Botulinum antitoxin compositions and methods
a technology of botulinum and compositions, applied in the field of botulinum antitoxin compositions and methods, can solve the problems of severe flaccid paralysis, serious threat to human safety, respiratory collapse and death,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
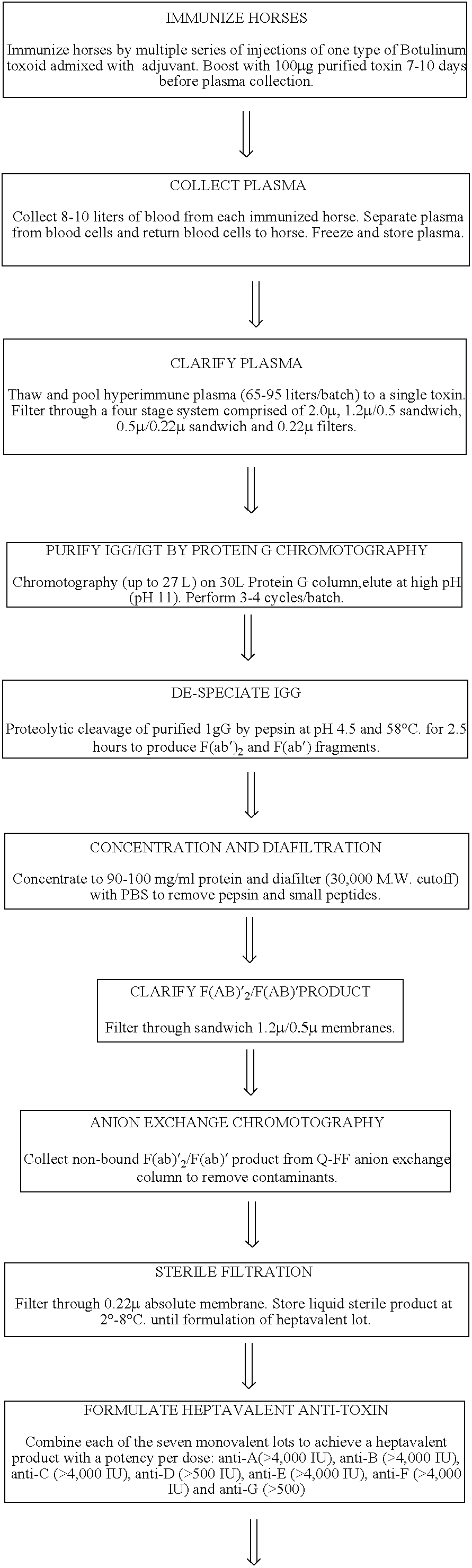
Immunization Regimen
In order to obtain high polyclonal antibody titers, we used a multi-step immunization procedure. Several horses were each immunized with a single botulinum serotype. First, the horses were immunized with the toxoid form, i.e., inactivated form of the toxin, of the specific toxin serotype to be used for that horse. The first inoculation contained 2 mg of toxoid in Complete Freund's Adjuvant injected intradermally using up to 30 sites at approximately 0.1 mL per site. After 14 days, the horses received a second inoculation of 0.5 mg toxoid in Incomplete Freund's Adjuvant injected intradermally at multiple sites at approximately 0.1 mL per site. After another 14 days, antitoxin titers were determined using a mouse neutralization assay and, if necessary, a subsequent priming dose of 0.5 mg of toxoid in RIBI's adjuvant system (MPL+TDM=CWS emulsion) was administered.
The injection site for the toxoid inoculations was a rectangular area measuring approximately 24×8 i...
example 2
Purification and De-Speciation of Equine Botulinum Antitoxins
Eighteen bags of frozen plasma, containing antitoxin to serotype B, were thawed, pooled together and clarified by filtration into a 110 L vessel. The total volume of plasma processed was 98 L. After clarification and rinsing of the filters with phosphate buffered solution (“PBS”), the volume of the sample was 103 L. This material was loaded onto 30 L protein G affinity columns in four cycles of 25.75 L per cycle. During each cycle, the antitoxin was eluted with 10 mM sodium bicarbonate / carbonate buffer in an average volume of 31.4 L. The total volume of antibody collected was 126.7 L with a protein concentration of 9.3 mg / mL. The antitoxin was collected into a 300 L vessel and diluted with 1M sodium acetate to a concentration of 50 mM sodium acetate, such that the pH was 4.5.
Pepsin was then added to the antibody solution at a concentration of 4% w / v and the temperature was adjusted to 58° C. After two hours, the digest...
example 3
Formulation of the Heptavalent Antitoxin
Various monovalent batches of each of the seven antitoxin serotypes were processed as described above. The batches were pooled under aseptic conditions and based on the titers of the individual batches of antitoxin, the following volumes were combined: A-7.38 L; B-18.57 L; C-18.18 L; D-4.86 L; E-3.11 L; F-24.82 L; and G-12.62 L. To this pool was added 120.79 L of phosphate buffered saline (PBS) and then 7.212 kilograms of solid lactose. The mixture was stirred for 16 hours at 2-8° C. The final lactose concentration was approximately 5%. The mixture was then filtered through a 0.22 micron filter and release tests described above were performed. This final purified bulk product was then vialed at approximately 23 mL (5-100 mL depending on the size of the lyophilization vial) per vial and lyophilized.
For lyophilization, the optimal cycle time is 96-110 hours (range could be 80-125 hours). The temperature of the trays when loading the vials in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com