Novel application of polypeptide Humanin
A function, polycystic ovary technology, applied in the direction of medical preparations containing active ingredients, peptide/protein components, microbial determination/inspection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
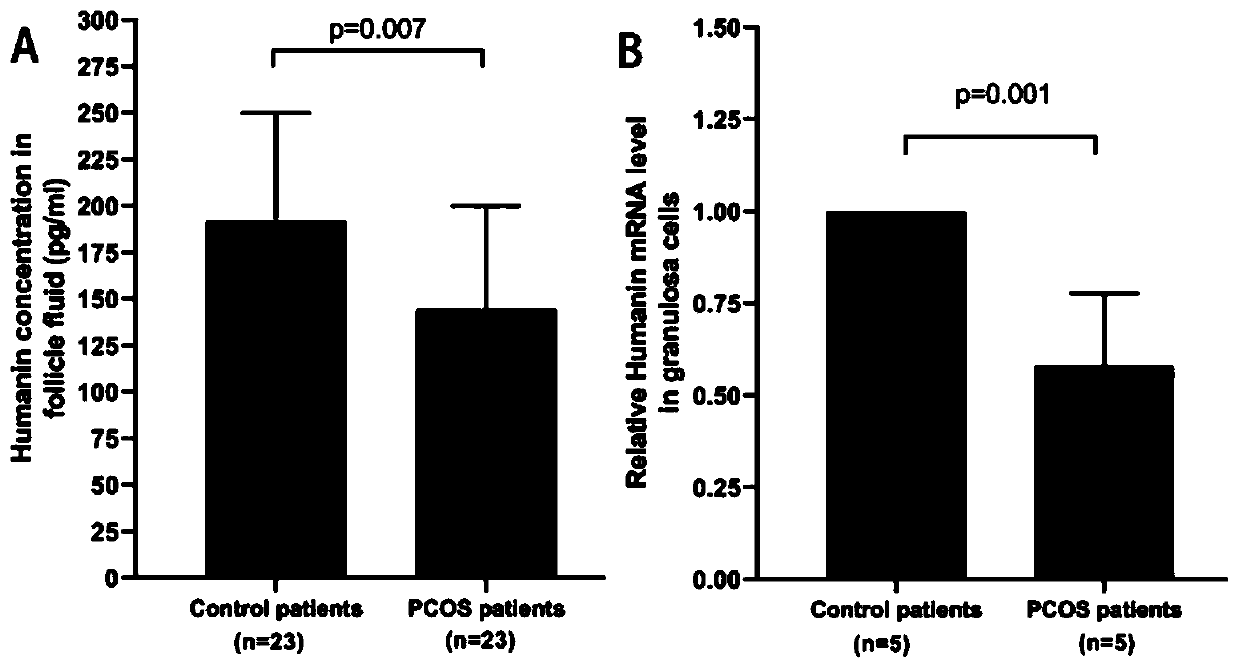
[0044] Example 1: Concentration of polypeptide Humanin in follicular fluid of PCOS patients and normal ovarian function population and expression in ovarian granulosa cells
[0045] Collect follicular fluid and ovarian granulosa cells from PCOS patients undergoing IVF / ICSI assisted pregnancy and from patients with non-ovarian factors receiving assisted reproductive technology (groups with normal ovarian function); they should first be approved by the ethics committee, and the patients should be fully informed and informed before collecting the samples. Sign the informed consent.
[0046] 1. Inclusion criteria for the PCOS group: ① Female, aged 22-35; ② Diagnosed with PCOS according to the Rotterdam criteria; ③ Patients with insulin resistance. Exclusion criteria: ① combined with other endocrine diseases, such as hyperthyroidism, hypothyroidism, etc.; ② combined with other ovarian diseases, such as chocolate cysts. The control group was patients who received assisted reproduct...
Embodiment 2
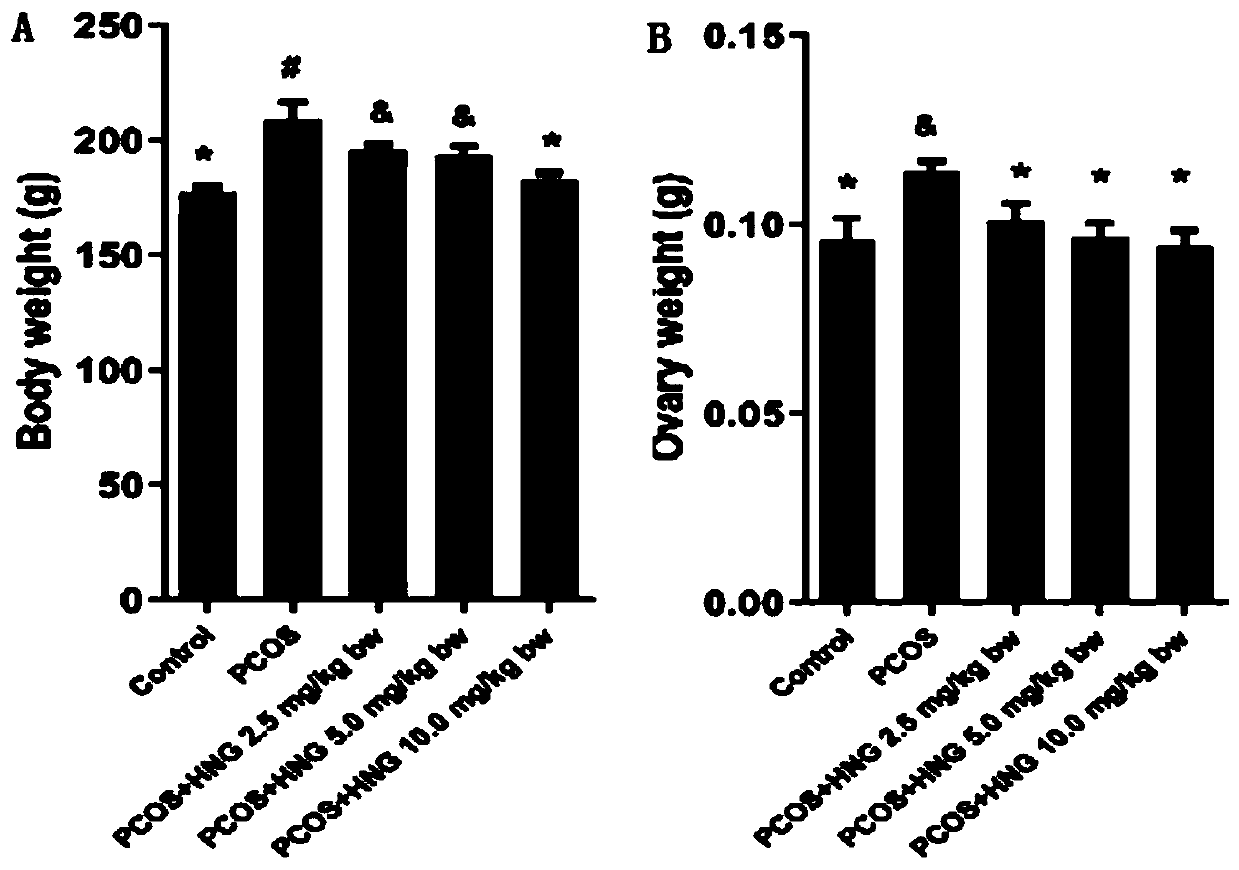
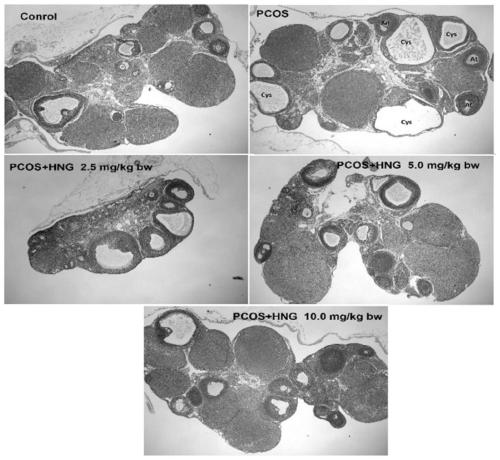
[0075] Example 2: Establishment of PCOS rat model and experiment on the effects of exogenous supplementary polypeptide Humanin (HNG) on oxidative stress, endocrine and ovulation status of PCOS rats
[0076] 1. Construction of PCOS rat model
[0077]Use DHEA (dehydroepiandrosterone) to establish a rat PCOS model; select 21-day-old weaned female SD rats (about 45g), inject DHEA subcutaneously, and the injection volume is 6mg / 100g body weight, and dissolve DHEA with 0.2mL injection oil , continuous injection for 20 days;
[0078] 2. Sixty 21-day-old SD rats were purchased from the Experimental Animal Center of Huazhong University of Science and Technology. The rats were randomly divided into 5 groups with 12 rats in each group. The specific groups are as follows:
[0079] A. PCOS rat model group
[0080] B. PCOS model + low dose HNG group
[0081] C. PCOS model + medium dose HNG group
[0082] D. PCOS model + high dose HNG group
[0083] E. Blank control group
[0084] The ...
Embodiment 3
[0107] Example 3: Construction of a cell model of oxidative stress injury, and the molecular mechanism experiment of polypeptide Humanin in alleviating oxidative stress 1. Identification of ovarian granulosa cell line COV434 and primary rat granulosa cells and verification experiment of Humanin expression in it
[0108] 1) Preparation of COV434 cell slides
[0109] a. Digest COV434 cells with trypsin infiltration method: Discard the medium, wash with PBS twice, add 300 μL of trypsin to the cell culture flask, completely infiltrate the cells, use a pipette to suck out the trypsin and discard it, and wait for the cells to digest into quicksand, add complete medium to stop digestion;
[0110] b. Inoculate the cells in a 24-well culture dish containing cell slides, and culture them in an incubator until the cells grow to the required experimental density;
[0111] 2) Extraction of primary rat granulosa cells and preparation of cell slides
[0112] a. 26-day-old SD rats were intr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com