Preparation method of pepsin-soluble high-purity superhelical-structured type-I collagen
A collagen and supercoil technology, applied in the field of preparation of high-purity type I collagen with low antigenicity, can solve the problems of cumbersome and complicated process, limited product purity, high production cost, etc., achieve simple and reasonable process flow, shorten purification time, The effect of shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
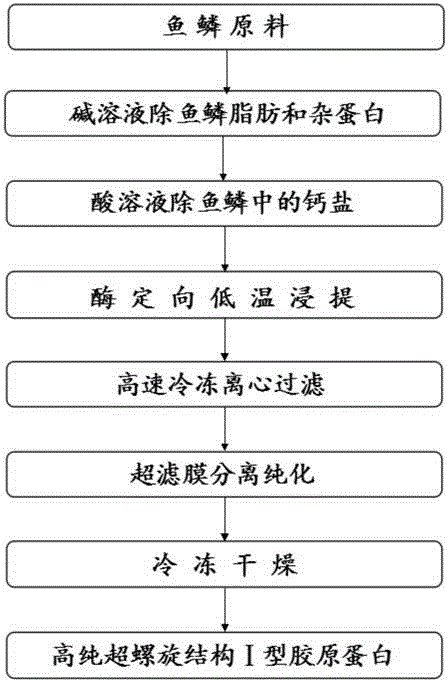
[0041] A preparation process of enzyme-soluble high-purity superhelical structure type I collagen, the steps are as follows:
[0042] (1) Raw material pretreatment
[0043] 2 kg of washed fish scales were pulverized first, then put into the reaction kettle, added an alkali solution 10 times the weight of the fish scales, stirred for 12 hours, washed with water, added an acid solution 10 times the weight of the fish scales, stirred for 12 hours, and then washed with water. Wherein the mass volume concentration of alkali solution is 3%, the mass volume concentration of acid solution is 5%, and pretreatment temperature is 25 ℃;
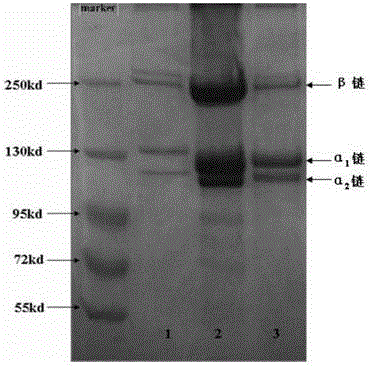
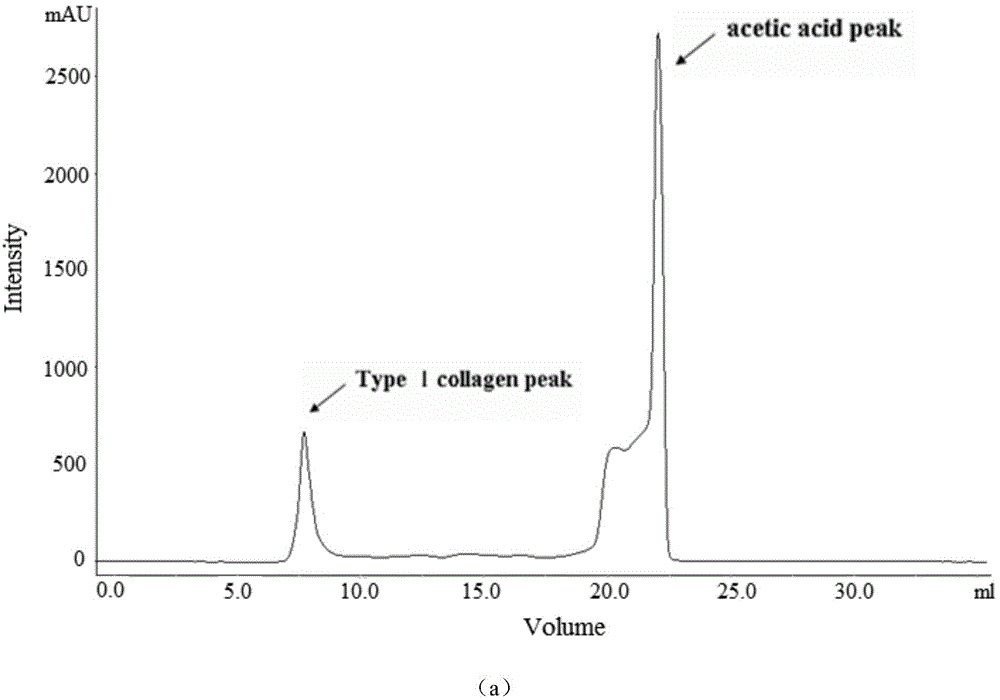
[0044] (2) Type Ⅰ collagen extraction
[0045] Add 0.1 mol / L of acetic acid, citric acid, oxalic acid or malic acid to the treated raw material as the extract solution, the extraction temperature is 4°C, the weight ratio of raw material to extractant is 1:10, and at the same time add 0.5% by weight of raw material The pepsin was stirred and extracted f...
Embodiment 2
[0051] A preparation process of enzyme-soluble high-purity superhelical structure type I collagen, the steps are as follows:
[0052] (1) Raw material pretreatment
[0053] Put 5 kg of washed fish scales into the reaction kettle, add an alkali solution 8 times the weight of the fish scales, stir for 10 hours, wash with water, add an acid solution 8 times the weight of the fish scales, stir for 10 hours, and then wash with water. The mass volume concentration is 2%, the mass volume concentration of the acid solution is 6%, and the pretreatment temperature is 20°C;
[0054] (2) Type Ⅰ collagen extraction
[0055] Add 0.25mol / L of acetic acid, citric acid, oxalic acid or malic acid to the treated raw material as the extracting solution, the extraction temperature is 4°C, the weight ratio of raw material to extractant is 1:10, and at the same time add 1% of raw material weight The pepsin was stirred and extracted for 12 hours. The extraction step could be repeated 3 times. The e...
Embodiment 3
[0061] A preparation process of enzyme-soluble high-purity superhelical structure type I collagen, the steps are as follows:
[0062] (1) Raw material pretreatment
[0063] Put 0.5 kg of washed fish scales into the reaction kettle, add an alkali solution 6 times the weight of the fish scales, stir for 4 hours, wash with water, add an acid solution 6 times the weight of the fish scales, stir for 12 hours, and then wash with water, the alkali solution The mass volume concentration of the acid solution is 1%, the mass volume concentration of the acid solution is 8%, and the pretreatment temperature is 15°C;
[0064] (2) Type Ⅰ collagen extraction
[0065] Add 1 mol / L of acetic acid, citric acid, oxalic acid or malic acid to the treated raw material as the extract solution, the extraction temperature is 8°C, the weight ratio of the raw material to the extractant is 1:10, and at the same time add 2% of the raw material weight The pepsin was stirred and extracted for 12 hours, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com