Method for inactivating viruses in process of preparing antitoxin and antiserum
An anti-serum and anti-toxin technology, applied in the direction of anti-viral immunoglobulin, serum immunoglobulin, anti-animal/human immunoglobulin, etc. Limit and other problems, to achieve the effect of short processing time, high purity and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
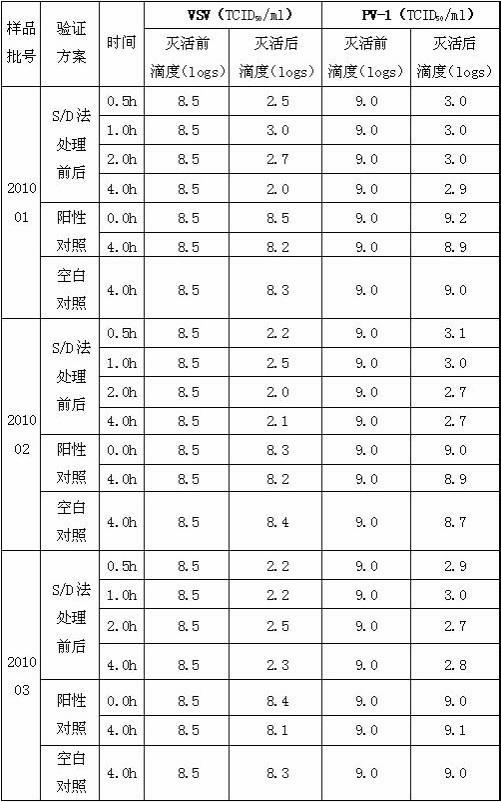
[0030] The method for inactivating virus in preparing tetanus antitoxin comprises the following steps:
[0031] A. After diluting the horse plasma raw material with water for injection to 2 times the volume, adjust the pH value of the diluent to 2.8 with 1N hydrochloric acid solution;
[0032] B. Add 0.1 g of pepsin to every 100 ml of the dilution in step A, and digest at 29°C for 3 hours to obtain a digestive juice;
[0033] C. Add 14g of ammonium sulfate to every 100ml of the digestion solution in step B, and adjust the pH value of the mixture to 4.8 with 1N sodium hydroxide solution, heat the mixture to 57°C and keep it for 30 minutes, then cool down to below 45°C, Separated to obtain supernatant;
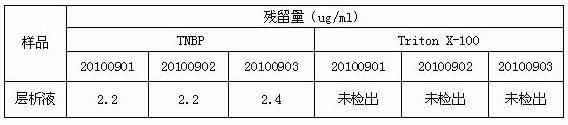
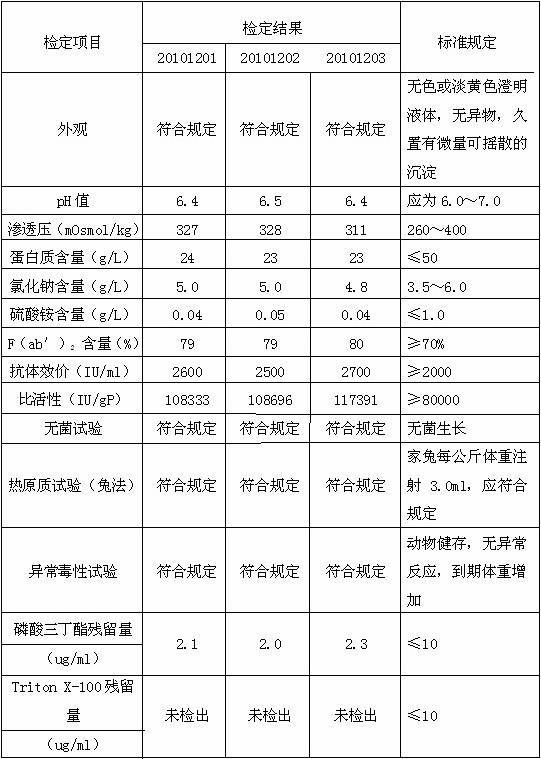
[0034] D, the supernatant of step C is diluted to contained protein concentration with water for injection and is 10 g / L, adds medical grade organic solvent tributyl phosphate and medical grade detergent Triton X-100 in this dilution, until Each 100ml of diluent contains 0.25g...
Embodiment 2
[0040] The method for inactivating virus in preparing anti-rabies serum comprises the following steps:
[0041] A. After diluting the plasma raw material with water for injection to 4 times its volume, adjust the pH value of the diluent to 4.0 with 4N hydrochloric acid solution;
[0042]B. Add 1 g of gastric enzyme to every 100 ml of the diluted liquid in step A, and digest at 31°C for 1 hour to obtain the digestive juice;
[0043] C. Add 16g of ammonium sulfate to every 100ml of the digestion solution in step B, and adjust the pH value of the mixture to 5.6 with 4N sodium hydroxide solution, heat the mixture to 59°C and keep it for 30 minutes, then cool down to below 45°C, Separated to obtain supernatant;
[0044] D, the supernatant of step C is diluted to contained protein concentration with water for injection and is 50 g / L, adds medical grade organic solvent tributyl phosphate and medical grade detergent Triton X-100 in this dilution, until Each 100ml of diluent contains...
Embodiment 3
[0050] The method for inactivating virus in preparing antivenom serum comprises the following steps:
[0051] A. After diluting the plasma raw material 3 times with 0.8% NaCl solution, adjust the pH value of the dilution solution to 3.2 with 3N hydrochloric acid solution;
[0052] B. Add 0.5 g of gastric enzyme to every 100 ml of the diluted solution in step A, and digest at 20°C for 22 hours to obtain a digestive solution;
[0053] C. Add 15g of ammonium sulfate to every 100ml of the digestion solution in step B, and adjust the pH value of the mixed solution to 5.0 with 3N sodium hydroxide solution, heat the mixed solution to 57°C and keep it for 30 minutes, then cool down to below 45°C, Separated to obtain supernatant;
[0054] D. Dilute the supernatant of step C with 0.8% NaCl solution to a protein concentration of 25 g / L, and add medical-grade organic solvent tributyl phosphate and medical-grade detergent to the diluted solution Polysorbate-80, until every 100ml of the d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com