A preparing method of high-purity antivenene
A technology for serum preparation and anti-snake venom, applied in the directions of anti-venom, antibodies, medical raw materials derived from mammals, etc., can solve the problems of large allergic side effects, difficult to further improve the purity, and complicated preparation process of snake venom wounds, so as to reduce allergies. Effects of Side Effects Risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
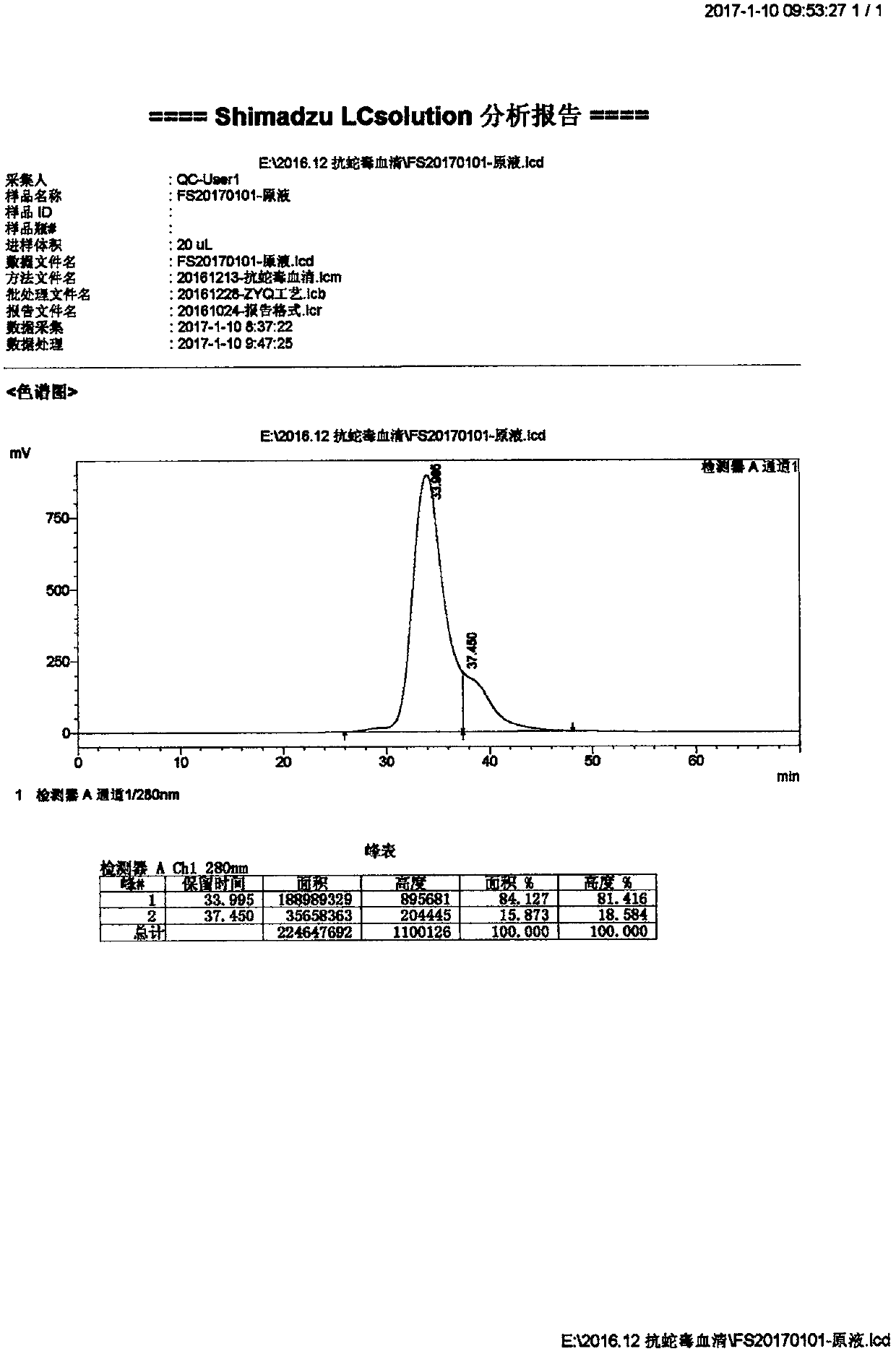
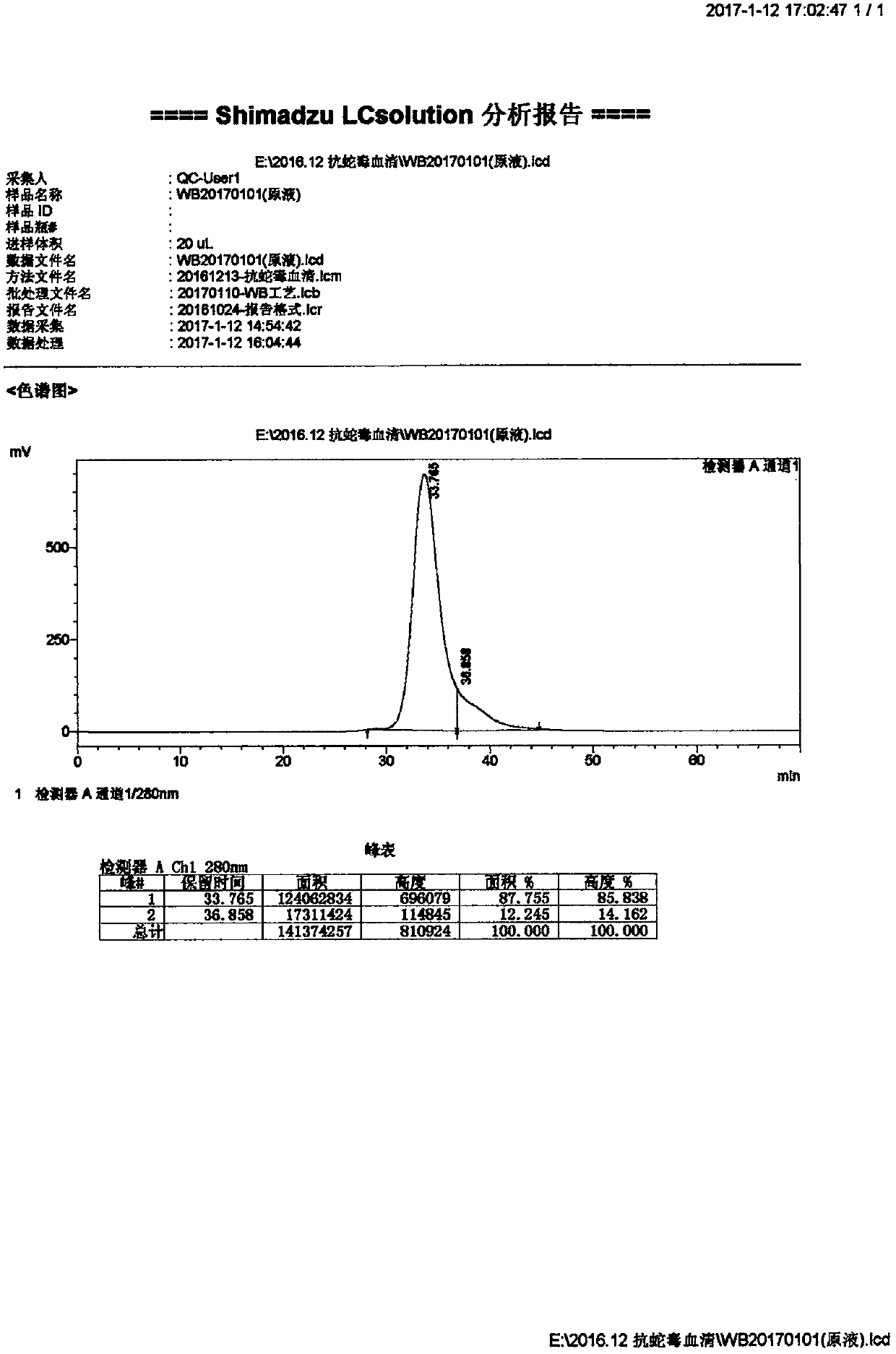
[0049] Product Name: Antivenom F(ab’) 2 High-purity antivenom preparations with a purity greater than 80%;
[0050] Material:
[0051] Puree: ultra-free horse plasma;
[0052] Diluent: water for injection;
[0053] αpH regulator: dilute hydrochloric acid 1mol / L;
[0054] βpH regulator: sodium hydroxide solution, 1mol / L;
[0055] γpH regulator: disodium hydrogen phosphate solution 0.5mol / L;
[0056] Enzymolysis agent: pepsin, specific activity 1:15000, 10mmmol / L, activated by hydrochloric acid;
[0057] Precipitant: octanoic acid, analytically pure;
[0058] Packing: ion exchange chromatography packing, anion-agarose DEAE-Sepharose;
[0059] Filtration membrane: polyethersulfone membrane 0.45μ;
[0060] Ultrafiltration membrane: polyethersulfone membrane 30kD;
[0061] Sterilization membrane: polyethersulfone film 0.2μ;
[0062] A buffer solution: 50mmol / L phosphate solution + 60mmol / L sodium chloride solution;
[0063] B buffer solution: 50mmol / L phosphate solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com