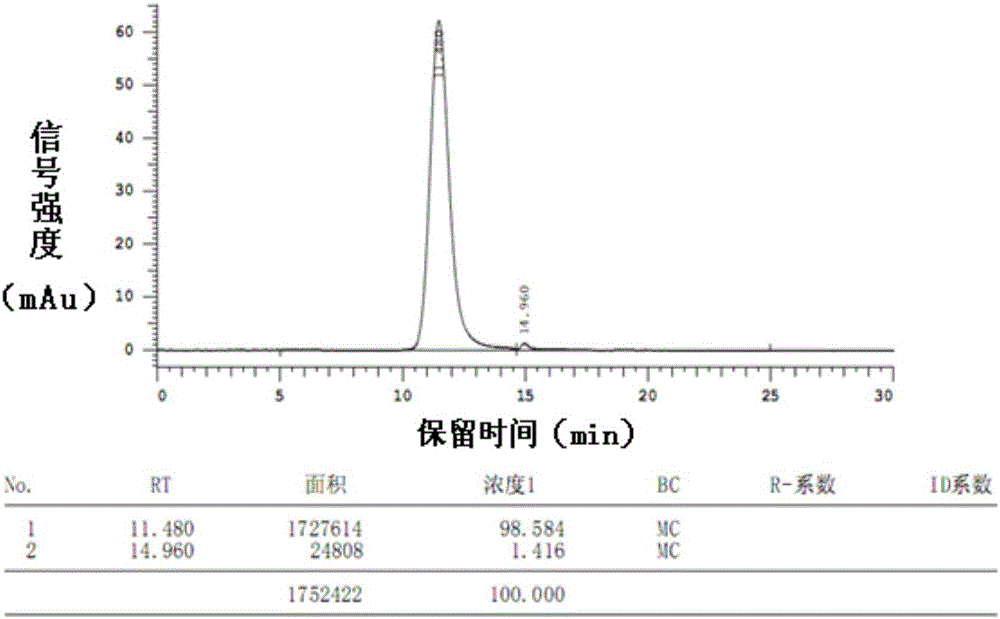
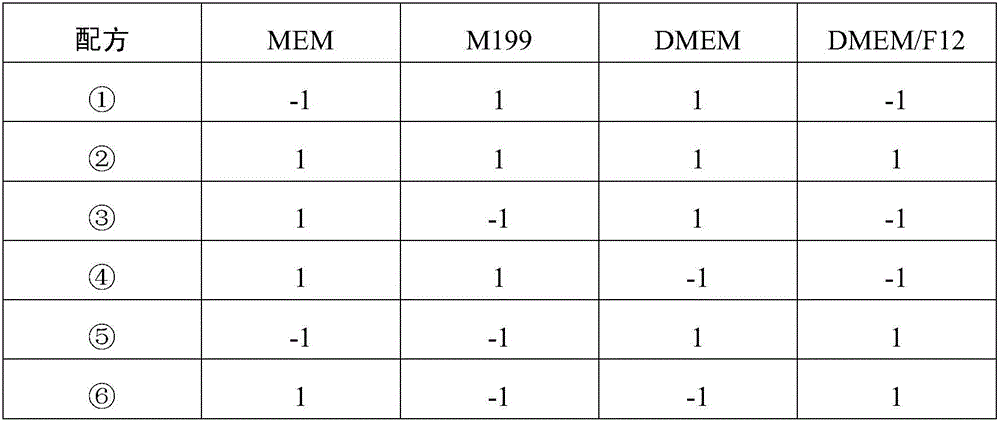
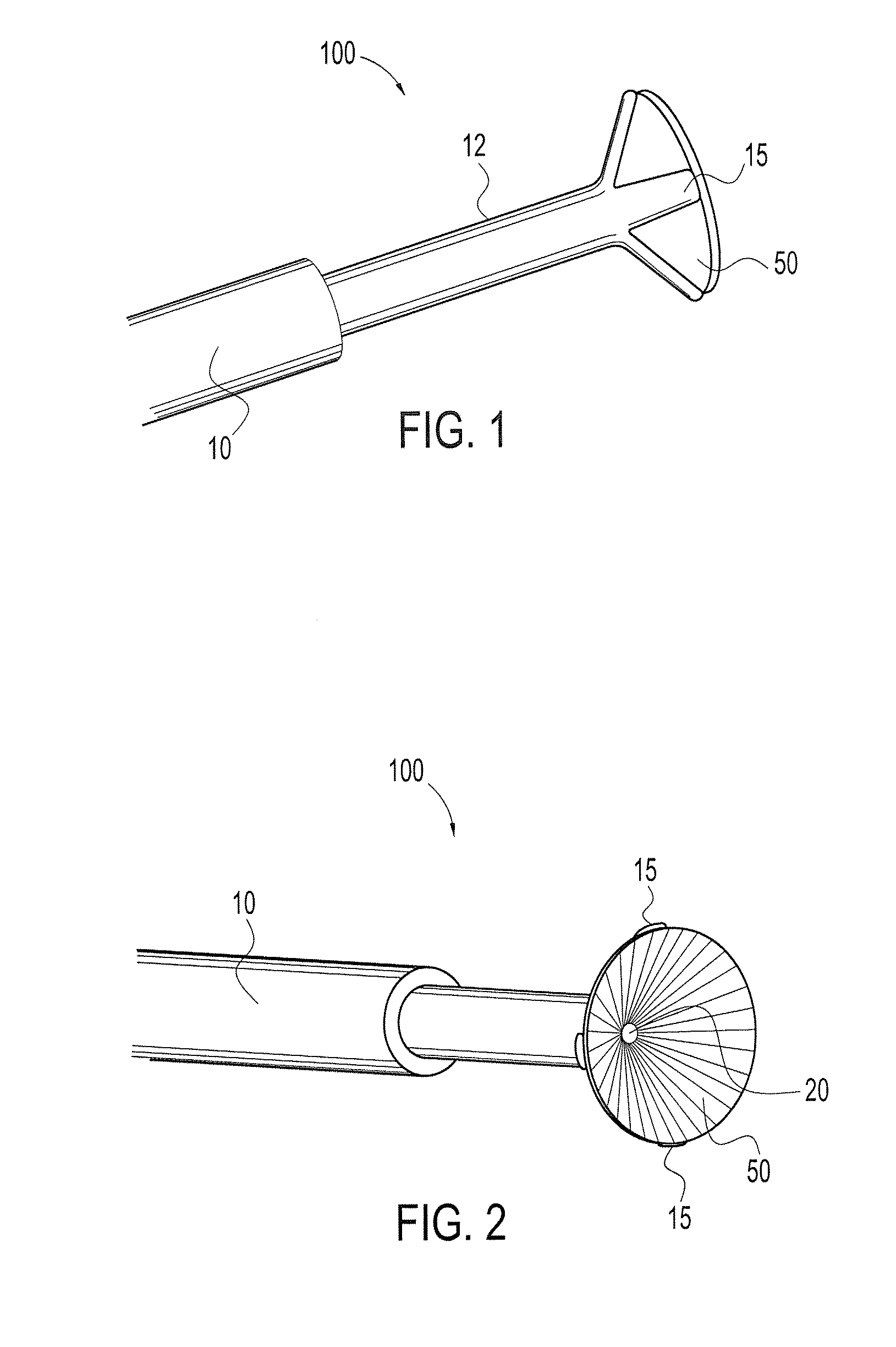
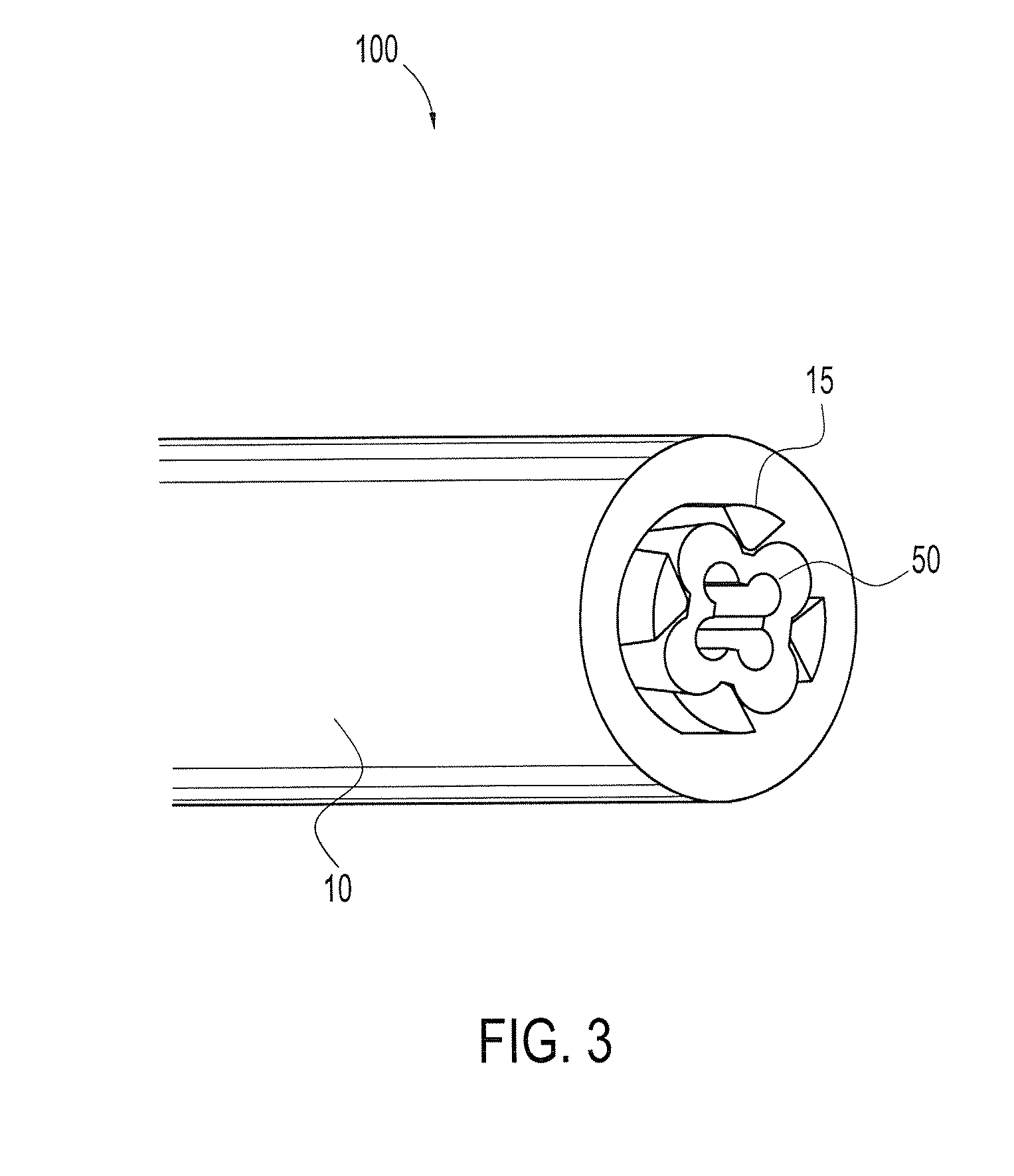
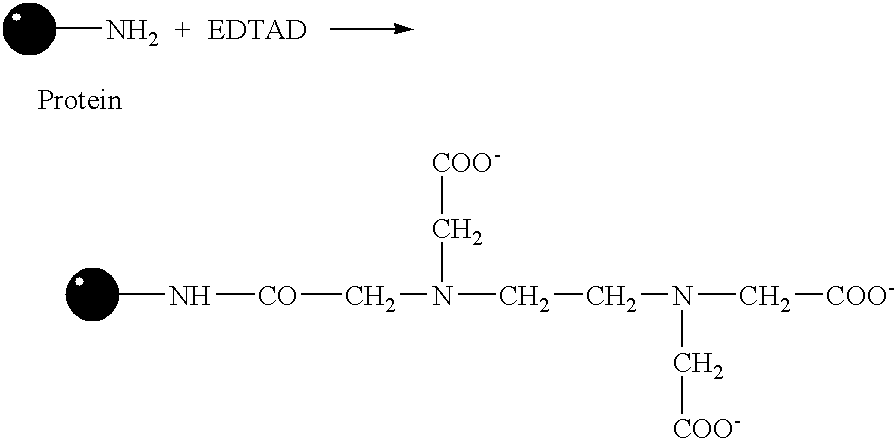
Patents
Literature
174 results about "Hybrid protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
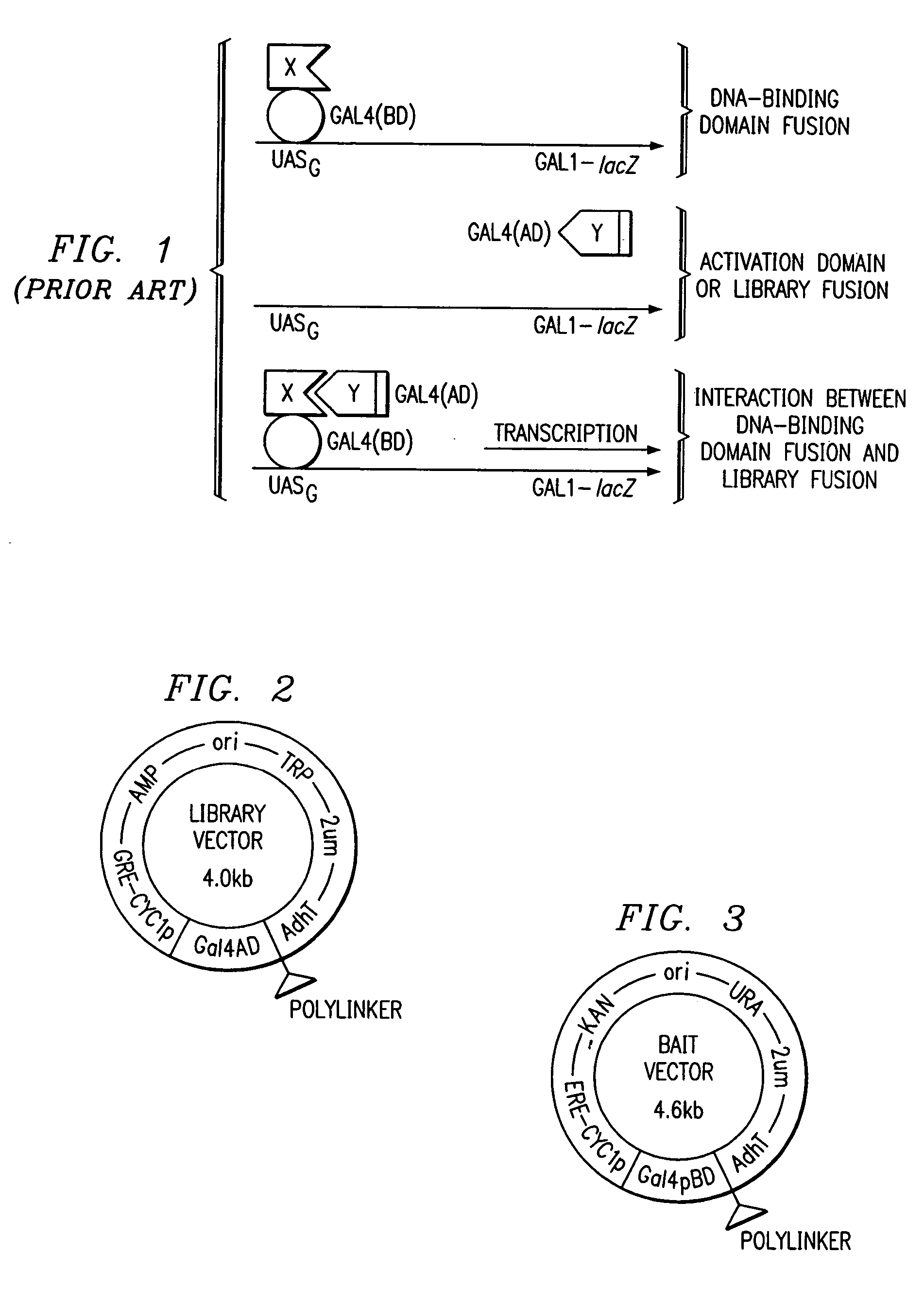
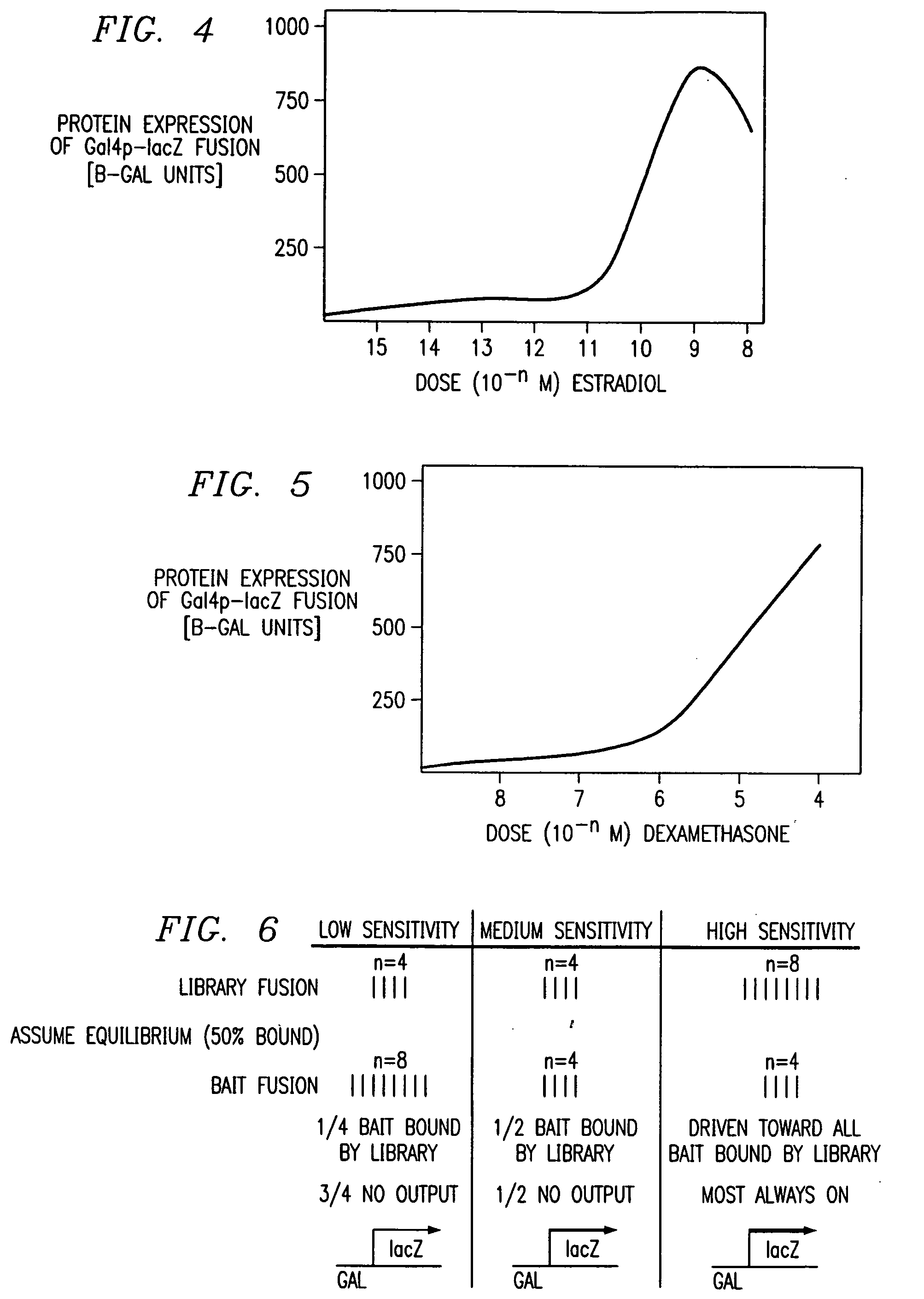
Two-hybrid screening (originally known as yeast two-hybrid system or Y2H) is a molecular biology technique used to discover protein–protein interactions (PPIs) and protein–DNA interactions by testing for physical interactions (such as binding) between two proteins or a single protein and a DNA molecule, respectively.
Gene recombination and hybrid protein development
InactiveUS20020045175A1Preserve stabilityPreserve desired propertyMicrobiological testing/measurementBiological testingBiopolymerHybrid protein
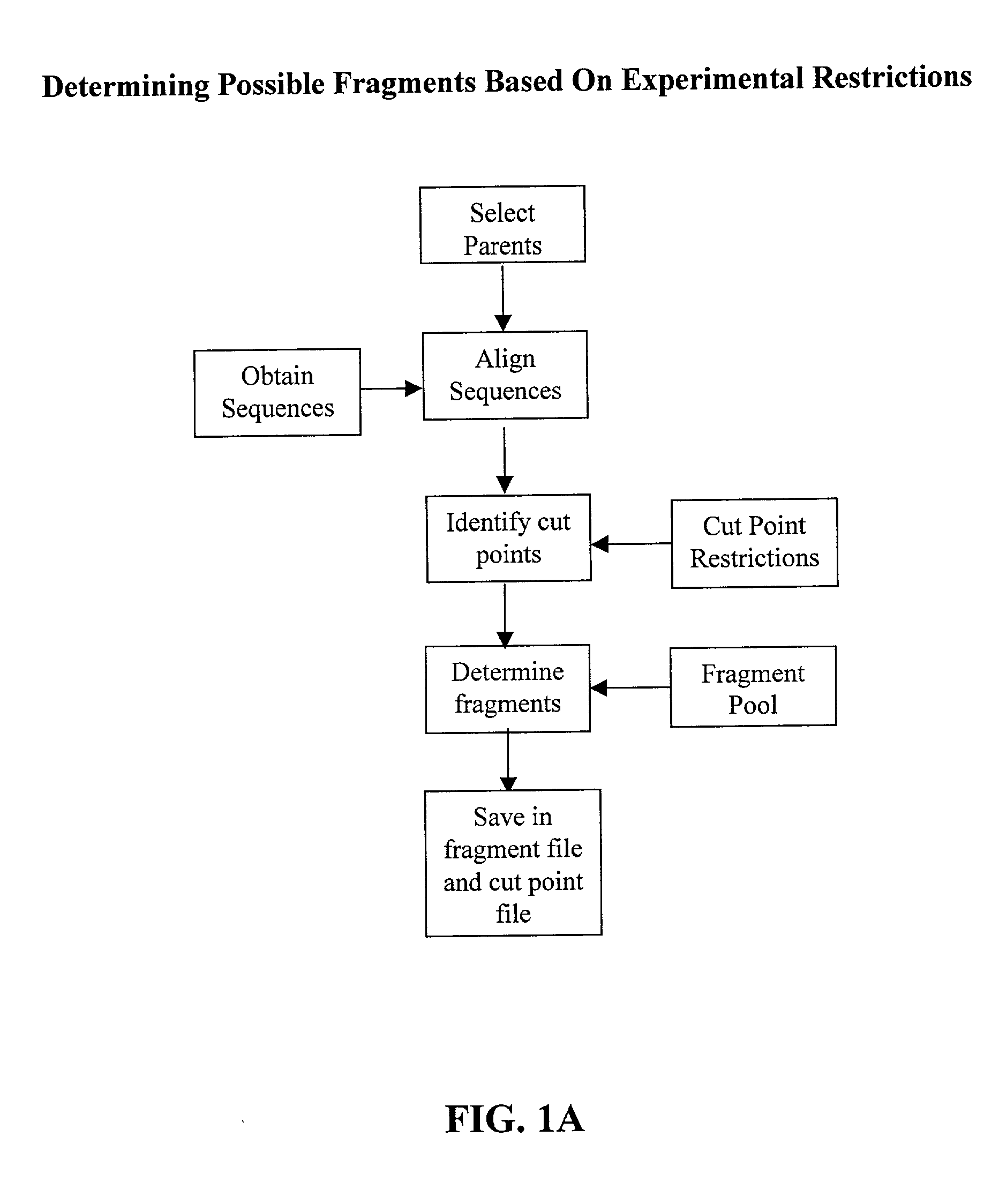
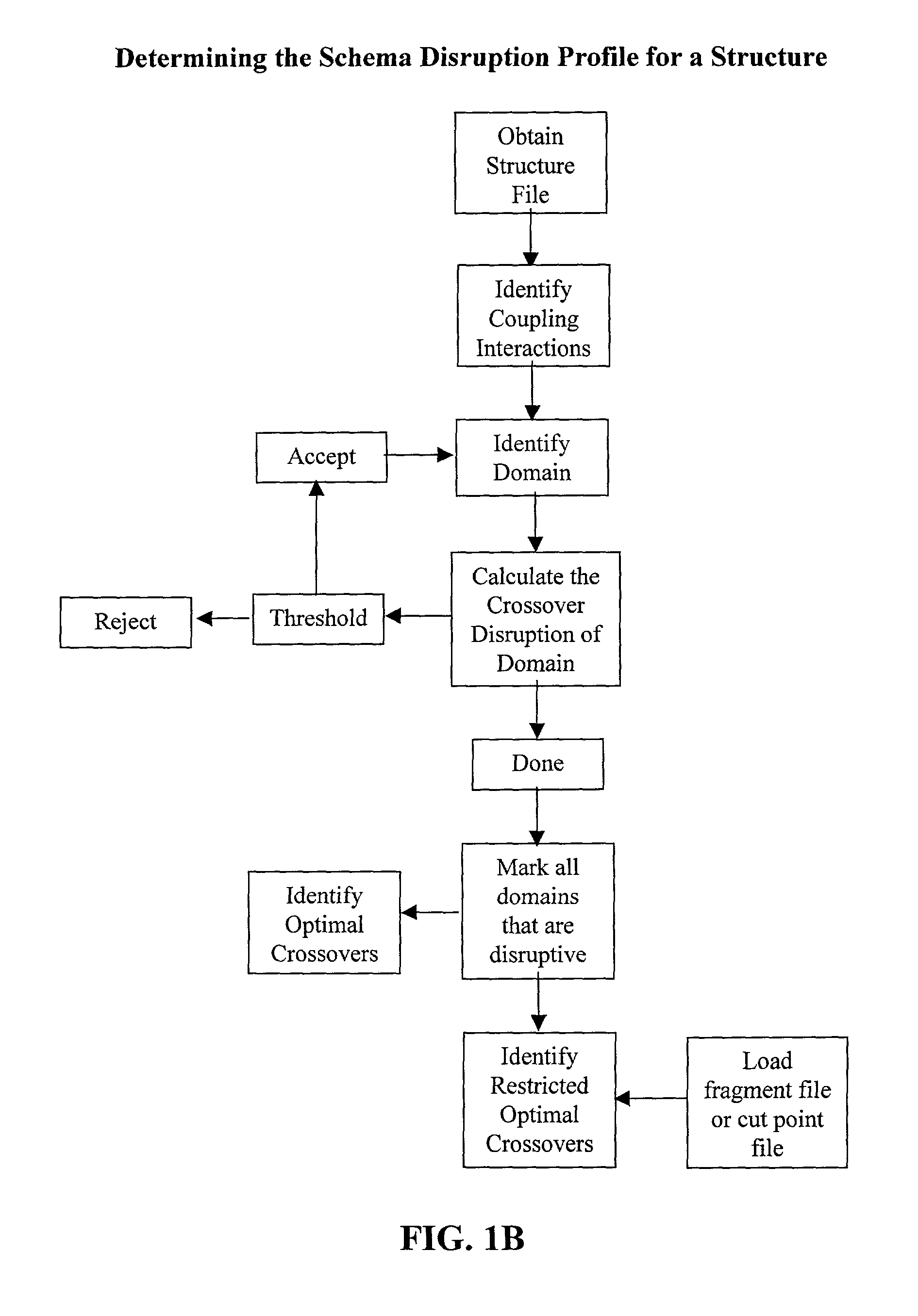
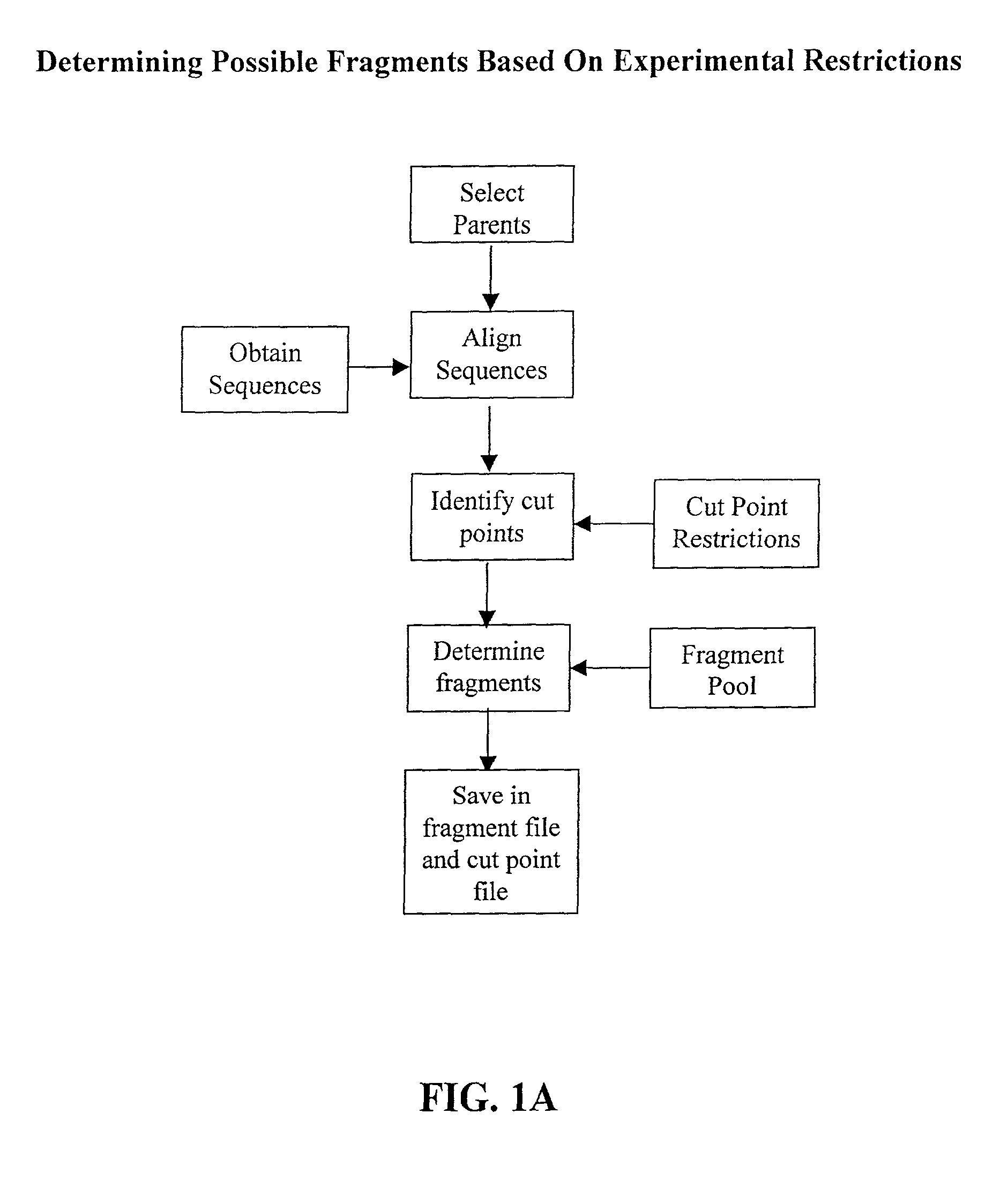
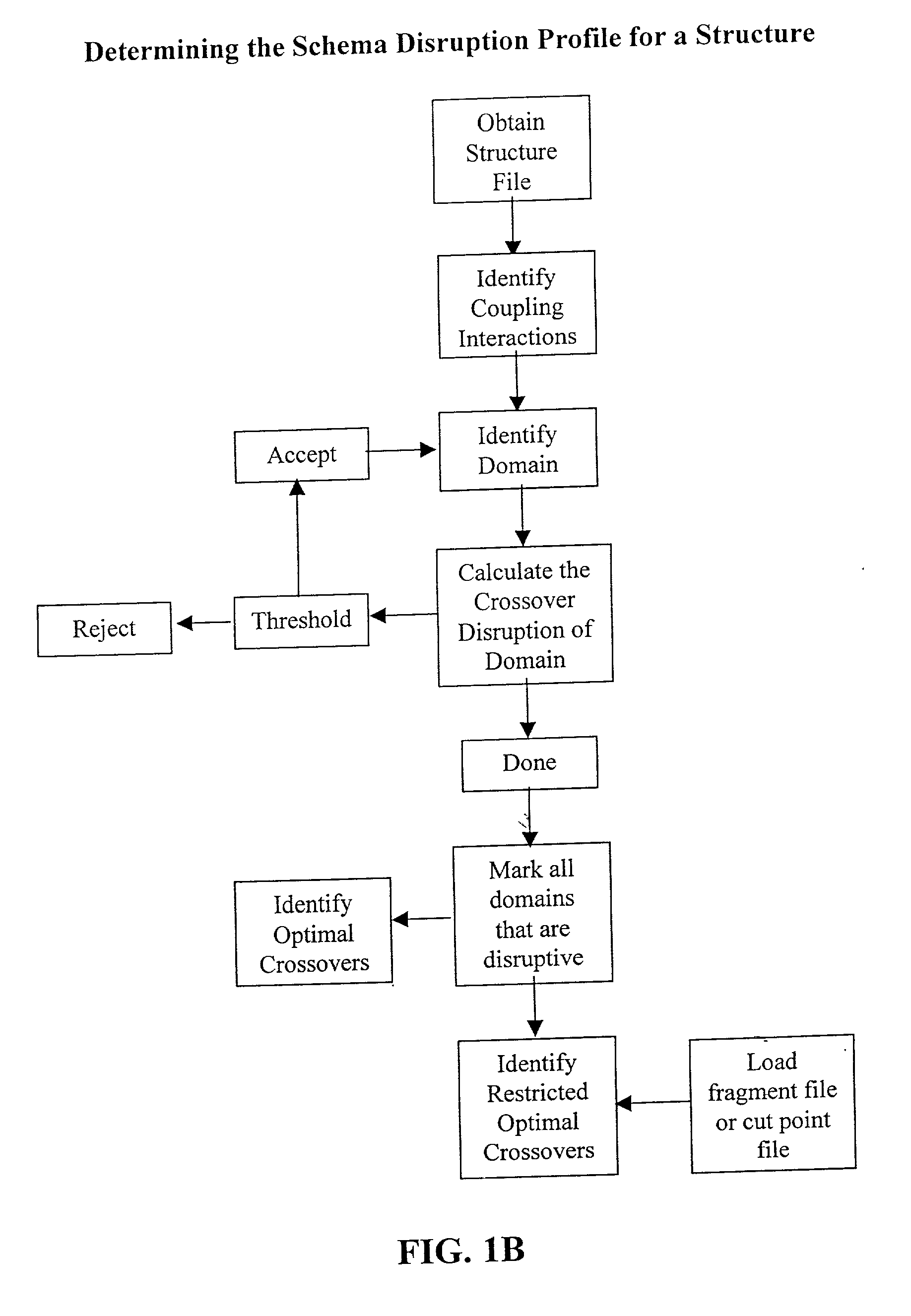
The invention relates to improved methods for directed evolution of polymers, including directed evolution of nucleic acids and proteins. Specifically, the methods of the invention include analytical methods for identifying "crossover locations" in a polymer. Crossovers at these locations are less likely to disrupt desirable properties of the protein, such as stability or functionality. The invention further provides improved methods for directed evolution wherein the polymer is selectively recombined at the identified "crossover locations". Crossover disruption profiles can be used to identify preferred crossover locations. Structural domains of a biopolymer can also be identified and analyzed, and domains can be organized into schema. Schema disruption profiles can be calculated, for example based on conformational energy or interatomic distances, and these can be used to identify preferred or candidate crossover locations. Computer systems for implementing analytical methods of the invention are also provided.
Owner:CALIFORNIA INST OF TECH
Inactivated Zika virus vaccine
ActiveCN105749268AEase the epidemicReduce the burden onSsRNA viruses positive-senseViral antigen ingredientsZika virusSide effect
The invention provides an inactivated Zika virus vaccine. The inactivated Zika virus vaccine is obtained by: performing ultrafiltration and concentration on Zika virus liquid after inactivation, centrifuging the concentrated virus liquid by adopting a sucrose density zone, performing ion exchange and concentration sterilization on a centrifugal product to obtain a Zika virus vaccine stock solution, diluting the vaccine stock solution until the total protein content is not more than 20mu g / ml, and adding an adjuvant to obtain a vaccine semi-finished product. The method for preparing the vaccine provided by the invention is simple, convenient and easy to operate, the cost is saved, the produced vaccine is suitable for Asian people, a unit dose of the Zika virus liquid is high in immunogenicity, the content of hybrid protein is low, the side effect after injection is small, and the safety is high, so that the vaccine is suitable for vaccination of fertile women before pregnancy, can avoid newborn Brazil microcephaly caused by infection of Zika virus, and is significant in social value and market efficiency.
Owner:SINOVAC RES & DEV
Methods of arthroscopic osteochondral resurfacing
Methods of arthroscopic resurfacing of anatomical tissue utilizing a biological component strengthened with glue or similar material. The biological component is selected from the group consisting of blood, blood components, PRP, bone marrow aspirate (BMA) and autologous conditioned plasma (ACP). The biological component / glue composition may be inserted (by injection and / or by employing a biologic resurfacing mold, for example) into, or in the vicinity of, the anatomical tissue. Upon insertion within, or contact with, the anatomical tissue, the biological component / glue composition is designed to coagulate and solidify (or partially solidify) within minutes, to advance healing or tissue growth of the anatomical tissue. The biological component / glue composition may optionally comprise components such as growth factors, antiseptic chemicals and / or antibiotics and / or electrolytes, or hormones or site-specific hybrid proteins, among others.
Owner:ARTHREX
Gene recombination and hybrid protein development
InactiveUS20030032059A1Preserve stability and desired propertyComputationally tractableBiological testingSequence analysisBiopolymerProtein insertion
The invention relates to improved methods for directed evolution of polymers, including directed evolution of nucleic acids and proteins. Specifically, the methods of the invention include analytical methods for identifying "crossover locations" in a polymer. Crossovers at these locations are less likely to disrupt desirable properties of the protein, such as stability or functionality. The invention further provides improved methods for directed evolution wherein the polymer is selectively recombined at the identified "crossover locations". Crossover disruption profiles can be used to identify preferred crossover locations. Structural domains of a biopolymer can also be identified and analyzed, and domains can be organized into schema. Schema disruption profiles can be calculated, for example based on conformational energy or interatomic distances, and these can be used to identify preferred or candidate crossover locations. Computer systems for implementing analytical methods of the invention are also provided.
Owner:WANG ZHEN GANG +3
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS6822076B2Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsTetanusBasophilia
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-lIe-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Protein-polysaccharide hybrid hydrogels
InactiveUS6821331B2Improve featuresIncrease contentProtein waste adhesivesOther chemical processesCross-linkHybrid protein
Disclosed is a hybrid protein-polysaccharide superabsorbent hydrogel. The hydrogel includes two interpenetrating matrices: a first matrix which is an acylated, cross-linked protein matrix, and a second matrix which is an anionic polysaccharidc matrix. The two matrices can be non-cross-linked, or the hydrogel can further include bridging moieties that covalently cross-linking the acylated, cross-linked protein matrix to the anionic polysaccharide matrix.
Owner:WISCONSIN ALUMNI RES FOUND
Preparation method of low molecular weight aquatic collagen peptide
InactiveCN102260727ALow Molecular Weight Collagen ContentLow molecular weight collagen content High molecular weight collagen contentPeptide preparation methodsFermentationFiltrationFreeze-drying
The invention discloses a method for preparing low-molecular-weight aquatic collagen peptides. Fresh fish scales are delimed with hydrochloric acid, fat and impurities are removed with caustic soda, and then hot water extraction is carried out to obtain a collagen solution, and three kinds of enzymes are used to separate enzyme Decompose collagen, then carry out activated carbon filtration and macroporous resin treatment, collect decolorization liquid, filter through ultrafiltration membrane, freeze-dry, the effective method of preparation molecular weight concentration, high recovery rate, good decolorization effect fish scale low molecular weight collagen, not only can Obtain high-content low-molecular-weight collagen peptides from fish skin and fish scales, remove pigments that affect the appearance of collagen products, and reduce the unique fishy smell of collagen, which can improve the product value of fish collagen products.
Owner:申铉日
Method of arthroscopic osteochondral resurfacing using PRP strengthened with fibrin glue
Methods of arthroscopic resurfacing of a joint utilizing a biological component strengthened with fibrin glue. The biological component is selected from the group consisting of PRP, bone marrow aspirate (BMA) and autologous conditioned plasma (ACP). The biological component / fibrin glue composition may be inserted (by injection or by employing a biologic resurfacing mold, for example) into a transosseous tunnel in the vicinity of the defect to be repaired. Upon insertion at the defect site, the biological component / fibrin glue composition is designed to coagulate and solidify within few minutes, to advance the healing of the damaged tissue and tissue growth. The biological component / fibrin glue composition may optionally comprise components such as growth factors, antiseptic chemicals and / or antibiotics and / or electrolytes, or hormones or site-specific hybrid proteins, among others.
Owner:ARTHREX
II-type collagen joint cartilage fluid and preparation method thereof
ActiveCN101810855APromotes self-healingImprove lubrication functionPeptide/protein ingredientsSkeletal disorderHazardous substanceHybrid protein
The invention provides II-type collagen joint cartilage fluid and a preparation method thereof. Bacteria, viruses and other hazardous substances of the joint cartilage are removed through degreasing-> serum removing-> surfactant treatment-> oxidant treatment-> disinfection and other links. Hybrid protein and other denatured protein of the cartilage are removed through acid hydrolysis, salt precipitation, dialysis and other processes, and high-purity and high-activity II-type collagen solution is prepared. Non-toxin and non-heat raw materials and auxiliary materials and isotonic solution are mixed in an appropriate ratio, and filled in a sterile non-heat disposable injector, to prepare II-type collagen joint cartilage fluid for disposable injection. Other components of the II-type collagen joint cartilage fluid comprise the auxiliary materials and the isotonic solution. The II-type collagen joint cartilage fluid can play the roles of lubrication, pain relieving and joint activity degree improving to the injured joints, fundamentally realizes the internal repair to the cartilage tissue, and is applicable to a patient with various joint cartilage injuries and all types of arthritis.
Owner:GUANGZHOU TRAUER BIOTECH
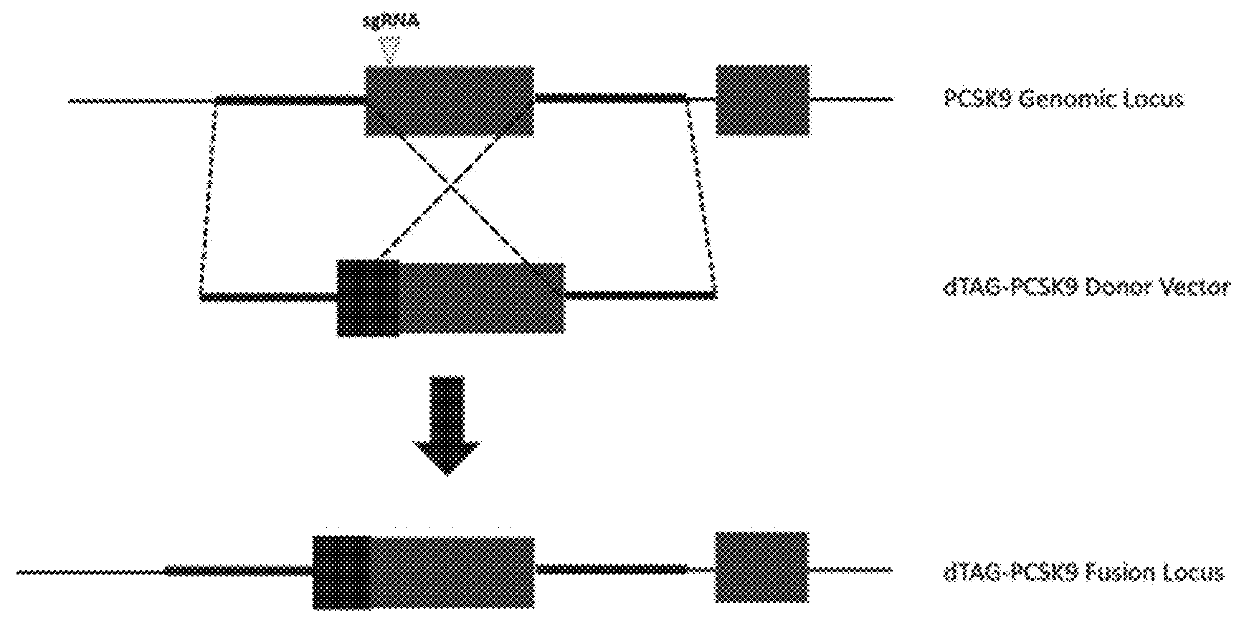
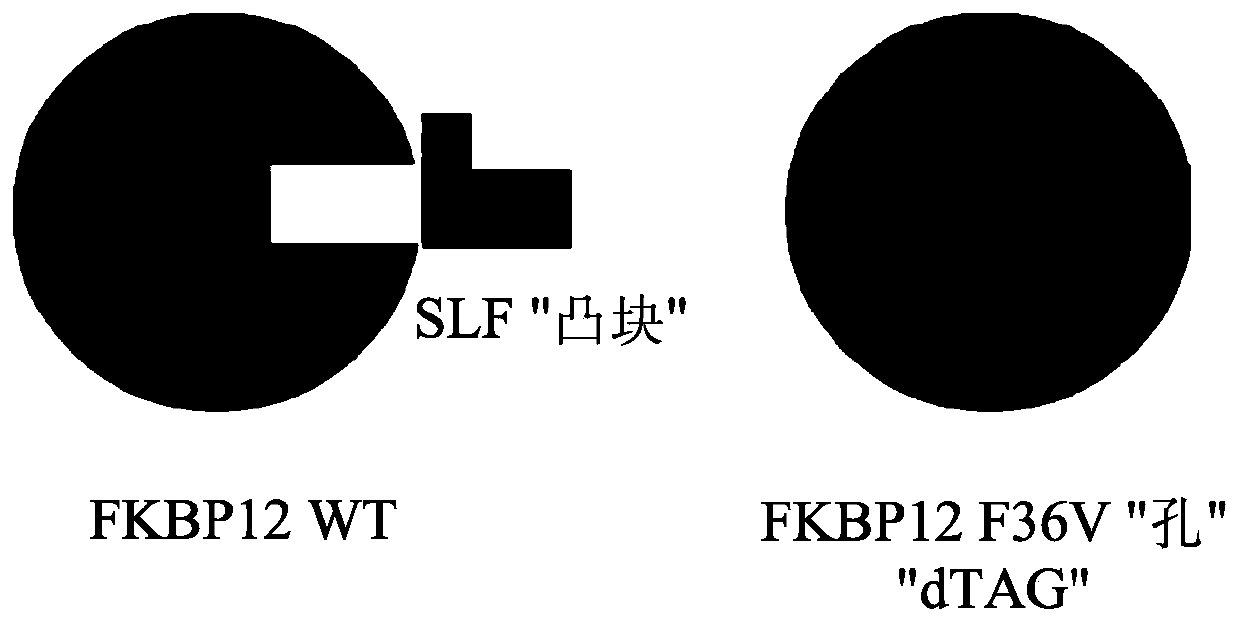
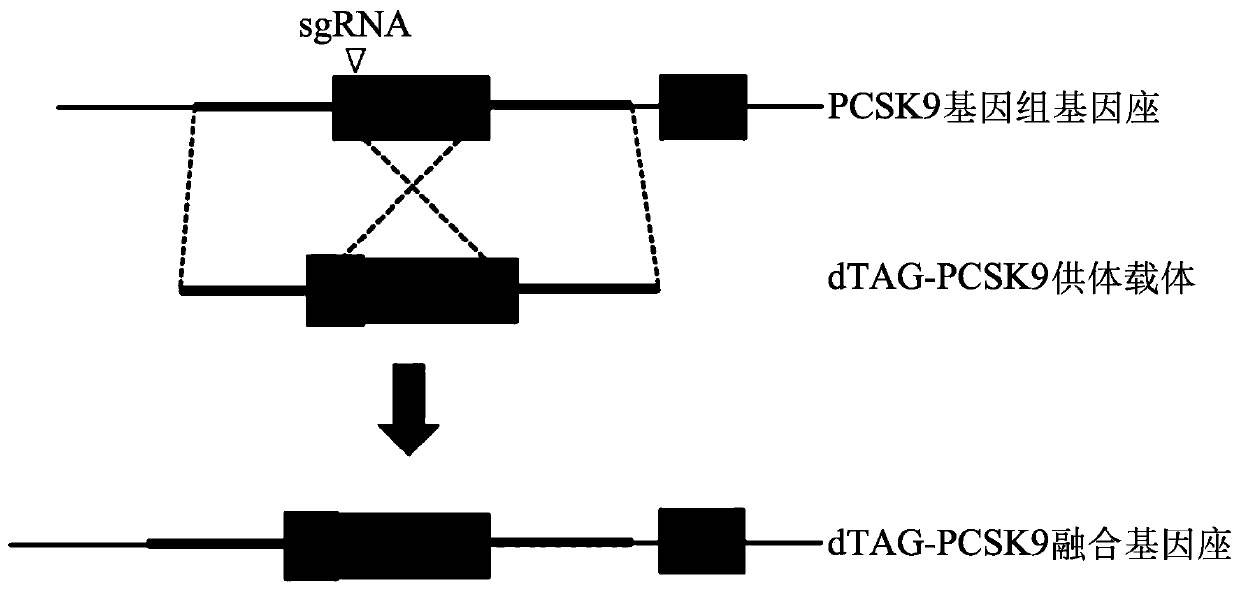
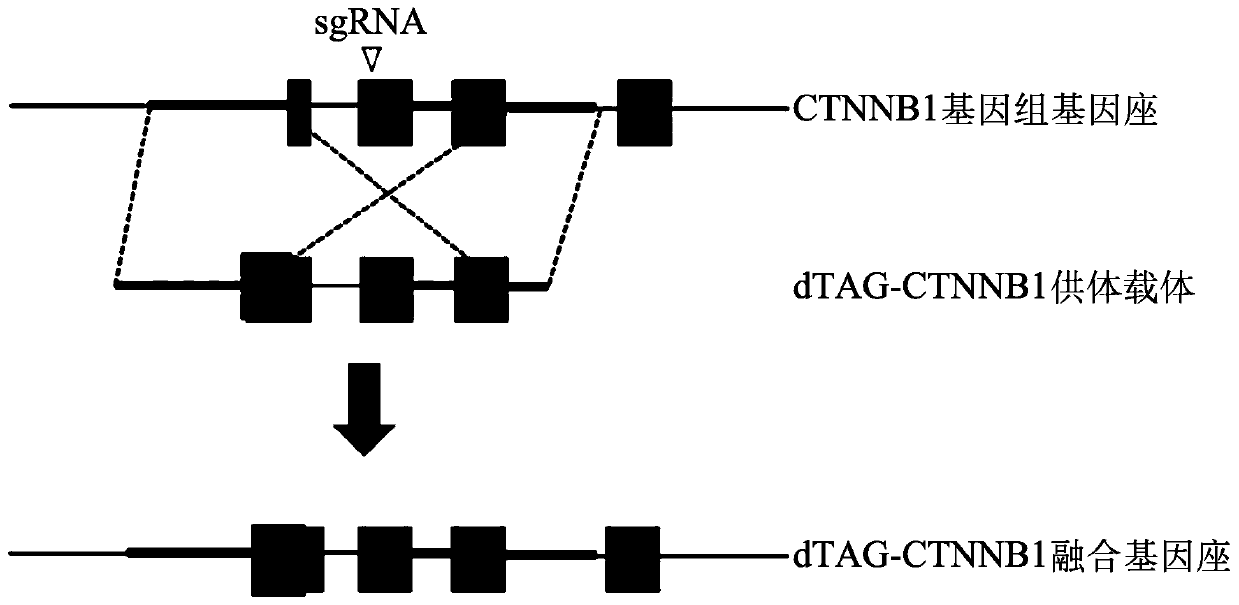
Tunable endogenous protein degradation
ActiveUS20180179522A1Negative consequencePermanently editing a gene can be avoidedOrganic active ingredientsFusion with degradation motifProtein targetNucleotide
Owner:DANA FARBER CANCER INST INC
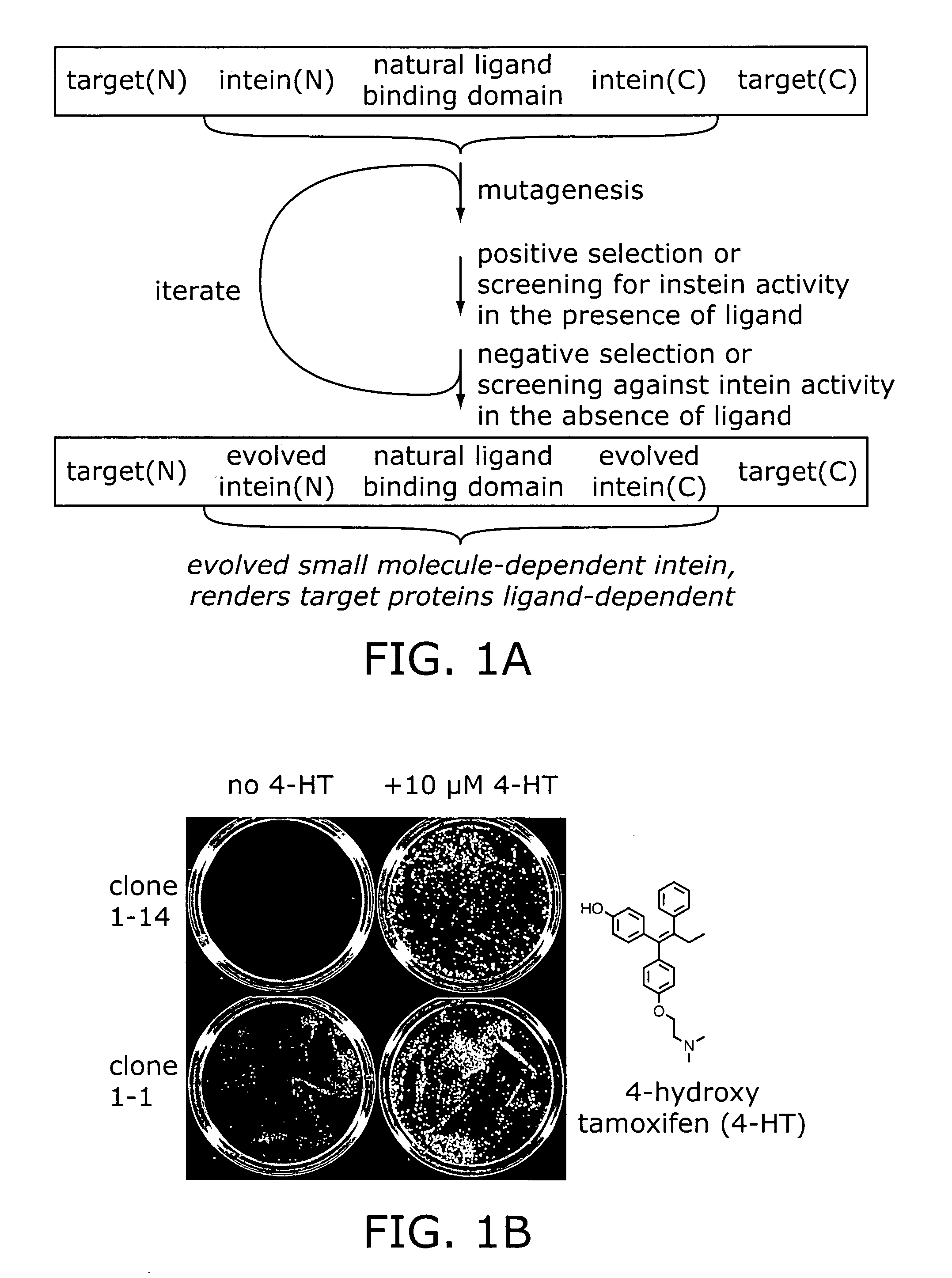
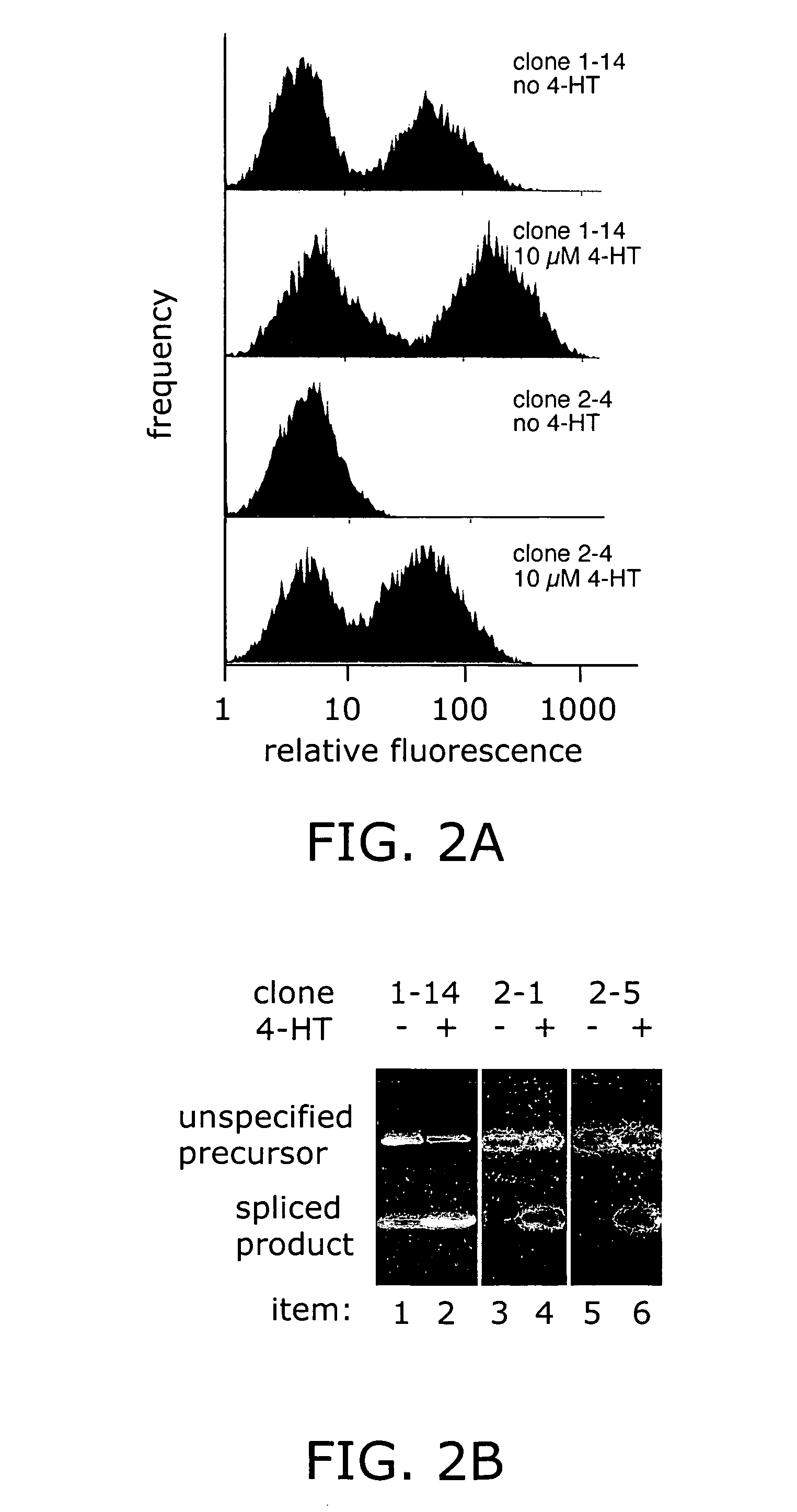
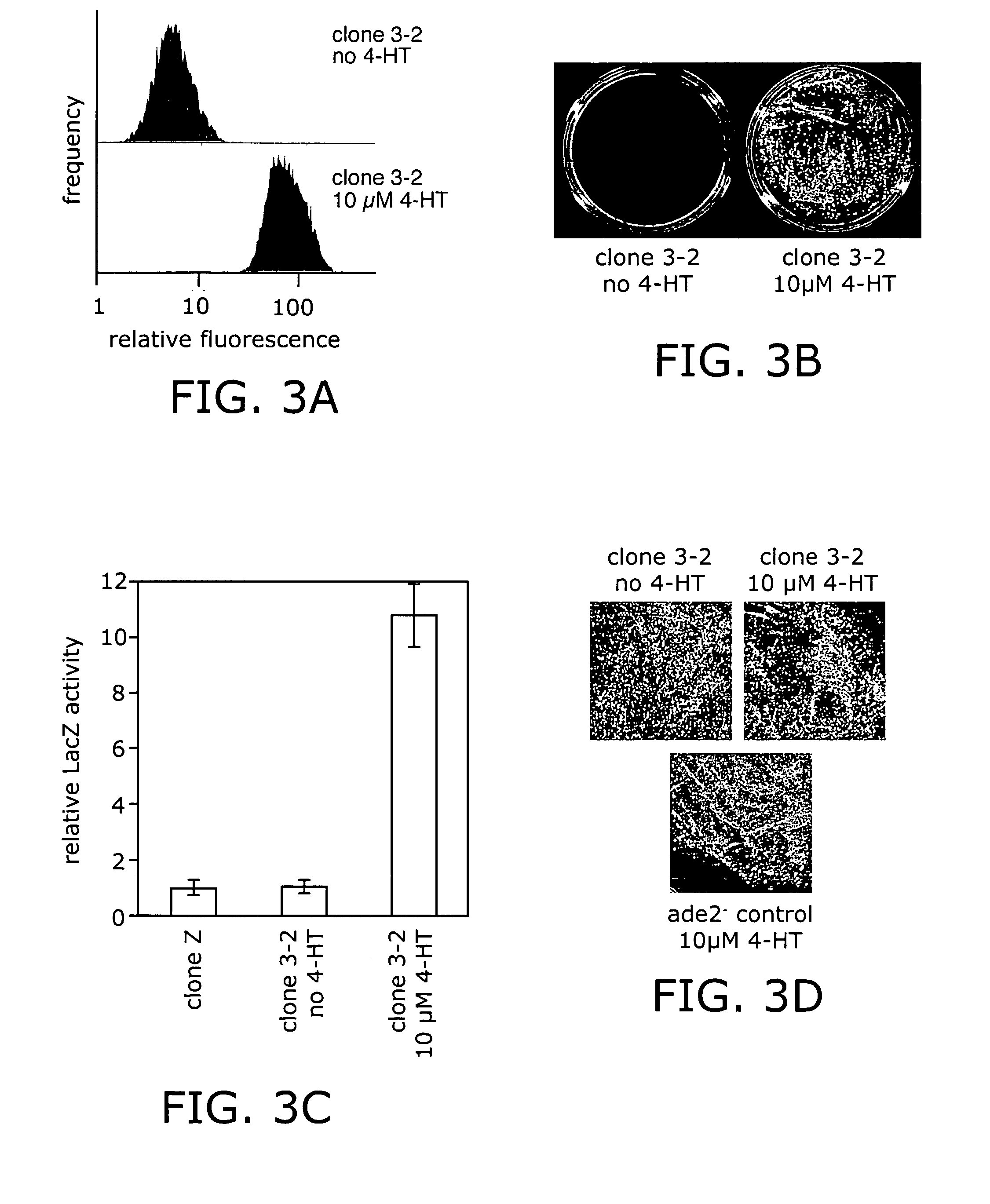
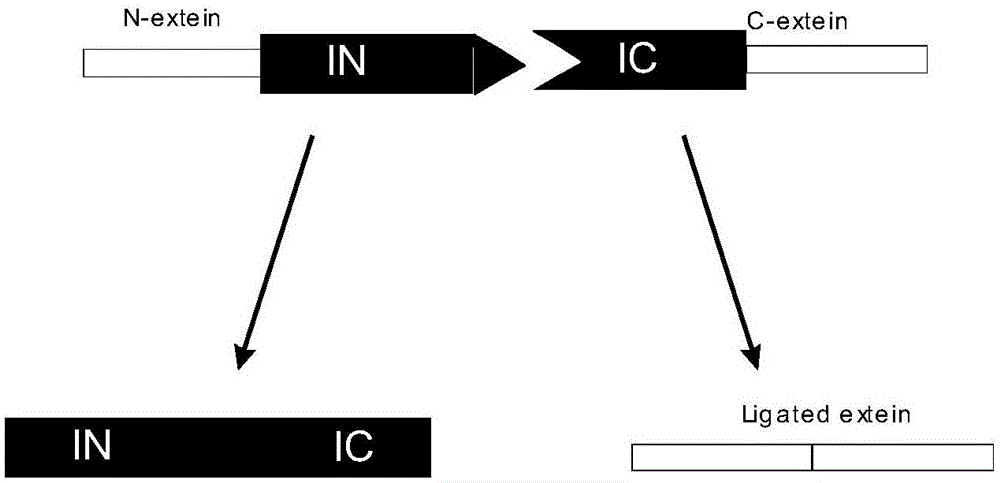
Ligand-dependent protein splicing
Ligand-dependent inteins allow for modulation of a protein's activity in vivo. Upon binding of the ligand to the ligand-dependent intein inserted into the protein of interest, the hybrid protein undergoes protein splicing removing the intein. The activity of the spliced protein is then restored. A 4-hydroxytamoxifen-dependent intein based on the M. tuberculosis RecA intein is prepared and demonstrated in a variety of exteins contexts. The invention provides a system for engineering other ligand-dependent inteins and using them, including the ligand-dependent inteins themselves, hybrid proteins with the inserted ligand-dependent inteins, polynucleotides encoding inteins and hybrid proteins, and engineered cells. Kits with the materials and reagents necessary for preparing and using ligand-dependent inteins are also included.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Process for extracellularly producing recombinant alpha-cyclodextrin glucosyltransferase
ActiveCN101831414AReduce pollutionImprove purification efficiencyTransferasesMicroorganism based processesHybrid proteinOxygen
The invention discloses a process for producing recombinant alpha-cyclodextrin glucosyltransferase in a fermenting way by culturing escherichia coli in high density through a temperature two-stage control strategy and a constant oxygen-dissolved and feed-supplemented batch technology, belonging to the technical field of fermentation engineering. The method comprises the following steps of: inoculating recombinant escherichia coli BL21(DE3) as a production strain at the inoculation quantity of 5-10 percent and fermenting in batch; starting adding a supplementing liquid in a flowing mode when dissolved oxygen rises to about 80-100 percent, ensuring that a strain body exponentially grows, controlling the temperature of the growth phrase of the strain body to be 33-37DEG C and maintaining the dissolved oxygen to 20-30 percent; adding 0.75-1.5 percent (mass volume percent) of glycine when the strain body OD600 reaches about 15-30; reducing the temperature to be 23-27DEG C and continuously supplementing 0.2-0.4g.l<-1>.h<-1> when the strain body OD600 reaches 45-60 and supplementing the supplementing liquid by adopting a gradient diminishing mode at the same time; and enabling the enzymeactivity of the extracellular alpha-cyclodextrin glucosyltransferase to reach 200-280U / ml by fermenting culture for 30-35 hours. By adopting the strategy to ferment, the invention realizes the high-efficiency extracellular expression of the alpha-cyclodextrin glucosyltransferase and greatly improves the production intensity. The invention has the advantages of wide source of raw materials, simple and feasible process and suitability for large-scale production; with effective extracellular expression, the pollution on host bacteria hybrid proteins can be greatly reduced and the purification efficiency of the protein can be improved. The invention lays a foundation for large-scale production of the alpha-cyclodextrin glucosyltransferase.
Owner:JIANGNAN UNIV
Preparation method of Lumbrokinase
The invention discloses a preparation method of Lumbrokinase, comprising the steps of grinding, deslagging, salting-out, centrifuging, ultra-filtrating, column chromatography isolating, ultra-filtrating, degerming, spray-drying and the like. The preparation method of Lumbrokinase provided by the invention is that ethanol is added as adjuvant to remove hybrid protein by physical settlement and ultrafiltration during the homogenate of earthworms, and then a column chromatography purification is performed so that the capacity of the column is reduced, thereby the yield of the Lumbrokinase is increased, the preparation cost is reduced, the waste fluid to be treated is decreased and the production capacity is increased greatly. The preparation method of Lumbrokinase has the characteristics of good quality controllability and stability of the production process.
Owner:QD BIO PHARMA
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS20030059912A1Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsADAMTS ProteinsPotassium
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-Ile-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Botulinum toxin assay with improved sensitivity
ActiveUS9526345B2High sensitivityLow sodiumMicrobiological testing/measurementBiological material analysisHybrid proteinToxin
Methods and compositions are provided where a transfected cell that produces a hybrid protein with a reporter-containing portion and a botulinum toxin cleavage site is contacted with a botulinum toxin at elevated temperatures and / or in media having a reduced sodium concentration. Kits that include such media and a botulinum toxin are also described.
Owner:BIOMADISON
Hybrid protein rapid enzymolysis method
InactiveCN101457247AShorten the timeReduce preprocessing stepsMaterial analysis by electric/magnetic meansFermentationProteinase activityProtein insertion
The invention relates to a method for fast enzymolysis of hybrid proteins comprising: providing protein samples to be enzymolysis; obtaining the denatured protein samples after the degeneration treatment of said protein samples; treating said denatured protein samples by means of protease enzymolysis and assistant with ultrasonic, obtaining the protein polypeptide fragments. The invention also provides a method for fast identifying the protein. The method of the invention can quickly and high efficiently implement the protein enzymolysis and identification with easy operation and high accuracy.
Owner:EAST CHINA UNIV OF SCI & TECH
Alkaline pectate lyase producing gene engineering bacteria and construction and use thereof
InactiveCN102191212AIncrease productionShort fermentation timeBacteriaMicroorganism based processesHybrid proteinGenetic engineering
The invention discloses alkaline pectate lyase producing gene engineering bacteria and construction and use thereof and belongs to the field of genetic engineering. In the invention, the Escherichia coli pel engineering bacteria capable of secreting and expressing pectinase Pel in vitro with high efficiency are constructed from an industrial prospective by using a totally synthetic Aspergillus nidulans pel gene. The invention also provides a method for producing pectinase by using the engineering bacteria. When the engineering bacteria are used, the fermentation time is short, and at 37 DEG C, the total fermentation lasts for 6 hours, wherein the early fermentation lasts for 4 hours and post fermentation lasts for 6 hours; the enzyme active yield is as high as 400U / mL; in addition, the produced pectinase can be secreted in vitro directly, hybrid proteins are reduced, and the purification is easy. Therefore, the engineering bacteria have better industrial application prospect.
Owner:YANCHENG TEACHERS UNIV
Methods of arthroscopic osteochondral resurfacing
ActiveUS20090060974A1Promote healingSkeletal disorderMammal material medical ingredientsBlood componentMedicine
Methods of arthroscopic resurfacing of anatomical tissue utilizing a biological component strengthened with glue or similar material. The biological component is selected from the group consisting of blood, blood components, PRP, bone marrow aspirate (BMA) and autologous conditioned plasma (ACP). The biological component / glue composition may be inserted (by injection and / or by employing a biologic resurfacing mold, for example) into, or in the vicinity of, the anatomical tissue. Upon insertion within, or contact with, the anatomical tissue, the biological component / glue composition is designed to coagulate and solidify (or partially solidify) within minutes, to advance healing or tissue growth of the anatomical tissue. The biological component / glue composition may optionally comprise components such as growth factors, antiseptic chemicals and / or antibiotics and / or electrolytes, or hormones or site-specific hybrid proteins, among others.
Owner:ARTHREX
Method and kit for detection of multiple protein interactions
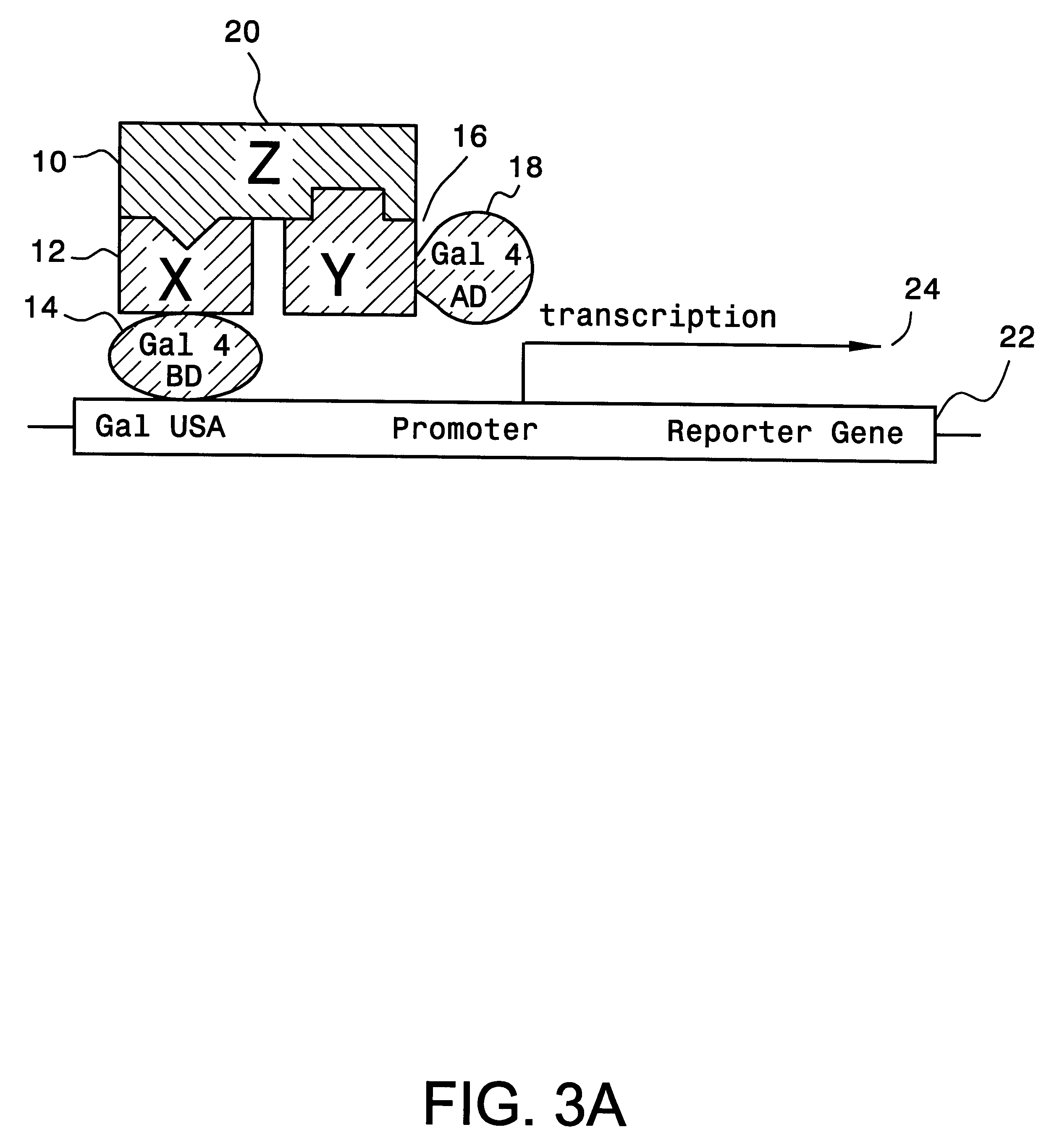
A method and a kit for detecting interactions between three or more proteins, in vivo, using reconstitution of the activity of a transcriptional activator is provided. Reconstitution of the transcriptional activator makes use of chimeric genes which express hybrid proteins. In one embodiment, three types of hybrid proteins are prepared. The first hybrid contains the DNA-binding domain of a transcriptional activator fused to the first test protein. The second hybrid protein contains a transcriptional activation domain fused to the second test protein. The third hybrid protein contains a nuclear localization peptide fused to a third test protein and mediates assembly of the three-protein complex involving the three hybrids. If the three test proteins are able to interact, they bring into close proximity the two domains of the transcriptional activator. This proximity is sufficient to cause transcription, which can be detected by the activity of a marker gene that contains a binding site for the DNA-binding domain.
Owner:GUILFORD PHARMACEUTICALS INC
Preparation method of pepsin-soluble high-purity superhelical-structured type-I collagen
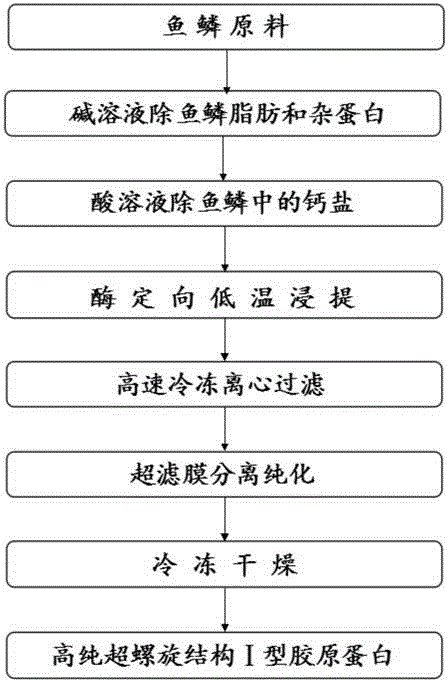
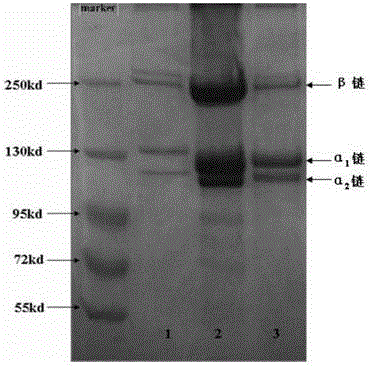
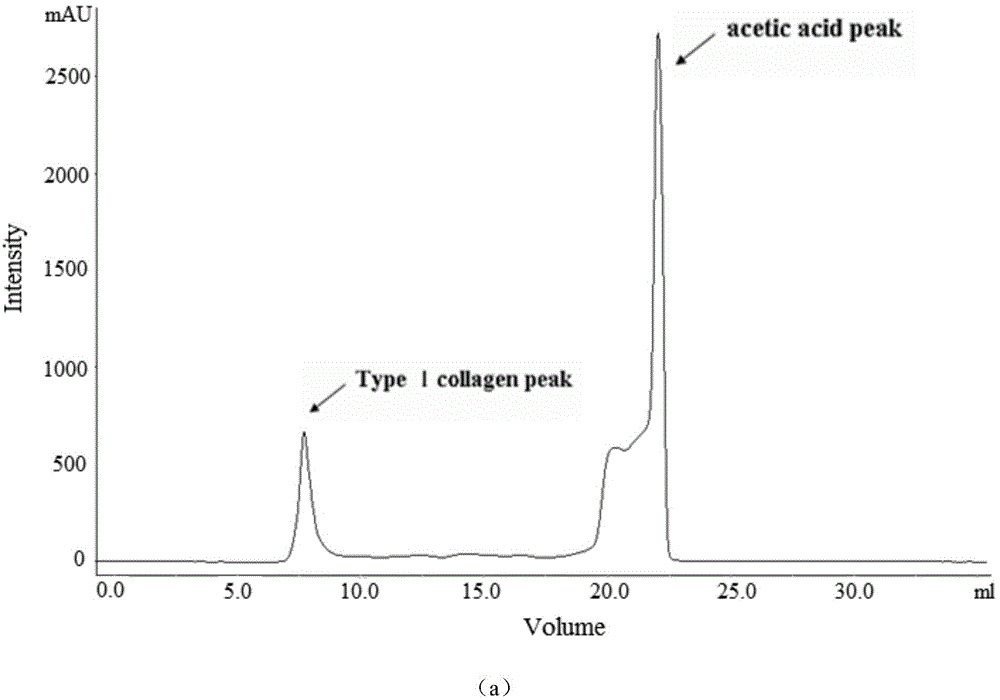
ActiveCN104152519AHigh yieldIntegrity guaranteedConnective tissue peptidesPeptide preparation methodsSeparation technologyFreeze-drying
The invention discloses a preparation method of a pepsin-soluble high-purity superhelical-structured type-I collagen, i.e. a low-antigenicity high-purity superhelical-structured type-I collagen. The preparation method is characterized by comprising the following steps: carrying out alkali treatment on scales which mainly contain the type-I collagen and serves as a raw material to remove hybrid proteins and fats, then carrying out acid treatment to remove calcium salts of the scales, adding a weak acid solution as an extraction agent, simultaneously adding pepsin, carrying out low-temperature stirring and leaching, and after the leaching is completed, carrying out refrigerated centrifugation so as to obtain crude leach liquor of the pepsin-soluble type-I collagen; purifying the crude leach liquor of the pepsin-soluble type-I collagen by using a membrane separation technology, and freeze-drying, so that the pepsin-soluble type-I collagen with a purity of not less than 90% and kept at a triple helix structure is obtained. A collagen product prepared by using the method disclosed by the invention is low in antigenicity, clear in configuration, and integral in triple helix structure; HPLC detection shows that the product is a type-I collagen with a purity of greater than or equal to 90%, and MALDI-TOF-MS detection shows that a mass spectrogram of the product has no impure peak, and the molecular weight is 288 kDa.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Method for improving separation efficiency, purity and biological specific activity of blood coagulation factor VIII and analog thereof by using affine aqueous two-phase system
InactiveCN102584933AHigh partition coefficientImprove separation efficiencyPeptide preparation methodsBlood coagulation factor VIIIPhosphate
The invention discloses a simple and quick method for improving separation efficiency, purity and biological specific activity of blood coagulation factor VIII and an analog thereof. The method is based on application of an affine aqueous two-phase technology, i.e., a characteristic hydrophilic macromolecular compound, preferably polyethylene glycol (PEG), forms a stable aqueous two-phase system with phosphate, glucan or other second component in aqueous solution; and one of core technologies is that: covalent linkage of the PEG and the blood coagulation factor VIII specific affine small peptide ligand is realized. The blood coagulation factor VIII is preferably located in a characteristic hydrophilic macromolecular compound phase connected to the affine small peptide ligand; and most of protein (or hybrid protein) from cell extract tends to move to another phase. By changing existence of target protein or polypeptide in one phase or another phase, the protein or polypeptide is efficiently purified with high purity.
Owner:汪志友
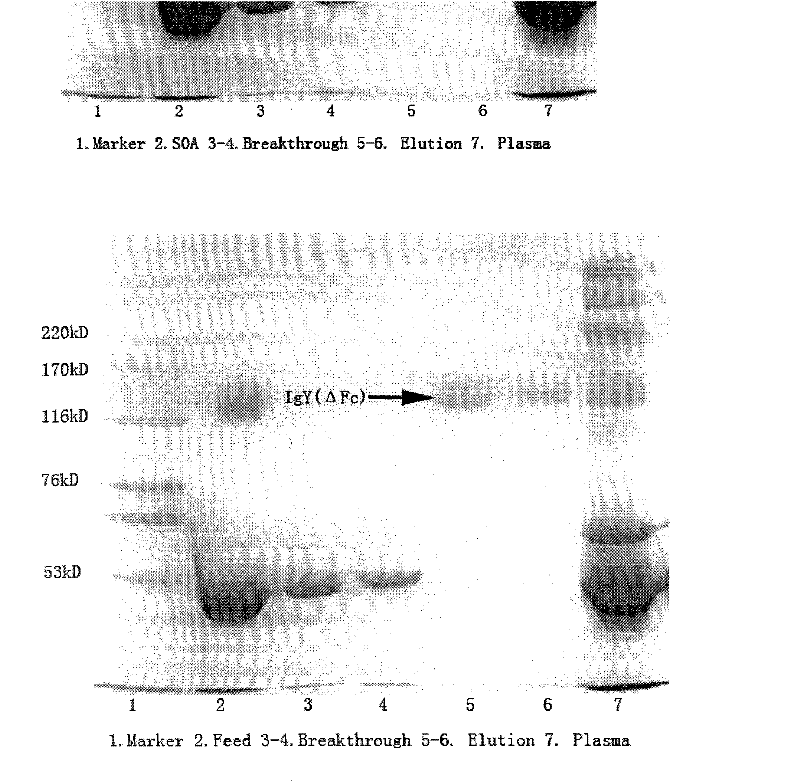
Method for separating immune globulin IgY(delta Fc) from goose blood
InactiveCN101899110BHigh purityImprove separation efficiencySerum immunoglobulinsPeptide preparation methodsOctanoic AcidsDesalination
The invention discloses a method for separating immune globulin IgY(delta Fc) from goose blood. The method comprises the following steps: 1) degreased plasm preparation, wherein fresh blood is selected to be centrifuged to remove erythrocyte, and then is degreased to obtain the degreased plasm; 2) octanoic acid precipitate, wherein octanoic acid is added to the plasm to reach a certain concentration, hybrid protein is precipitated and removed, and is centrifugally separated to obtain supernate; 3) column chromatography, wherein the supernate is separated by a chromatographic column which is filled with hybrid mode adsorbent to collect elution peak; and 4) desalination and drying, wherein the collected fluid is desalinated, refrigerated and dried to obtain the immune globulin IgY(delta Fc)with purity of over 95 percent. The method is characterized in that a new separation process is designed, the immune globulin IgY (delta Fc) can be separated from the goose blood; and the key of the method is that the immune globulin IgY (detal Fc) can be directly extracted from the supernate which is precipitated from the octanoic acid. The method has the advantages of simple operation steps andhigh separation and purification factors, and can be popularized and applied to treatment of blood of other waterfowls.
Owner:ZHEJIANG UNIV
Protein-polysaccharide hybrid hydrogels
InactiveUS20040200386A1Improve featuresIncrease contentProtein waste adhesivesOther chemical processesCross-linkHybrid protein
Disclosed is a hybrid protein-polysaccharide superabsorbent hydrogel. The hydrogel includes two interpenetrating matrices: a first matrix which is an acylated, cross-linked protein matrix, and a second matrix which is an anionic polysaccharide matrix. The two matrices can be non-cross-linked, or the hydrogel can further include bridging moieties that covalently cross-linking the acylated, cross-linked protein matrix to the anionic polysaccharide matrix.
Owner:WISCONSIN ALUMNI RES FOUND
Expression and preparation methods for bivalent bispecific antibody and hybrid protein
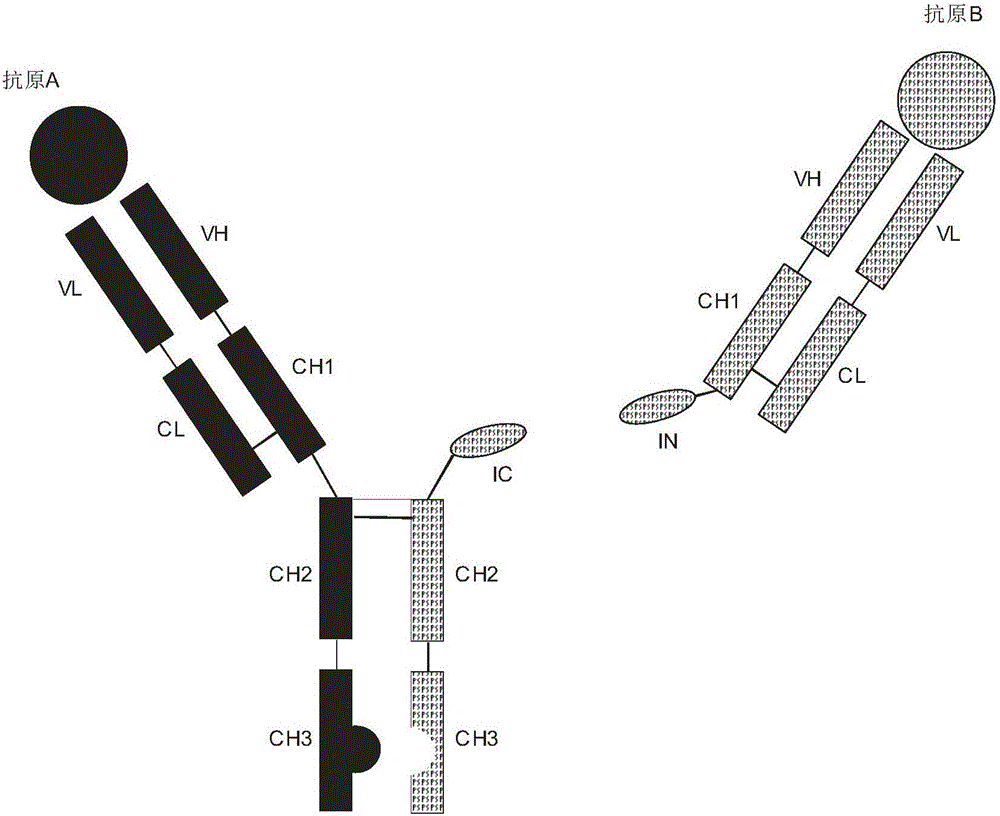
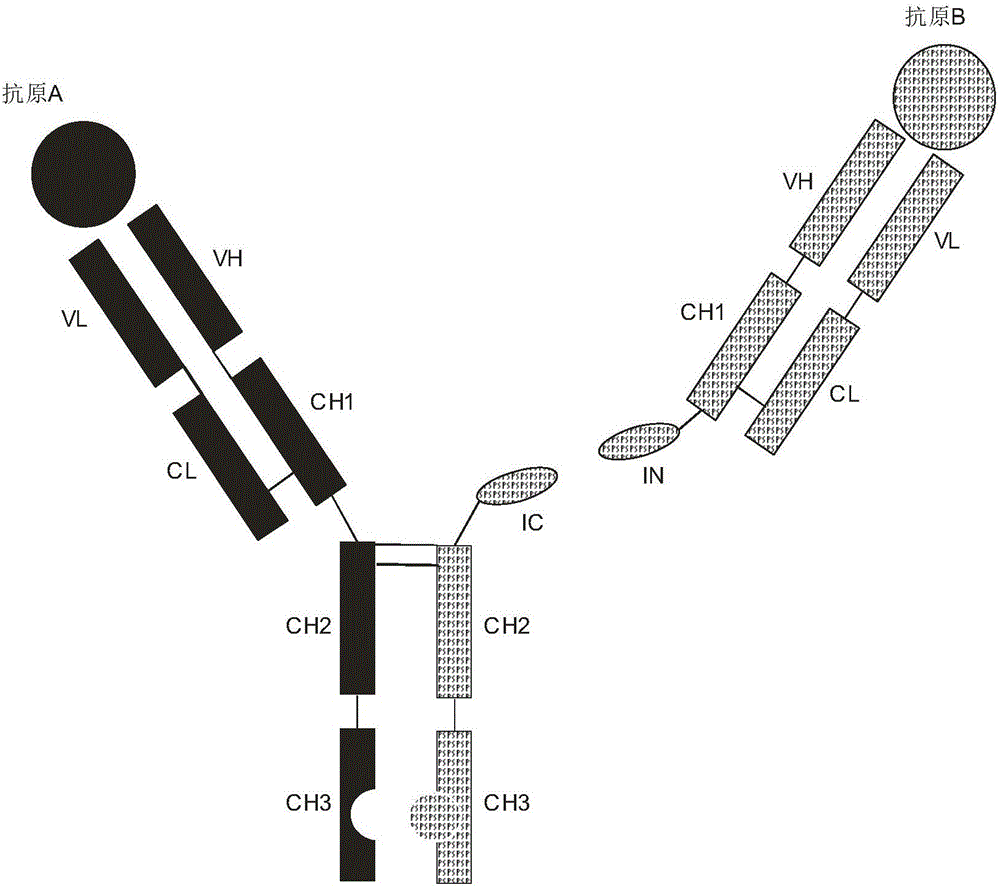
ActiveCN106397599AExtended half-lifeThe structure is complete and stableHybrid immunoglobulinsPolypeptide with affinity tagEucaryotic cellIntein
The invention discloses expression and preparation methods for a bivalent bispecific antibody and a hybrid protein and belongs to the technical field of biology. According to the methods, the aim of preparing products, i.e., the bivalent bispecific antibody and the immune hybrid protein thereof are prepared through expressing all parts of the bivalent bispecific antibody and the immune hybrid protein thereof separately in appropriate procaryotic or eucaryotic cell systems, carrying out high-performance affinity-chromatography purification and separation, and then, carrying out splicing in vitro under the condition of intein splicing. The methods have high production efficiency, are wide in applicable application range and facilitate the separation and purification of the products. The products prepared by using the methods have biological activities, i.e., bivalent bispecific immunoaffinity and cytotoxicity. The methods are novel methods for preparing biological drugs for directional attack on cancers or other diseases.
Owner:SHANGHAI JIAO TONG UNIV +2
Tunable endogenous protein degradation with heterobifunctional compounds
Owner:DANA FARBER CANCER INST INC
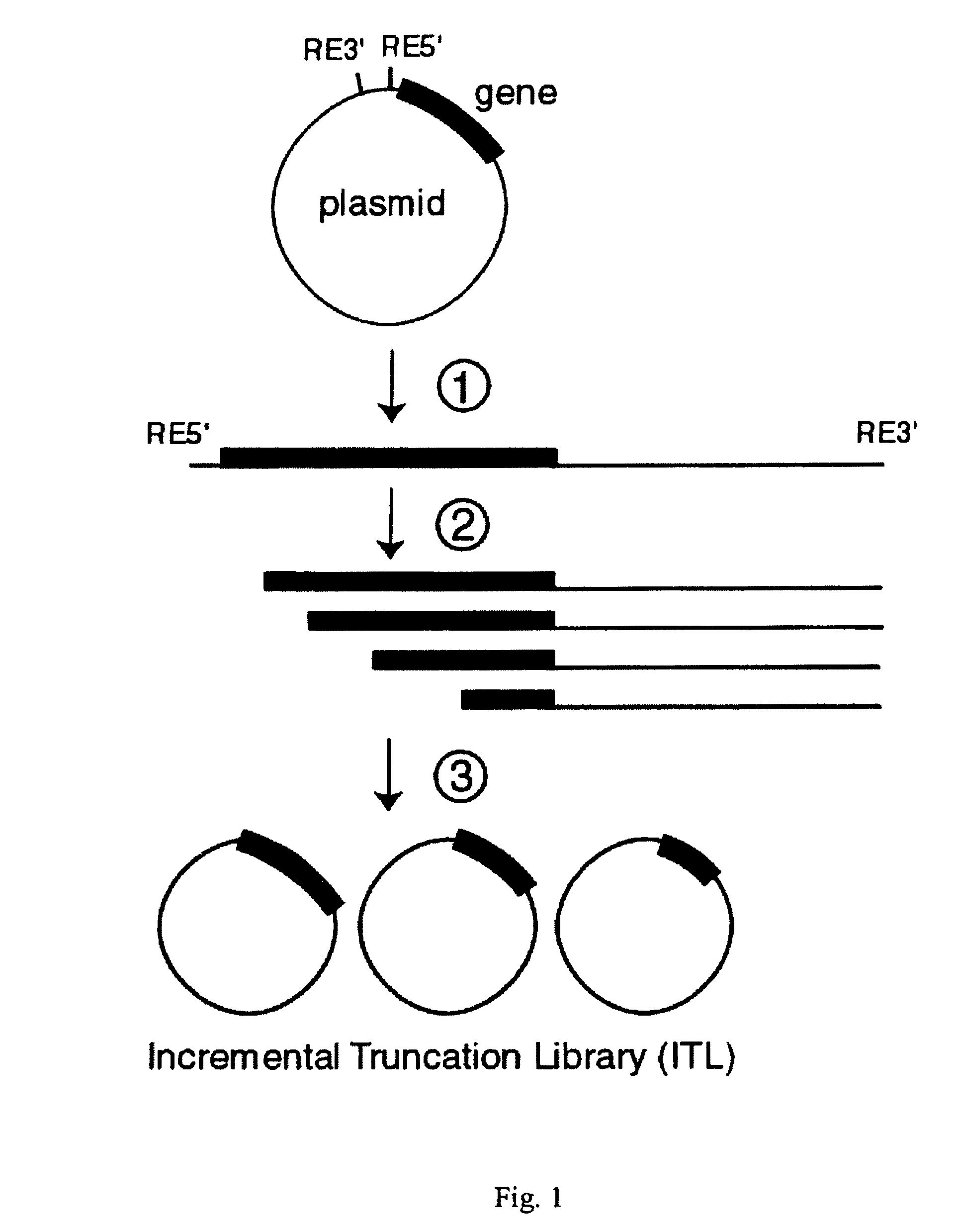
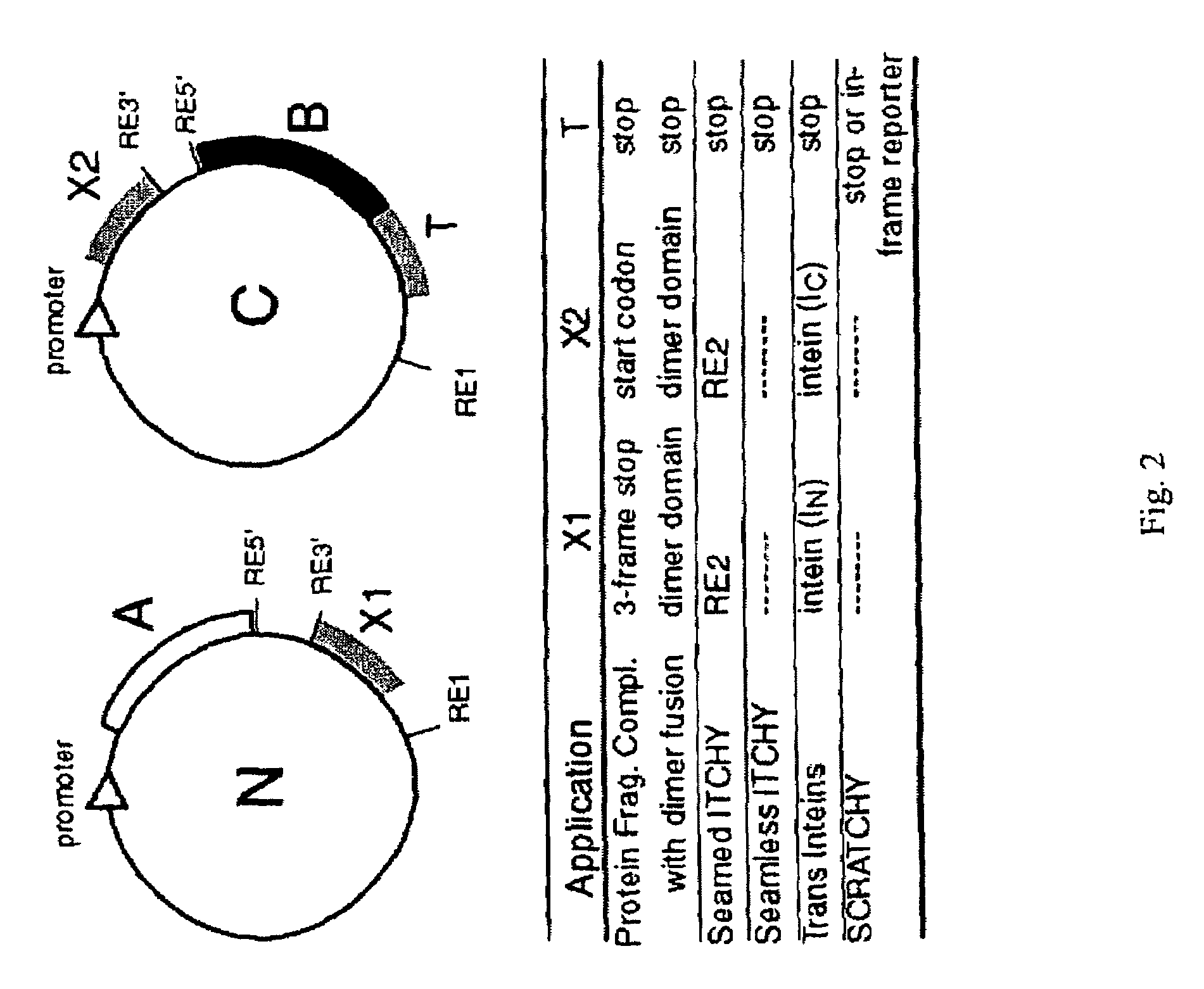
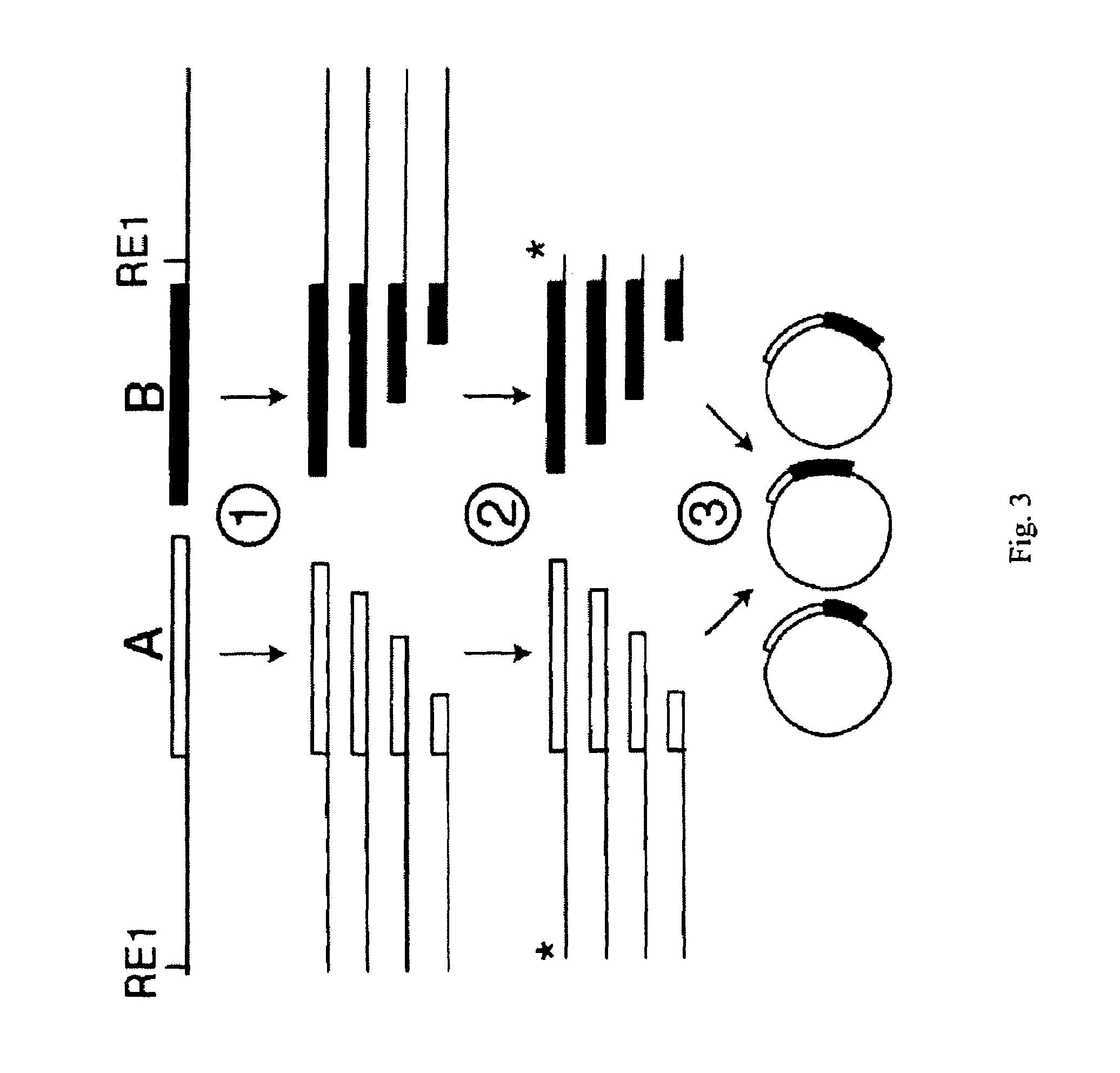
Incrementally truncated nucleic acids and methods of making same
Owner:PENN STATE RES FOUND
Adjustable sensitivity, genetic molecular interaction systems, including protein-protein interaction systems for detection and analysis
InactiveUS20040180325A1Microbiological testing/measurementBiological testingInteraction systemsGenetic molecular
A method for detecting interactions between first and second interacting molecules a variable sensitivity. This variable sensitivity may be obtained by providing for the overexpression of either a bait hybrid protein containing a DNA binding domain (desensitization) or a prey hybrid protein containing the DNA activation domain for a reporter gene (enhanced sensitivity). The use of exogenous activators of one or the other according to the needs of a particular system is readily accomplished.
Owner:HYBRIGEN
Post-extraction process for producing antibacterial peptide through bacillus subtilis
ActiveCN104130318ADry fastHigh activityDepsipeptidesPeptide preparation methodsSolubilityHybrid protein
The invention relates to a post-extraction process for producing antibacterial peptide through bacillus subtilis and belongs to the technical field of biology. The process comprises the following steps: by taking bacillus subtilis as a production strain, preparing a finished antibacterial peptide product through centrifugation, membrane separation and spray drying treatment of fermentation liquor, centrifuging by adopting two continuous centrifugal machines, performing classification and membrane separation, removing the thallus and hybrid proteins to the greatest degree, thus obtaining the antibacterial peptide with high activity. By utilizing heat resistance of the antibacterial peptide, the antibacterial peptide is rapidly dried through a spray drying technology, the preparation efficiency of the antibacterial peptide is improved, and maltodextrin serving as an auxiliary material has the advantages of high solubility and high stability, is unsuitable for moisture absorption and deterioration and contains rich trace elements and mineral substances. The separated and purified antibacterial peptide is high in activity, low in cost, simple in process and low in post-treatment loss.
Owner:山东仙普爱瑞科技股份有限公司
Method for separating purified gamma-polyglutamic acid from fermentation liquor
The invention discloses a method for separating purified gamma-polyglutamic acid from fermentation liquor. The method comprises the following steps of: firstly, diluting the fermentation liquor by using water and regulating the pH value to 2.8-3.2; carrying out plate filter for degerming, and regulating the pH value of filter liquor to 6.9-7.3; carrying out alcohol precipitation and removing water in the precipitate; centrifuging to separate out the precipitate as well as decompressing and drying to obtain a crude gamma-polyglutamic acid product; dissolving again and regulating the pH value to 6.9-7.1; adding protease, preserving heat and removing hybrid protein; regulating the pH value to 2.9-3.1 and ultra-filtering to remove pigment and micromolecule impurities; regulating the pH value to 6.9-7.1 and carrying out alcohol precipitation; and decompressing and drying to obtain a finished high-purity gamma-polyglutamic acid product. In the invention, the purposes of separating and purifying high-purity gamma-polyglutamic acid are achieved through regulating the pH values, centrifuging, precipitating and filtering in multiple steps. The method has the advantages of high purity, high yield, low cost and environmental protection.
Owner:浙江赛灵特医药科技有限公司
Low-serum culture medium suitable for growth of Vero cells
InactiveCN108103007APromote growthImprove securityCulture processArtificial cell constructsQuality cultureSide effect
The invention provides a low-serum culture medium suitable for the growth of Vero cells. The dosage of calf serum for conventional culture is reduced to 2 percent from 10 percent (which is the contentof a component V), and conventionally added animal derived components are replaced with plant derived components and recombinant protein. According to the low-serum culture medium provided by the invention, the dosage of the serum is reduced, so that the cost is reduced, and the possibility that the serum in the culture medium carries exogenous pathogenic bacterium pollution is reduced; meanwhile, a lot of hybrid proteins in the serum are reduced and separation and purification of down-stream products are facilitated; plant hydrolyzed protein and recombinant endothelial growth factors are used for replacing the animal derived components so that the side effect of the animal derived components is reduced and the safety of biological products is improved. According to the culture medium provided by the invention, the Vero cells grow well and the cost is reduced; the exogenous pathogenic bacterium pollution is reduced, a post-period purification technology is reduced and the stability ofproducing cell products is improved; one good-quality culture medium is provided for producing the biological products which take the Vero cells as virus hosts or expression vectors.
Owner:宁波高新区绿元汇生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com