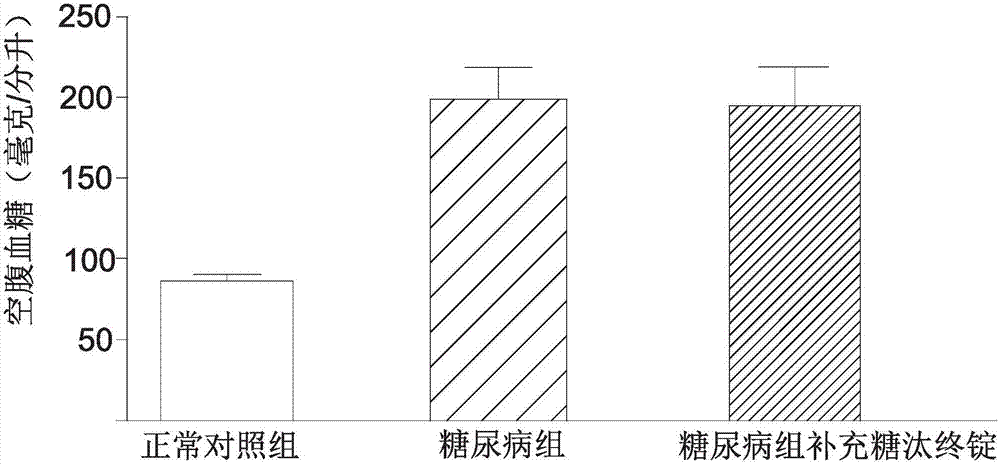
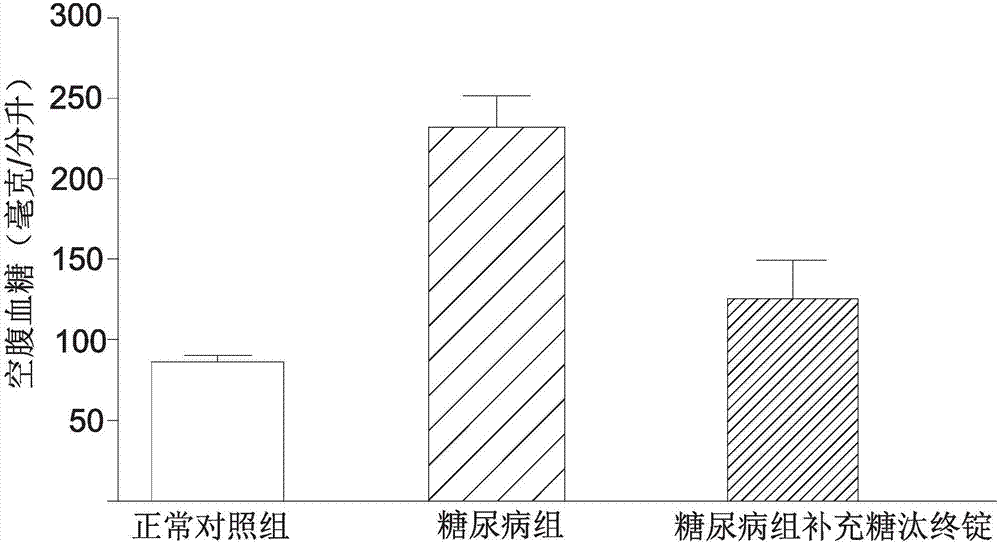
InactiveCN107281262AImproved fasting blood sugar levelsImprove blood sugar regulation lozengesHeavy metal active ingredientsOrganic active ingredientsYeastBitter gourd
The invention provides a Tangtaizhongding composition with a blood glucose regulating function. A lozenge is jointly formed by grouped polymerization and mixing of a bitter gourd extract, an olive extract, a cortex cinnamomi extract, zinc gluconate and yeast chromium. Through the clear display by the animal experiment results, the composition of the Tangtaizhongding compound blood glucose regulating lozenge provided by the invention has the obvious relieving effect on the fasting blood-glucose value of diabetes patients; further, the Tangtaizhongding compound blood glucose regulating composition provided by the invention has the obvious relieving effect.
Owner:汎宸企业股份有限公司
microRNA sequence for early diagnosis of type 2 diabetes and application of microRNA sequence
PendingCN111073973AEasy to synthesizeReduce utilizationMicrobiological testing/measurementDNA/RNA fragmentationMedicineGenetics
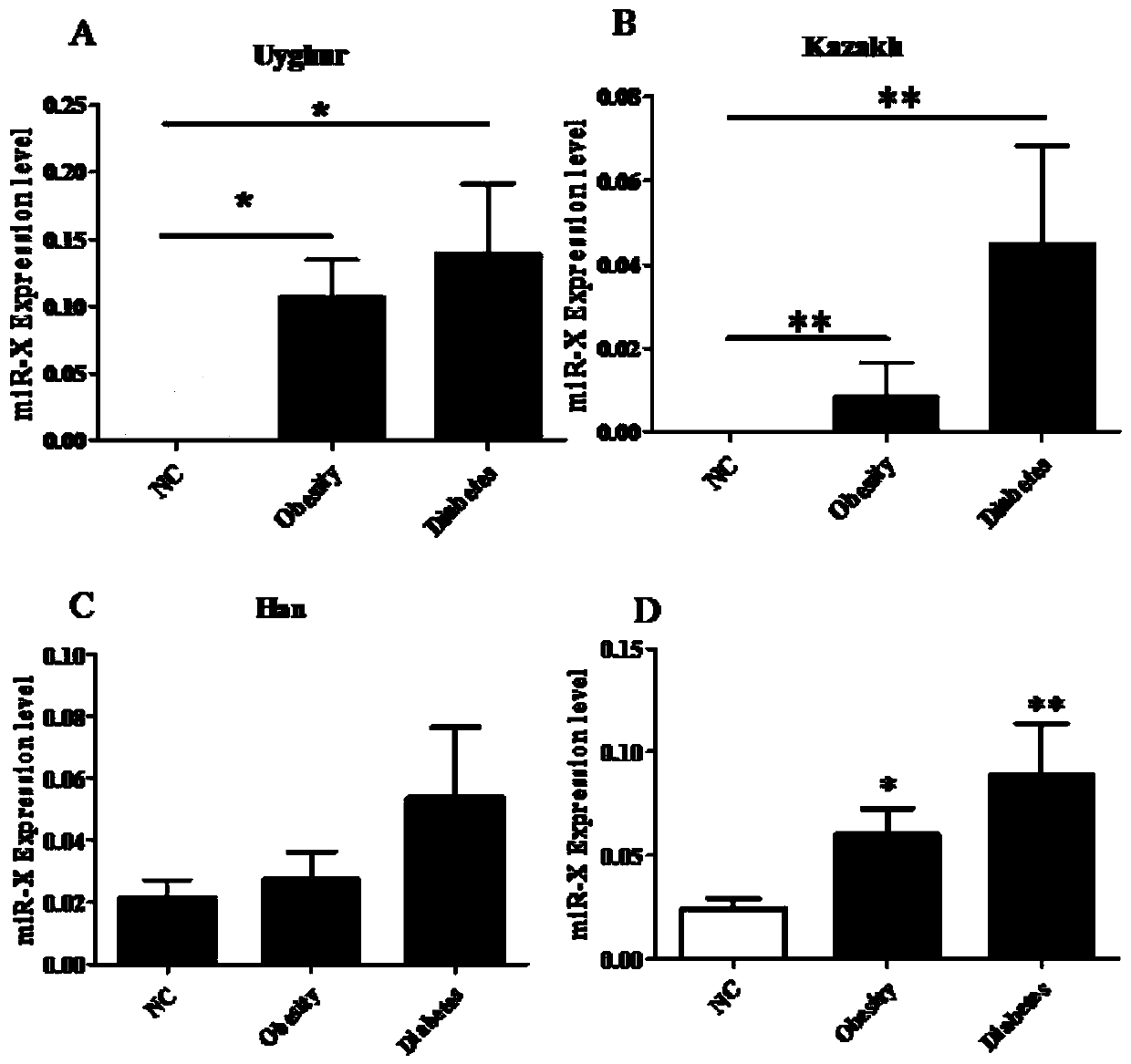
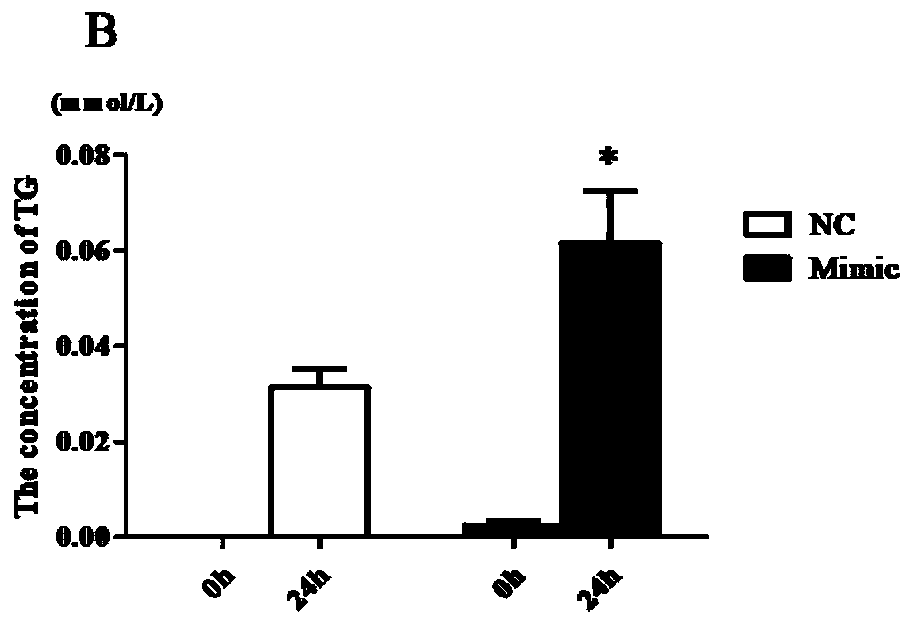
The invention discloses a microRNA sequence for early diagnosis of type 2 diabetes and an application of the microRNA sequence, and belongs to the technical field of medicine. The sequence is microRNA-X, the microRNA sequence is shown as SEQ:ID:NO:1, and the sequence of a design primer is shown as SEQ:ID:NO:2-SEQ:ID:NO:3. According to the invention, serum of 562 Uyghur individuals is collected, analysis is performed by using an Illumina Infinium Global Screening Array-24 v1.0 (GSA) Bead Chip technology, and the microRNA sequence significantly positively correlated with fasting blood glucose isfound.
Owner:SHIHEZI UNIVERSITY
Preparation method of bitter gourd health-caring food with auxiliary blood-glucose-reducing effect
ActiveCN108936662AImprove eating experienceImprove taste and flavorMetabolism disorderNatural extract food ingredientsAlcoholAdditive ingredient
The invention provides a preparation method of a bitter gourd health-caring food with an auxiliary blood-glucose-reducing effect. The bitter gourd health-caring food with the auxiliary blood-glucose-reducing effect is prepared by rationally compounding an alcohol extract of bitter gourds with a water extract composition of fructus momordicae, mulberry fruits, stigmata maydis and stevia; the bittergourd health-caring food specifically comprises the following ingredients in parts by weight: 60-70 parts of the alcohol extract of the bitter gourds, and 30-40 parts of the water extract compositionof the fructus momordicae, the mulberry fruits, the stigmata maydis and the stevia; and the water extract composition is prepared from the fructus momordicae, the mulberry fruits, the stigmata maydisand the stevia at mass ratio of (30-35) to (20-25) to (5-8) to (5-8). The bitter gourd health-caring food with the auxiliary blood-glucose-reducing effect is capable of significantly reducing fastingblood glucose; compared with the prior art, the bitter gourd health-caring food with the auxiliary blood-glucose-reducing effect has equivalent or better auxiliary blood-glucose-reducing effects, andproduces synergistic effects. In addition, the raw materials all have the characteristics of being rich in resources, wide in distribution, low in cost, easy to obtain and the like; and the production processes are relatively simple and relatively low in operation cost. Thus, the bitter gourd health-caring food with the auxiliary blood-glucose-reducing effect is more suitable for ordinary consumers and high in safety.
Owner:广州汝丽多食品科技有限公司
Application of pharmaceutical composition to preparation of medicines for treating type 2 diabetes mellitus
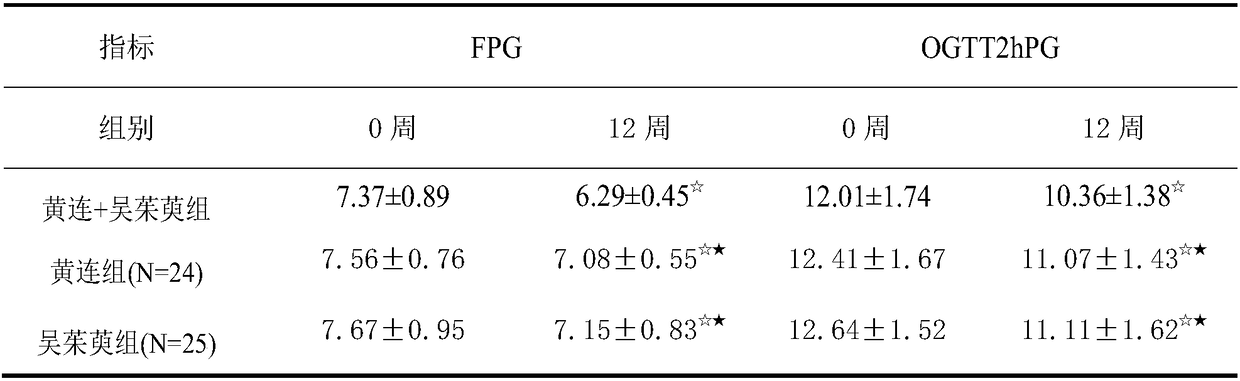
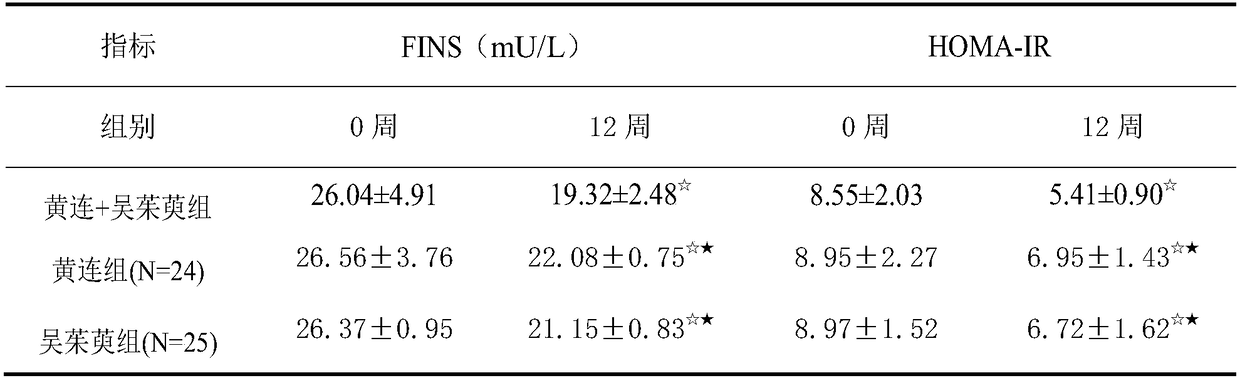
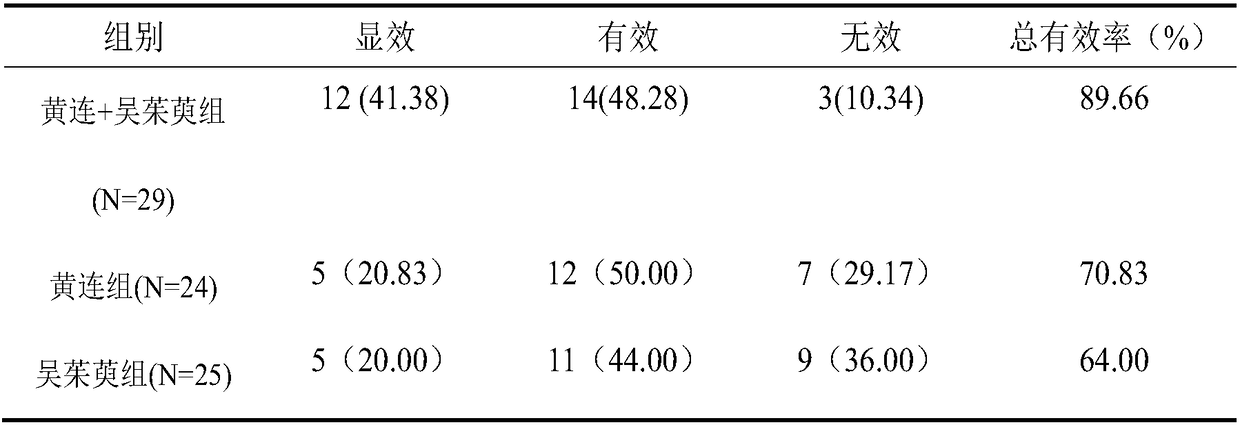
InactiveCN108619289AImprove fasting blood glucose (FPG)Improved Fasting Insulin (FINS)Metabolism disorderEndocrine system disorderGlycemicActive ingredient
The invention provides application of a pharmaceutical composition to preparation of medicines for treating type 2 diabetes mellitus, and the pharmaceutical composition is prepared from the followingraw materials by a weight ratio: 2-10 parts of rhizoma coptidis and 1-10 parts of fructus evodiae. The composition provided by the invention can effectively improve the levels of fasting plasma glucose (FPG), fasting insulin (FINS) and OGTT2h plasma glucose (OGTT2hPG) in patients with the type 2 diabetes mellitus, and does not have an adverse effect; therefore, a new choice is provided for clinical medication.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
Tea drink with effects of reducing blood glucose and proteinuria for sub-health population
InactiveCN108524790ALower blood sugarDispersion deliveryMetabolism disorderNormal blood glucoseAdditive ingredient
The invention discloses a tea drink with effects of reducing blood glucose and proteinuria for sub-health population. The active ingredients of the tea drink are prepared from the following raw medicinal materials: corn stigma, hemp leaves, lophatherum gracile, lycium barbarum and liquorice. The tea drink disclosed by the invention has a certain effect of reducing blood glucose and proteinuria, but the reduction scale is not too large. Therefore, the tea drink is particularly applicable to the sub-health population having sustained fasting blood-glucose equivalent to a normal blood glucose value or slightly higher than the normal blood glucose value, and the blood glucose index of the sub-health population can be reduced from a high critical point to a normal range.
Owner:广西菩提愿投资有限公司
Tibetan medicine composition for treating diabetes and preparation method of Tibetan medicine composition
InactiveCN106620085AIncreased sensitivityReduce high blood sugarMetabolism disorderGranular deliveryBeta-cell FunctionSide effect
The invention relates to Tibetan medicine composition for treating diabetes and a preparation method of the Tibetan medicine composition. The composition is prepared from flowers of scindapsus aureus, sibiraea augustata and crocus sativus and in the form of capsules, granules, tablets or pills prepared by using a pharmaceutically acceptable carrier or excipient. The composition has the effects of clearing heat, removing toxins, dispersing stagnation and dredging collaterals and is applicable to patients suffered from impaired glucose regulation and early diabetes patients. Preliminary experiment research indicates that the composition can lower blood glucose, reduce insulin resistance, increase sensitivity of insulin, protect beta cell functions, reduce serum total cholesterol and serum triglyceride and has no toxic and side effects. Clinical research indicates that the composition can reduce fasting blood glucose and postprandial blood glucose and has no toxic and side effects.
Owner:西藏藏医药大学
Application of l-arabinose in the preparation of medicines or health products
ActiveCN103156865BLower total cholesterolLower triglyceridesOrganic active ingredientsMetabolism disorderDigestionBlood uric acid
Owner:GUANGXI INST OF BOTANY THE CHINESE ACAD OF SCI +3
Verifying the construction method of food glycemic index animal experimental model and the animal model constructed
ActiveCN106135133BEfficient verificationValidate cost-effectiveOrganic active ingredientsAnimal husbandryHuman bodyIndividual animal
The invention discloses a construction method of an animal experimental model for verifying a food blood glucose generation index and the animal model constructed through the method. The construction method comprises the steps that at least ten female rats and at least ten male rats are selected from wistar rats and SD rats, the rats are not fed on food and fed on water for 10 hours to 16 hours, and the intragastric administration food amount is calculated through weighing; the fasting blood glucose is detected by adopting a Roche glucometer, blood glucose values obtained 30 min, 60 min, 90 min and 120 min after intragastric administration of glucose, maltose, soft sugar, saccharose, lactose and fructose is conducted are detected, and the area under a food blood glucose response curve of all time detection points of each rat in each group is calculated by adopting an integral method; a glucose experiment result is taken as the GI standard 100, and GI values of other food are calculated according to the following formula: GI=100*the area under a blood glucose response curve of the food to be verified / the area of a blood glucose response curve of glucose; trend comparison is conducted on the sequence of the food GI values verified from the rats and the sequence of the GI values verified from a human body, and 7-10-week-age SD male rats with the weight of 210 g to 350 g are taken as the animal experimental model for verifying the food blood glucose generation index.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com