Patents
Literature
56 results about "Chagas disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
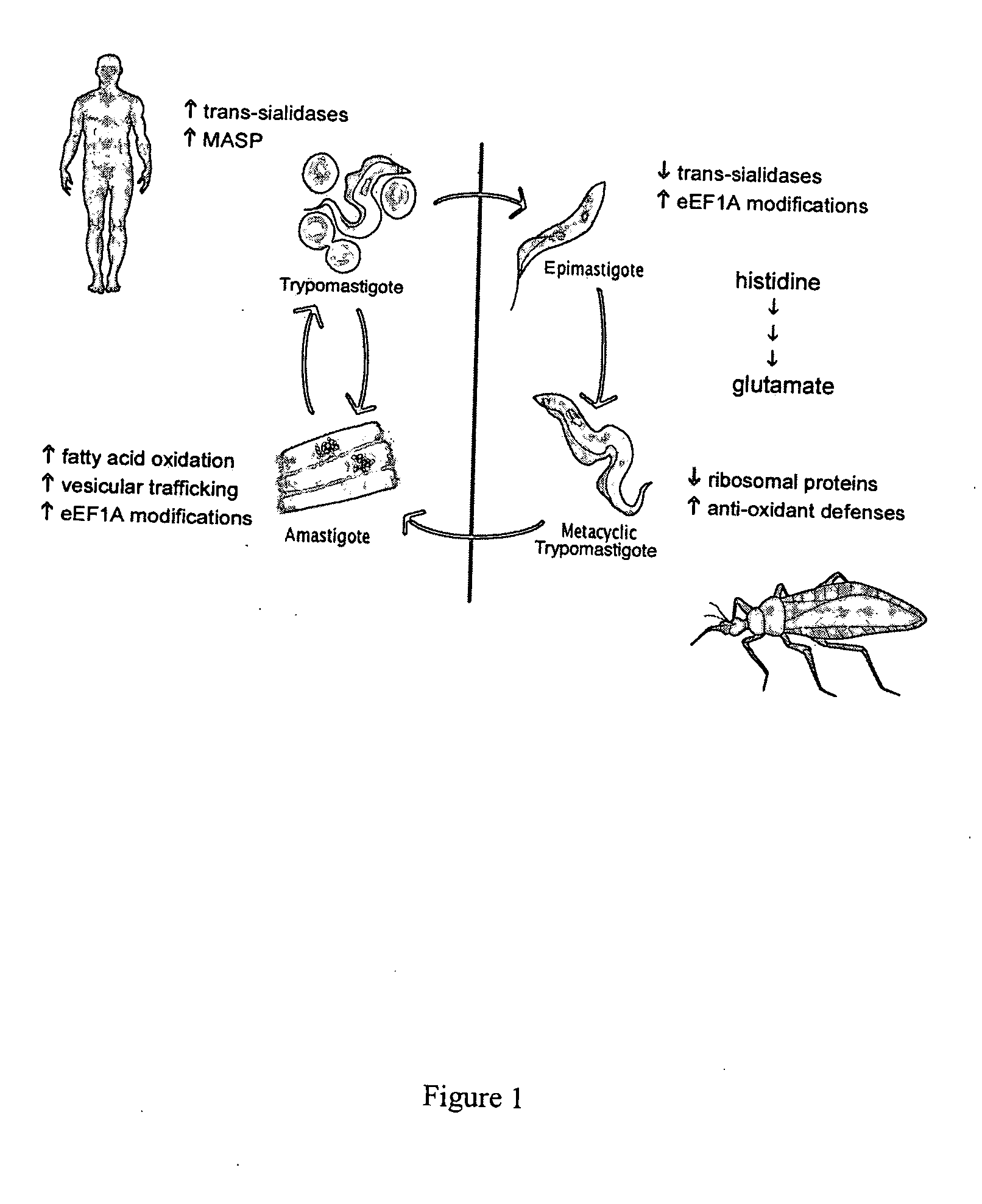
An infectious disease caused by the parasite Trypanosoma cruzi.
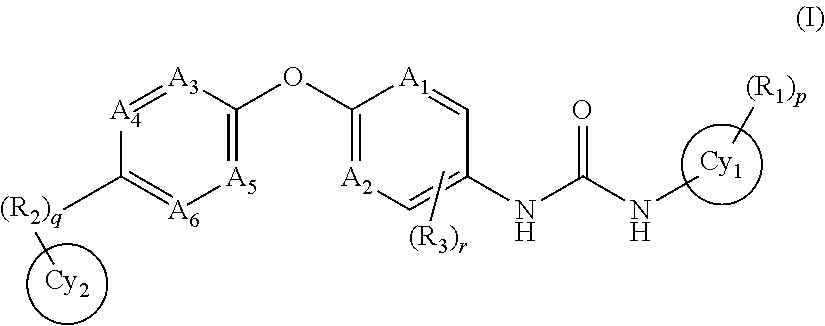
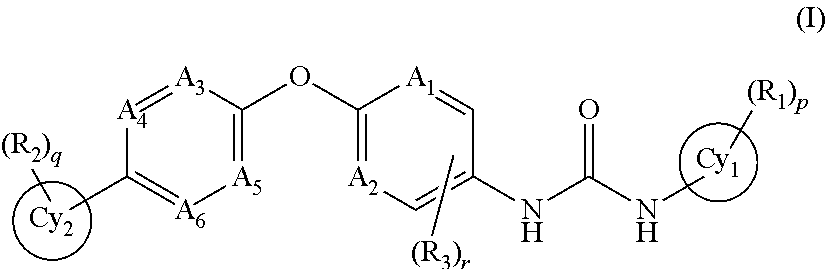
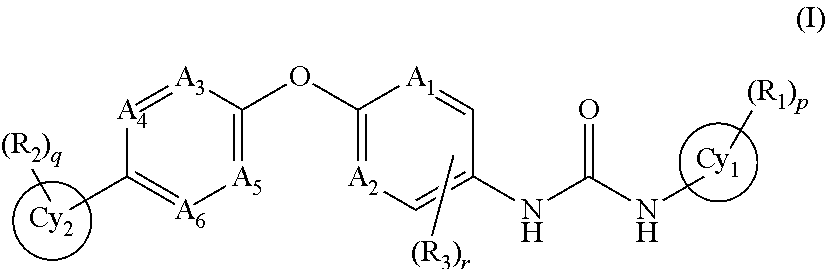
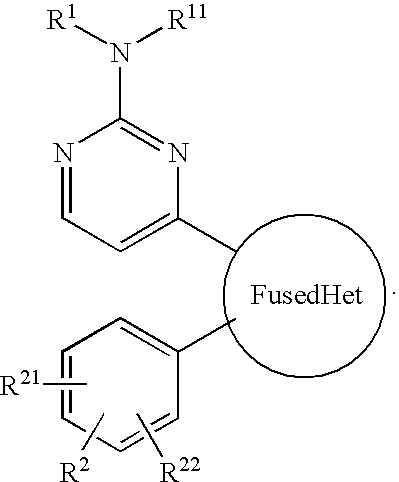
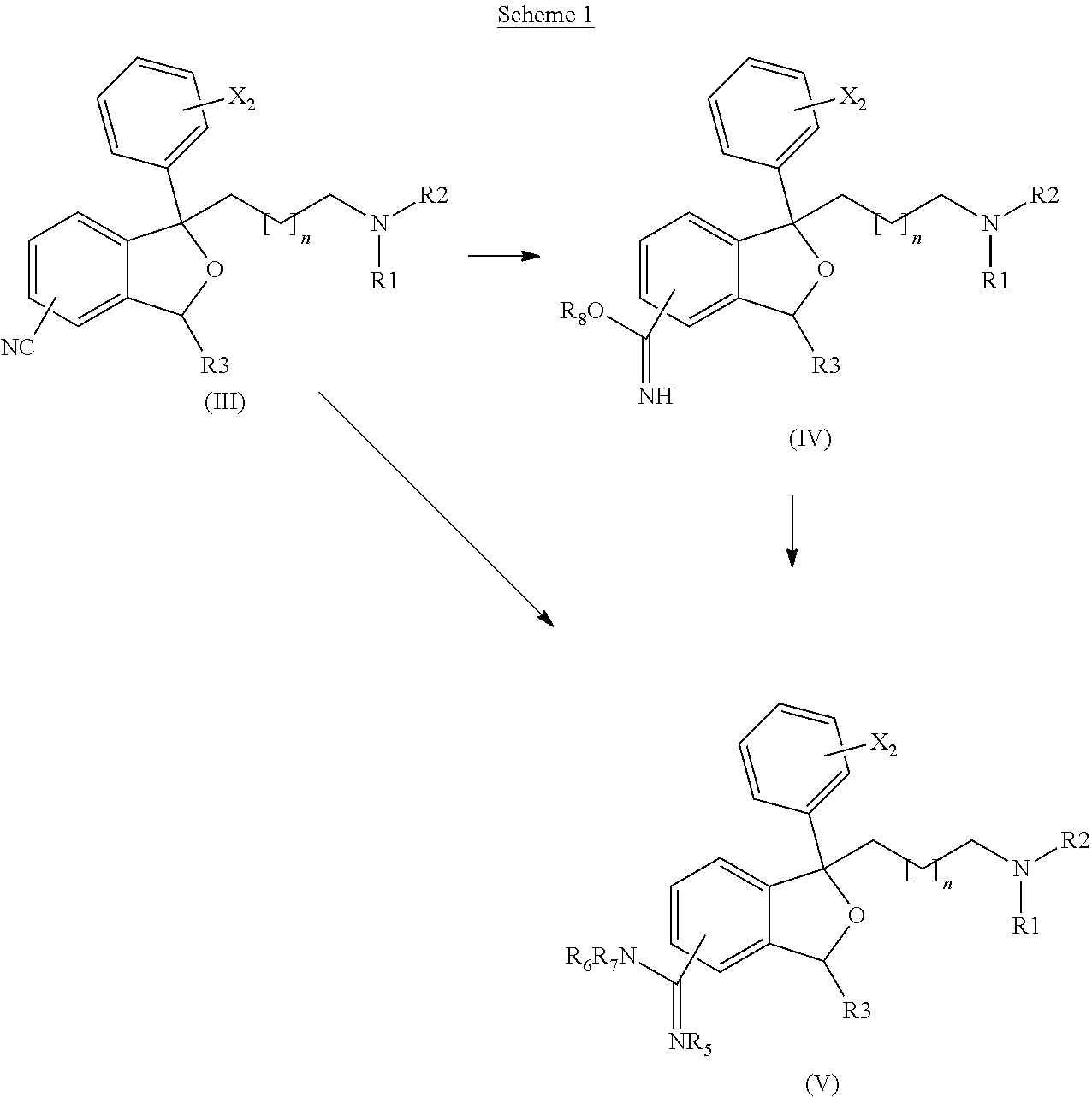
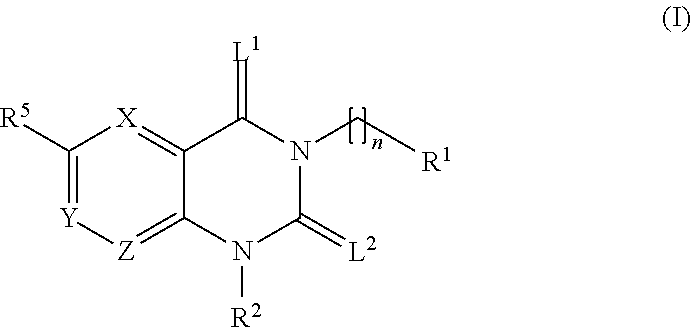
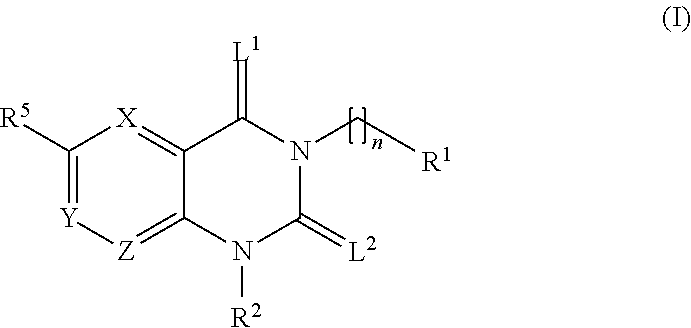
Trk-INHIBITING COMPOUND
ActiveUS20160000783A1Excellent kinase selectivityInhibits NGF vascular hyper permeabilityBiocideOrganic chemistryChagas diseaseBULK ACTIVE INGREDIENT
The present invention provides a drug containing a compound having Trk-inhibiting activity as an active ingredient in prophylaxis and / or therapy for Trk-related diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease. A compound represented by the general formula (I), wherein all symbols represent the same meanings as described in the specification, a salt thereof, an N-oxide thereof, a solvate thereof or a prodrug thereof is useful as a drug component having Trk-inhibiting activity in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease.
Owner:ONO PHARMA CO LTD
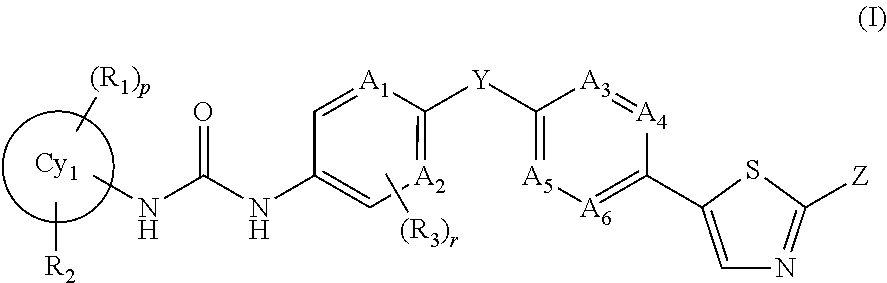
Trk-inhibiting compound
ActiveUS9242977B2Safe prophylactic and therapeutic agentHigh selectivityNervous disorderOrganic chemistryChagas diseaseInflammatory bowel disease
An object of the present invention is to provide a drug containing a compound having Trk-inhibiting activity as an active ingredient in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease. A compound represented by the general formula (I):(wherein all symbols represent the same meanings as described in the specification), a salt thereof, an N-oxide thereof, a solvate thereof or a prodrug thereof is useful as a drug component having Trk-inhibiting activity in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease.
Owner:ONO PHARMA CO LTD
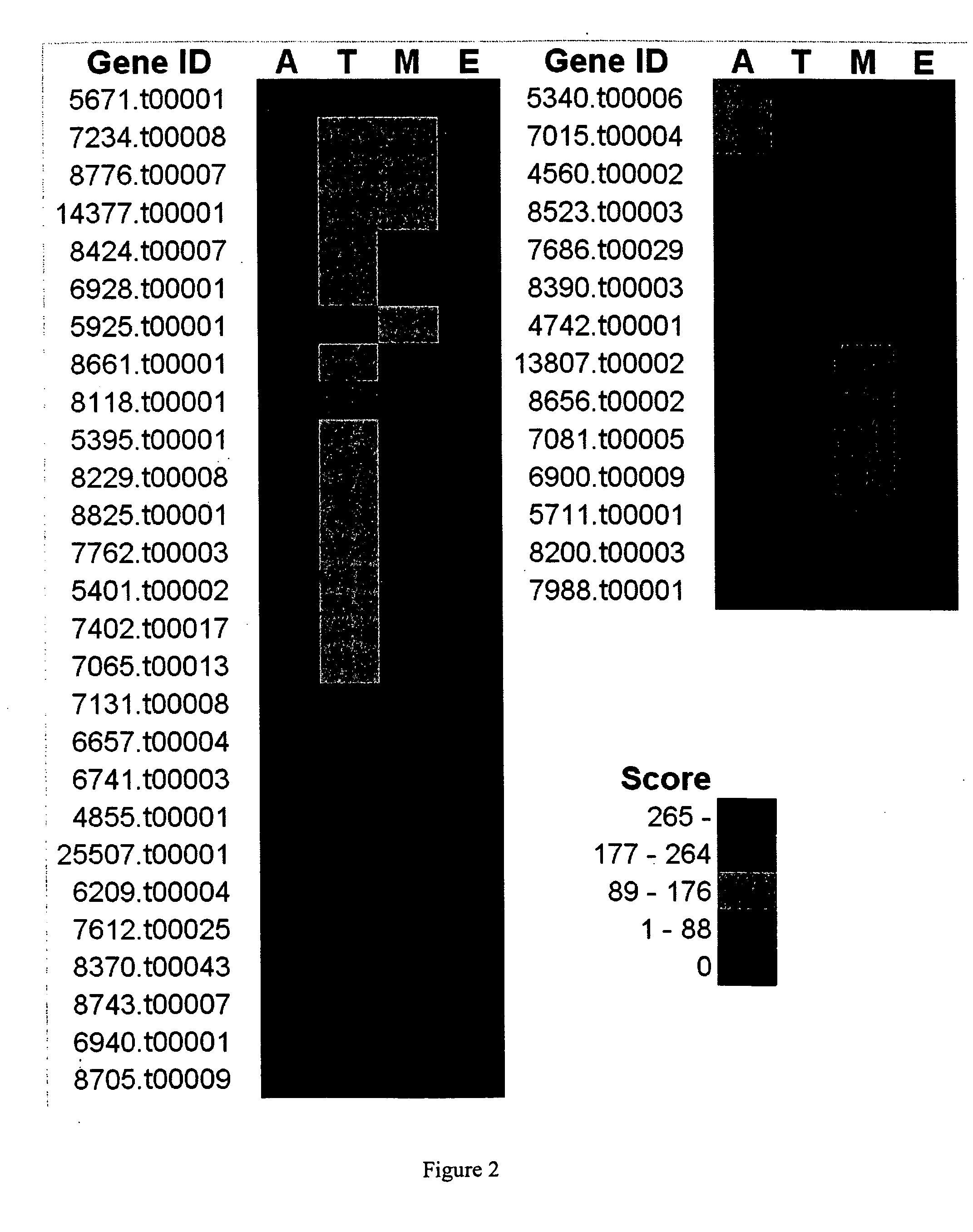
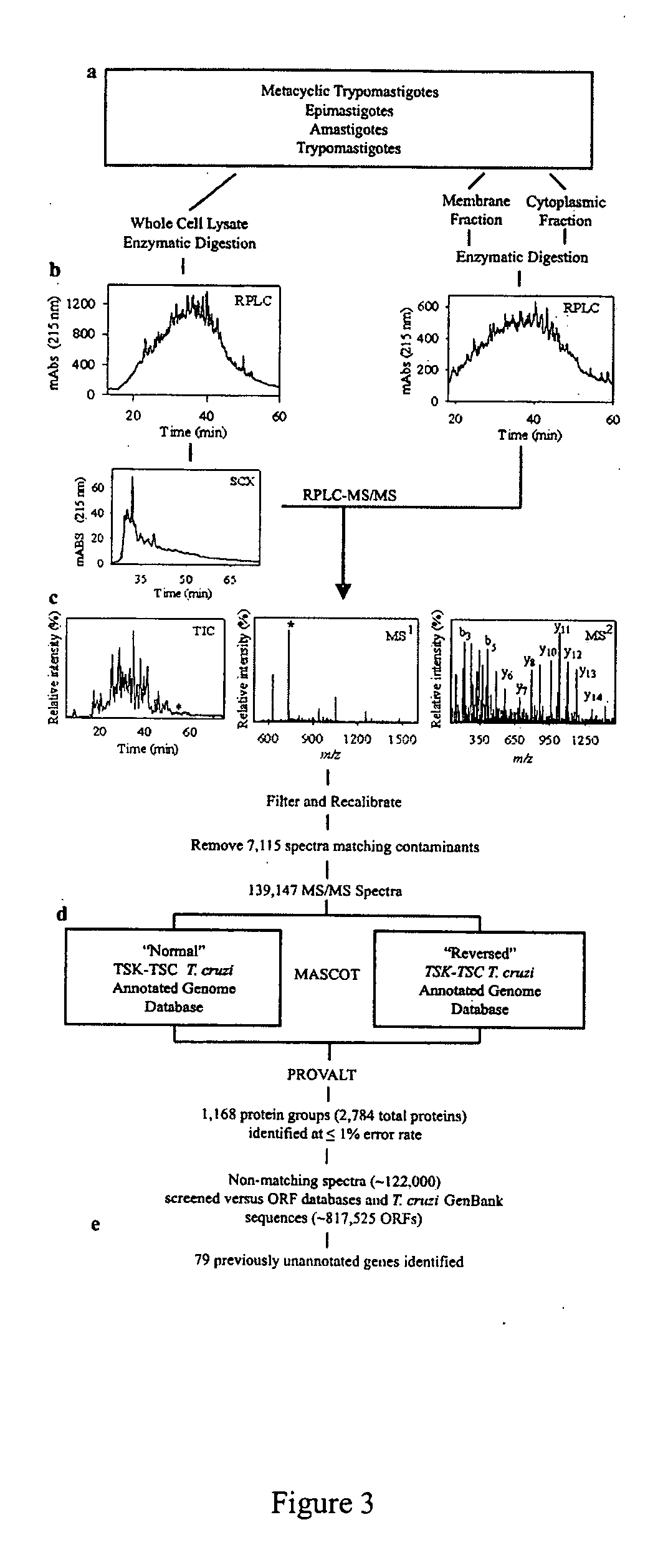
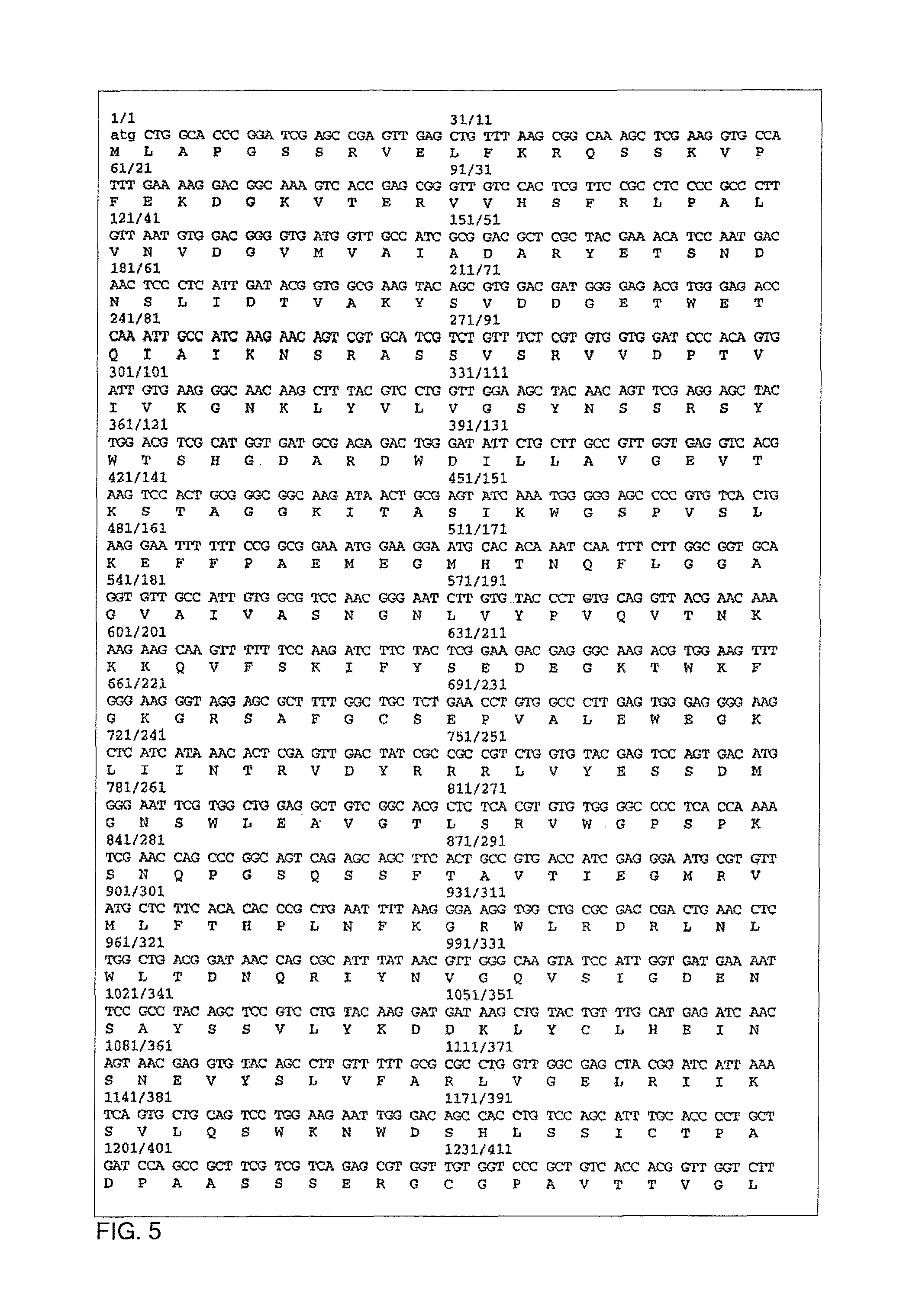
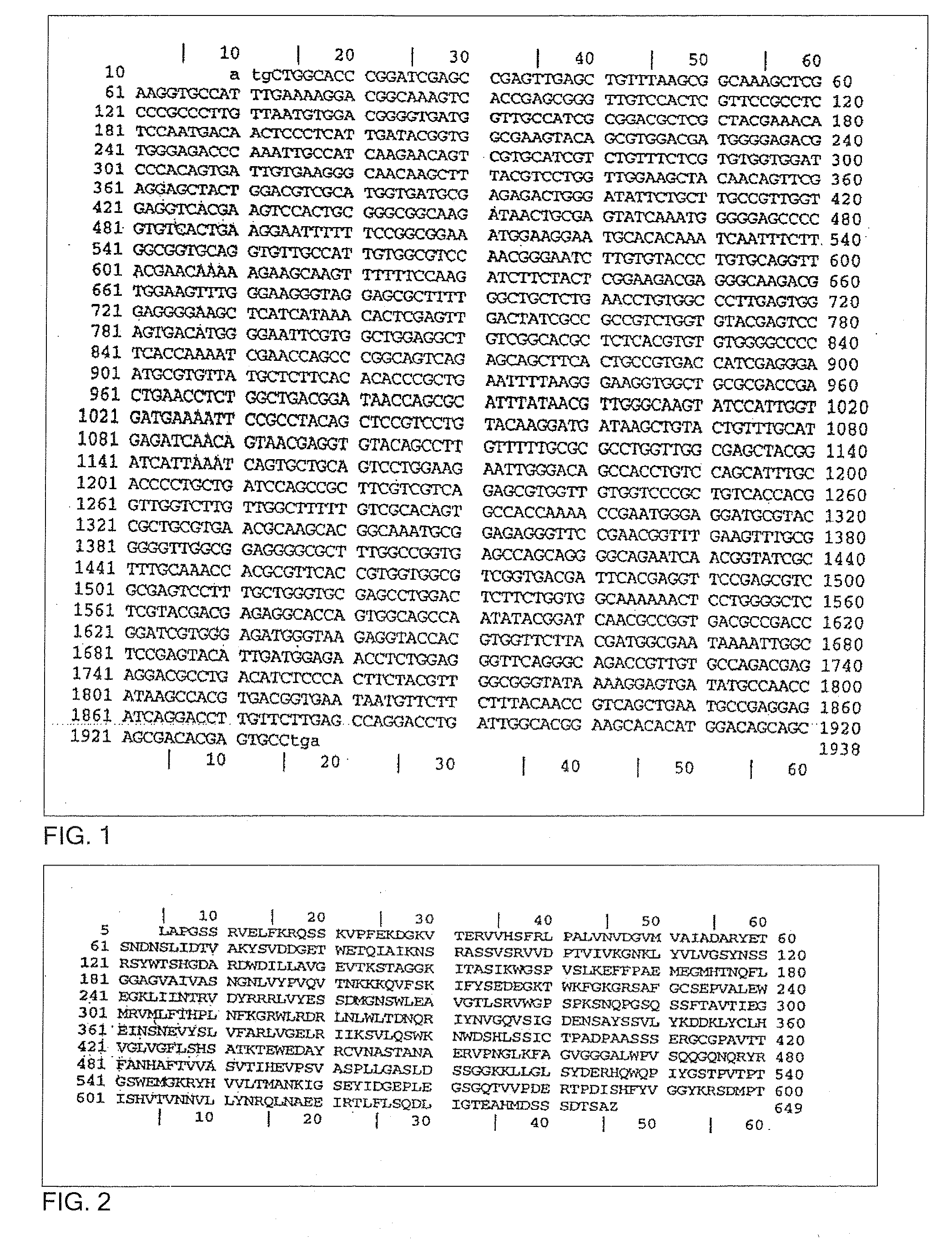
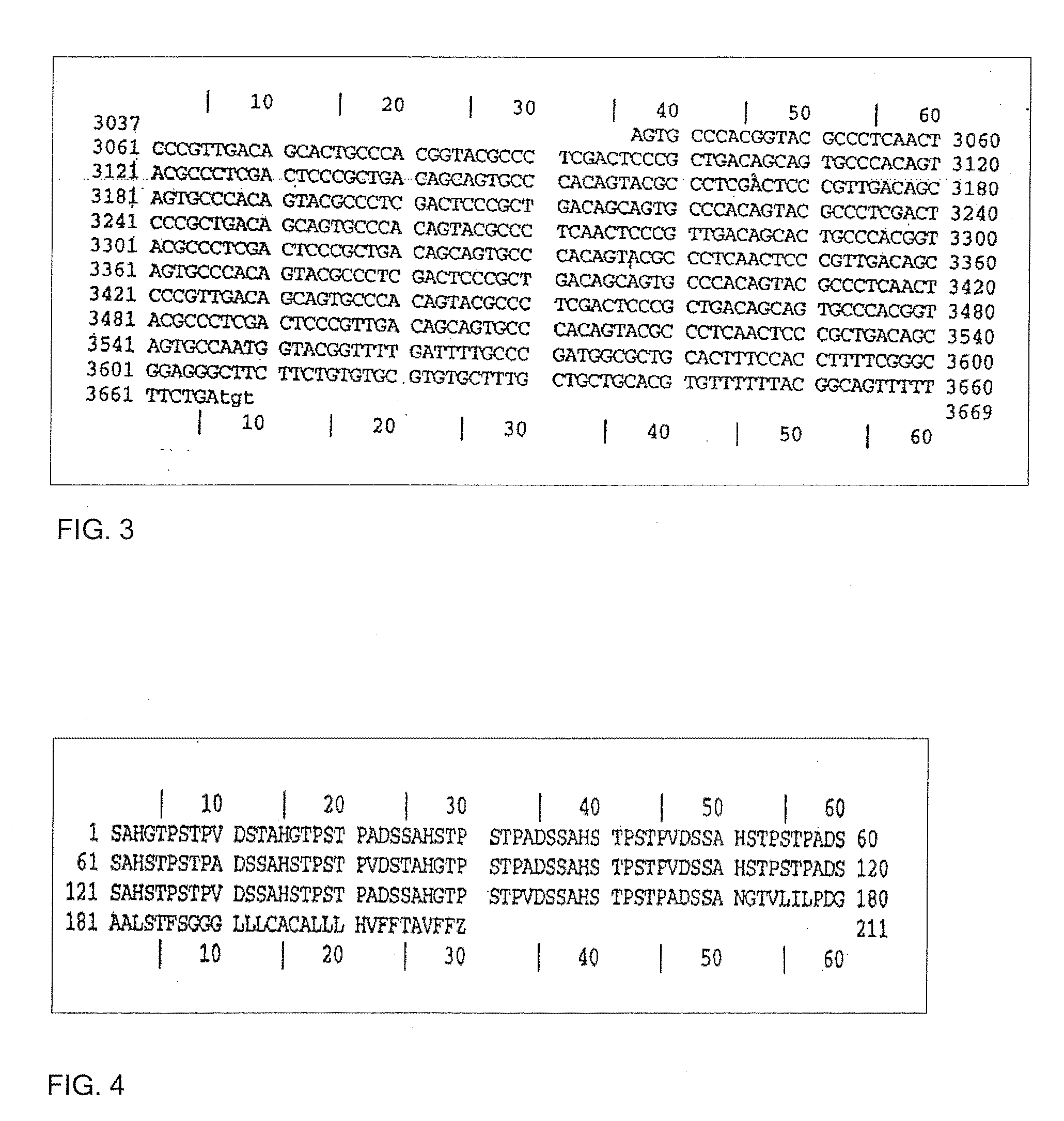
Trypanosoma cruzi proteome compositions and methods
Molecular targets are identified in T. cruzi suitable for use in diagnosis of Chagas disease, drug development, and vaccines, including live vaccines.
Owner:GEORGIA RESERACH FOUND INC UNIV OF
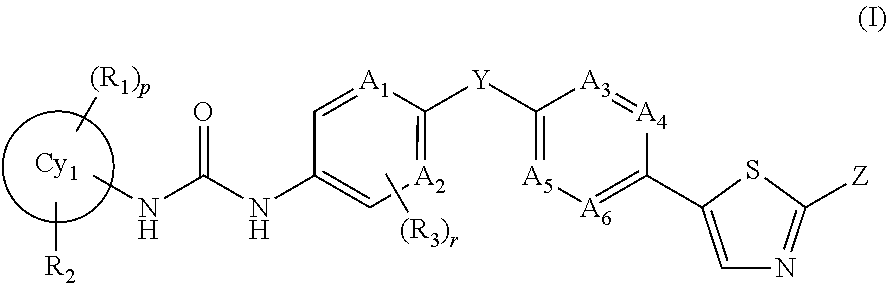
Trk-inhibiting compound
ActiveUS20160280684A1High selectivityAvoid osmosisOrganic active ingredientsOrganic chemistryInflammatory Bowel DiseasesChagas disease
The present invention provides a drug containing a compound having Trk-inhibiting activity as an active ingredient in prophylaxis and / or therapy for Trk-related diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease. A compound represented by the general formula (I), wherein all symbols represent the same meanings as described in the specification, a salt thereof, an N-oxide thereof, a solvate thereof or a prodrug thereof is useful as a drug component having Trk-inhibiting activity in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease.
Owner:ONO PHARMA CO LTD
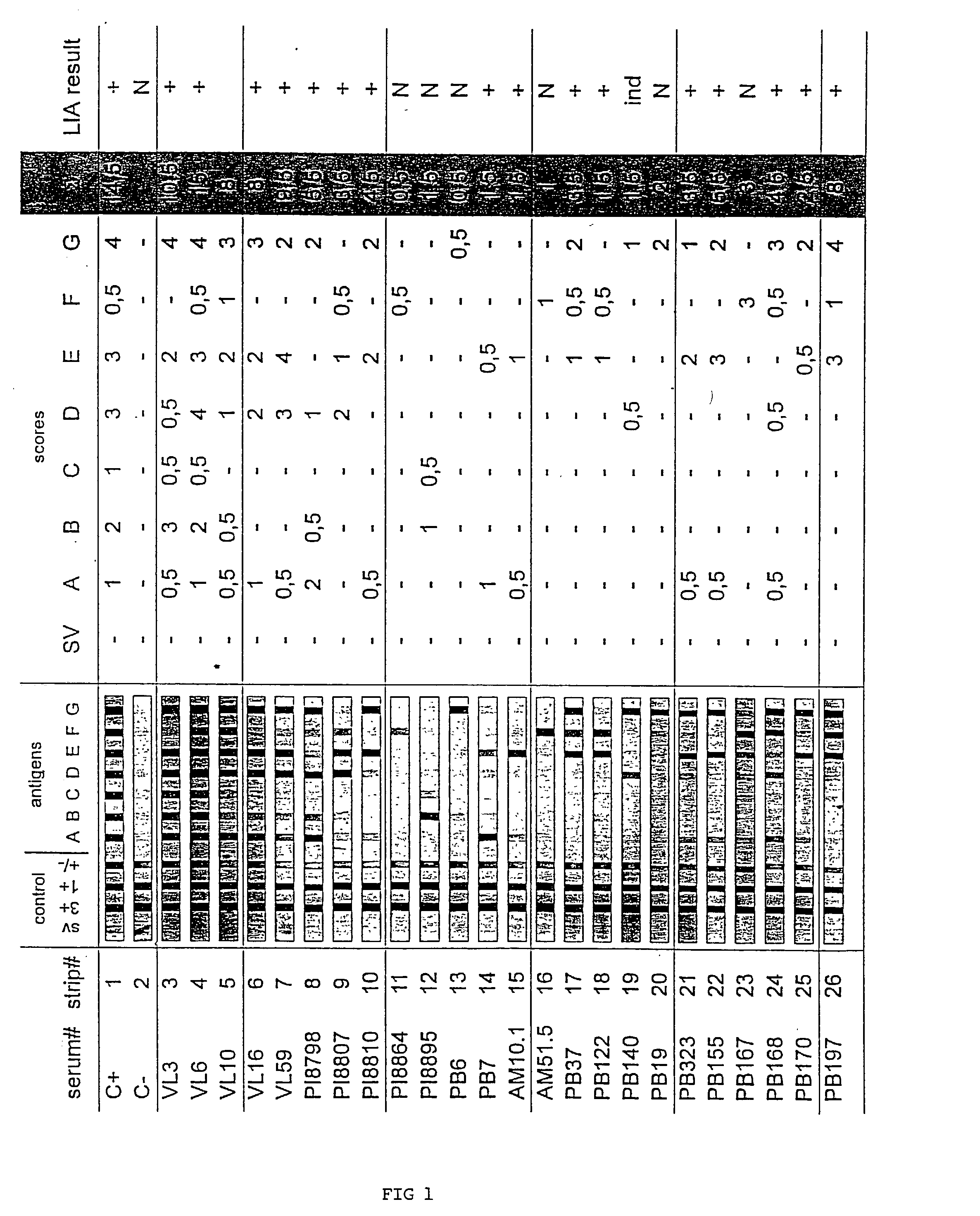
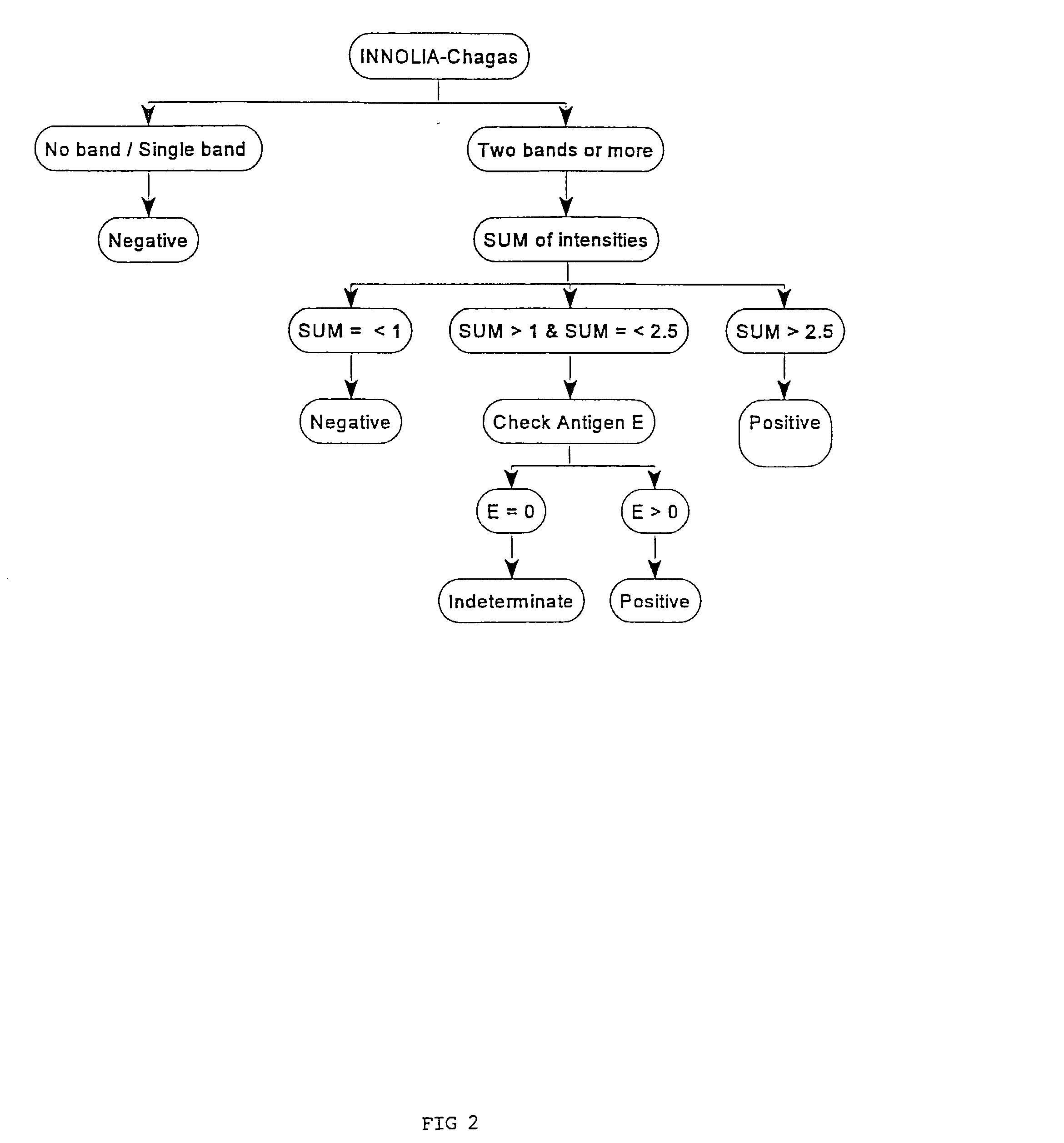
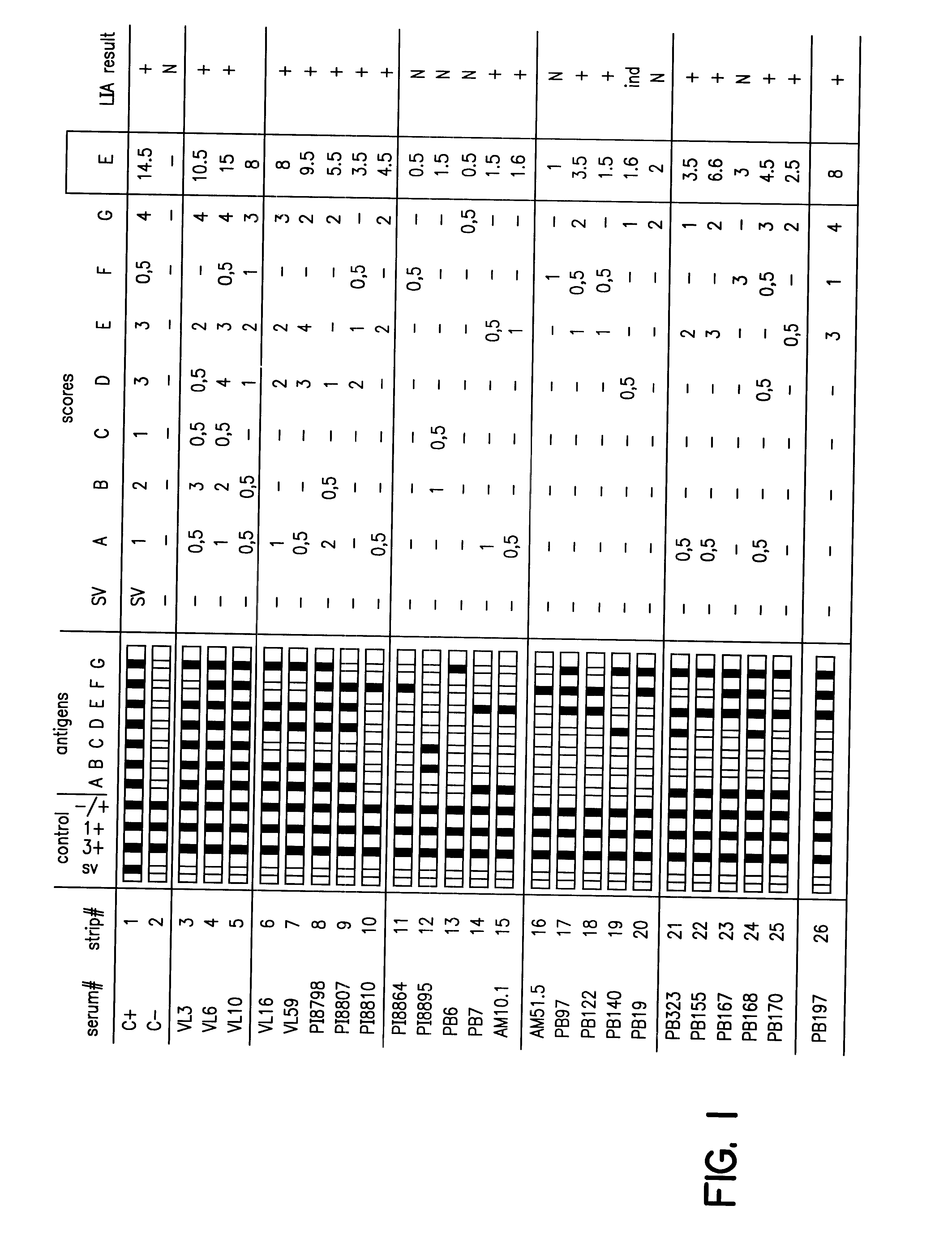
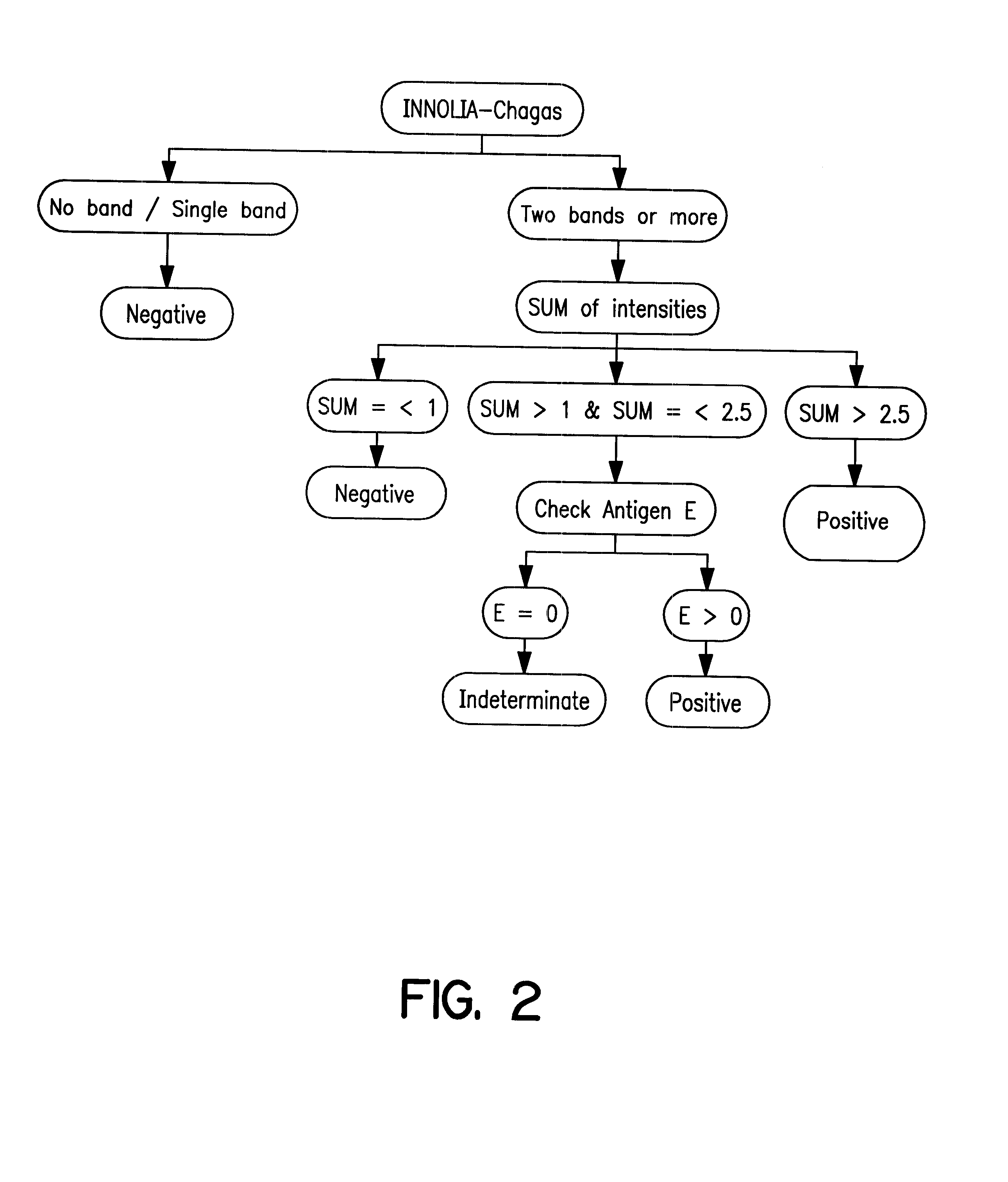
Antigens and immunoassays for diagnosing Chagas' disease
Transfusion of contaminated blood has become the major route of transmission for Chagas' disease. Current screening tests are insensitive and yield conflicting results, while confirmatory assays do not exist. The present invention relates to antigens and their use for serological diagnosis of Chagas' disease. More specifically, the present invention concerns assays which are able to reliably and accurately detect the presence of antibodies to various specific antigens of Trypanosoma cruzi in a highly sensitive and specific manner.
Owner:INNOGENETICS NV
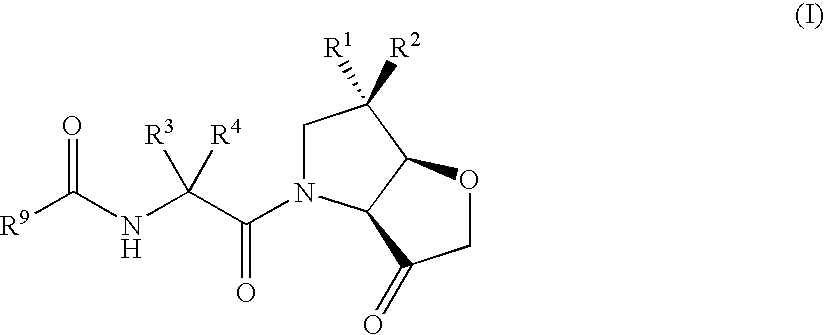
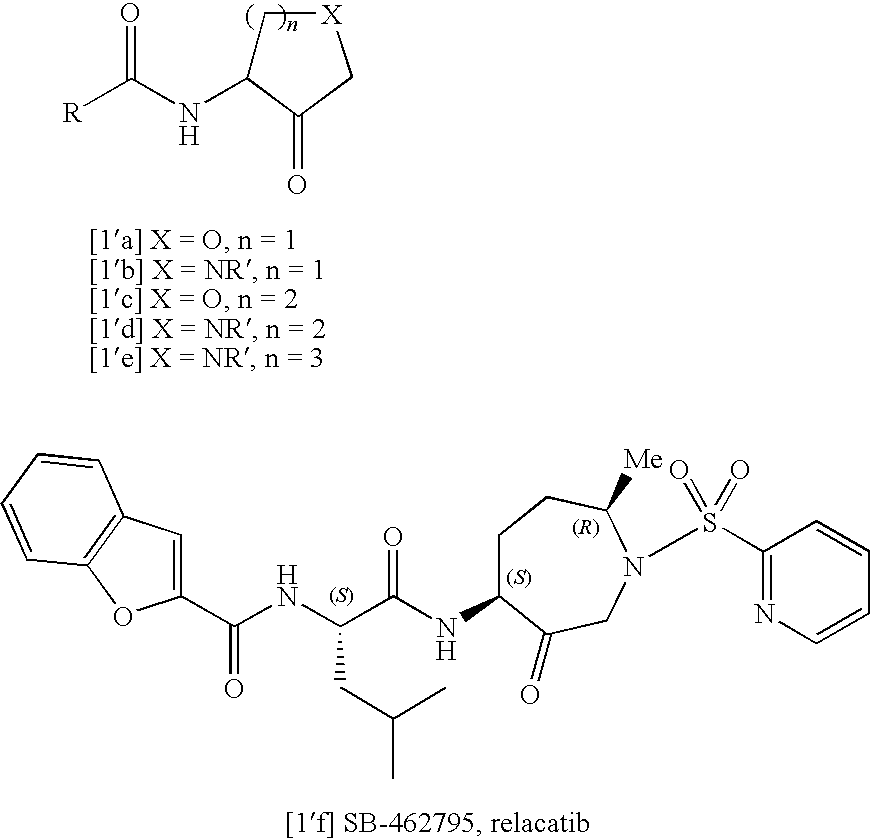
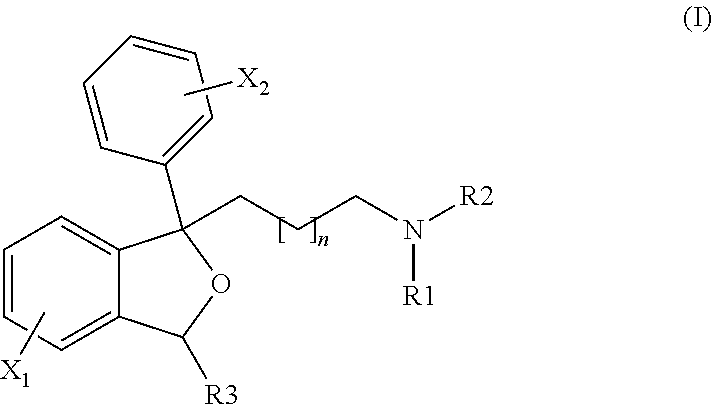
Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors
InactiveUS20100010009A1Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryGingival diseaseChagas disease
The present invention relates to compounds of formula (1), and pharmaceutically acceptable salts thereof, A compound of formula (I), or a pharmaceutically acceptable salt, hydrate, complex or pro-drug thereof (I), wherein: one of R1 and R2 is H, and the other is selected from F and Cl, or R1 and R2 are both F; R3 is selected from cyclopentyl and cyclohexyl; R4 is an optionally substituted 5- or 6-membered monocyclic or an 8- to 10-membered bicyclic aryl or heteroaryl ring which includes up to four heteroatoms. The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:GRUNENTHAL GMBH
Antigens and immunoassays for diagnosing Chagas' disease
Transfusion of contaminated blood has become the major route of transmission for Chagas' disease. Current screening tests are insensitive and yield conflicting results, while confirmatory assays do not exist. The present invention relates to antigens and their use for serological diagnosis of Chagas' disease. More specifically, the present invention concerns assays which are able to reliably and accurately detect the presence of antibodies to various specific antigens of Trypanosoma cruzi in a highly sensitive and specific manner.
Owner:INNOGENETICS NV
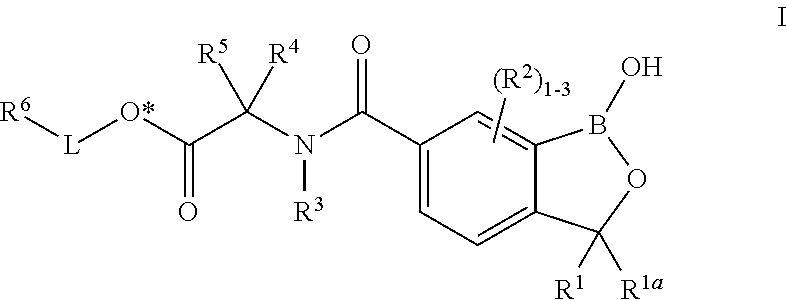
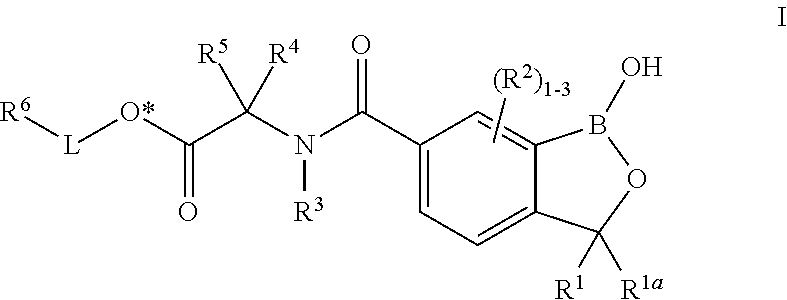
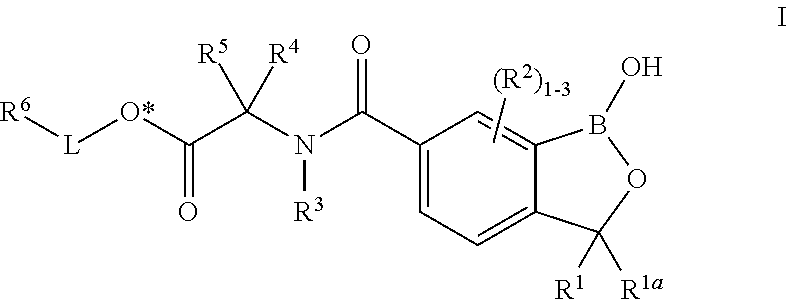
Oxaborole esters and uses thereof
ActiveUS20170327519A1Boron compound active ingredientsGroup 3/13 element organic compoundsSouth American trypanosomiasisMedicine
The present invention provides oxaborole ester compounds and compositions thereof which are useful to treat diseases associated with parasites, such as Chagas Disease and African Animal Trypanosomosis.
Owner:ANACOR PHARMA INC
Flavin protein of trypanosoma cruzi, method of screening vermicide with the use of the same and diagnostic
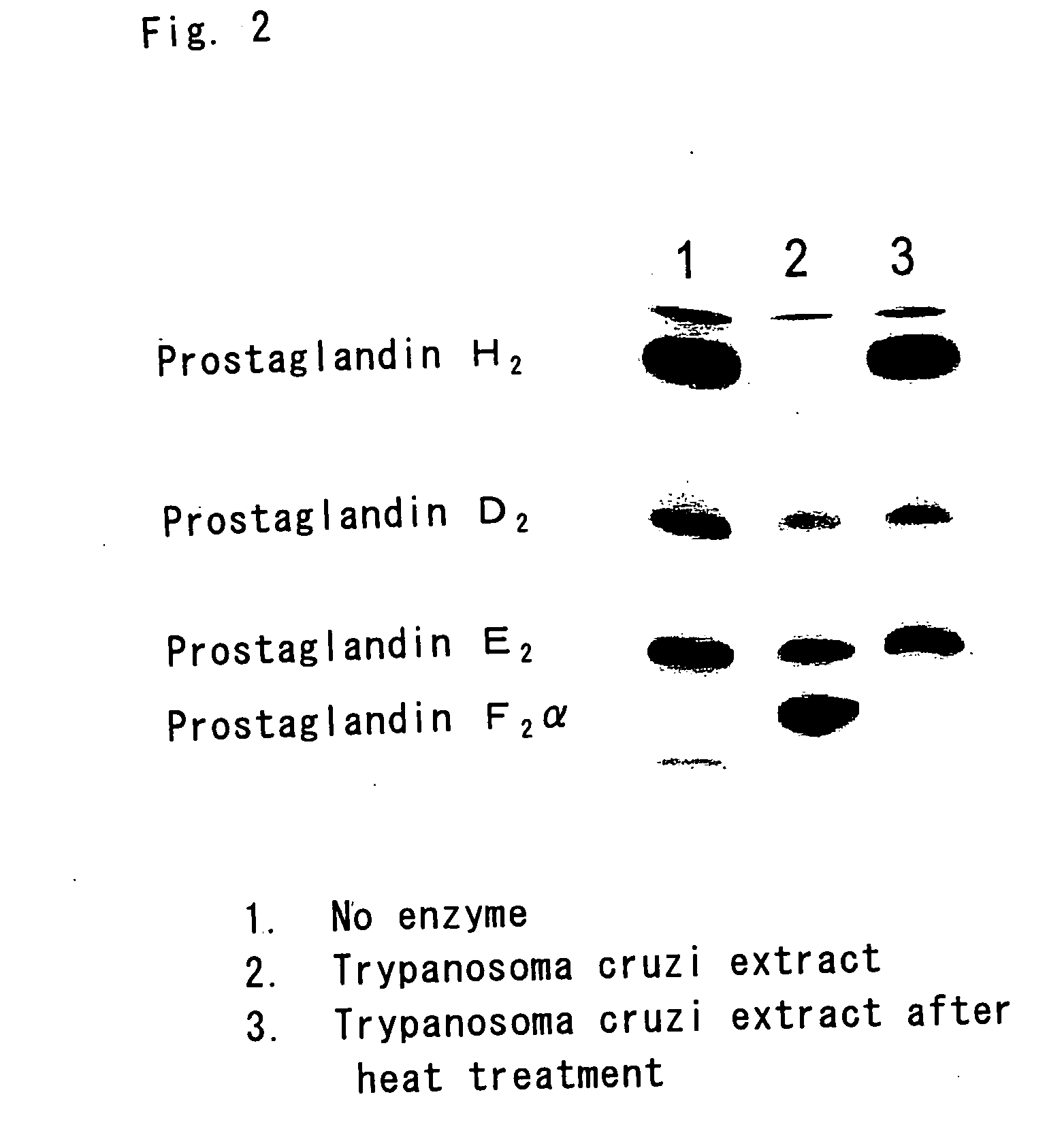
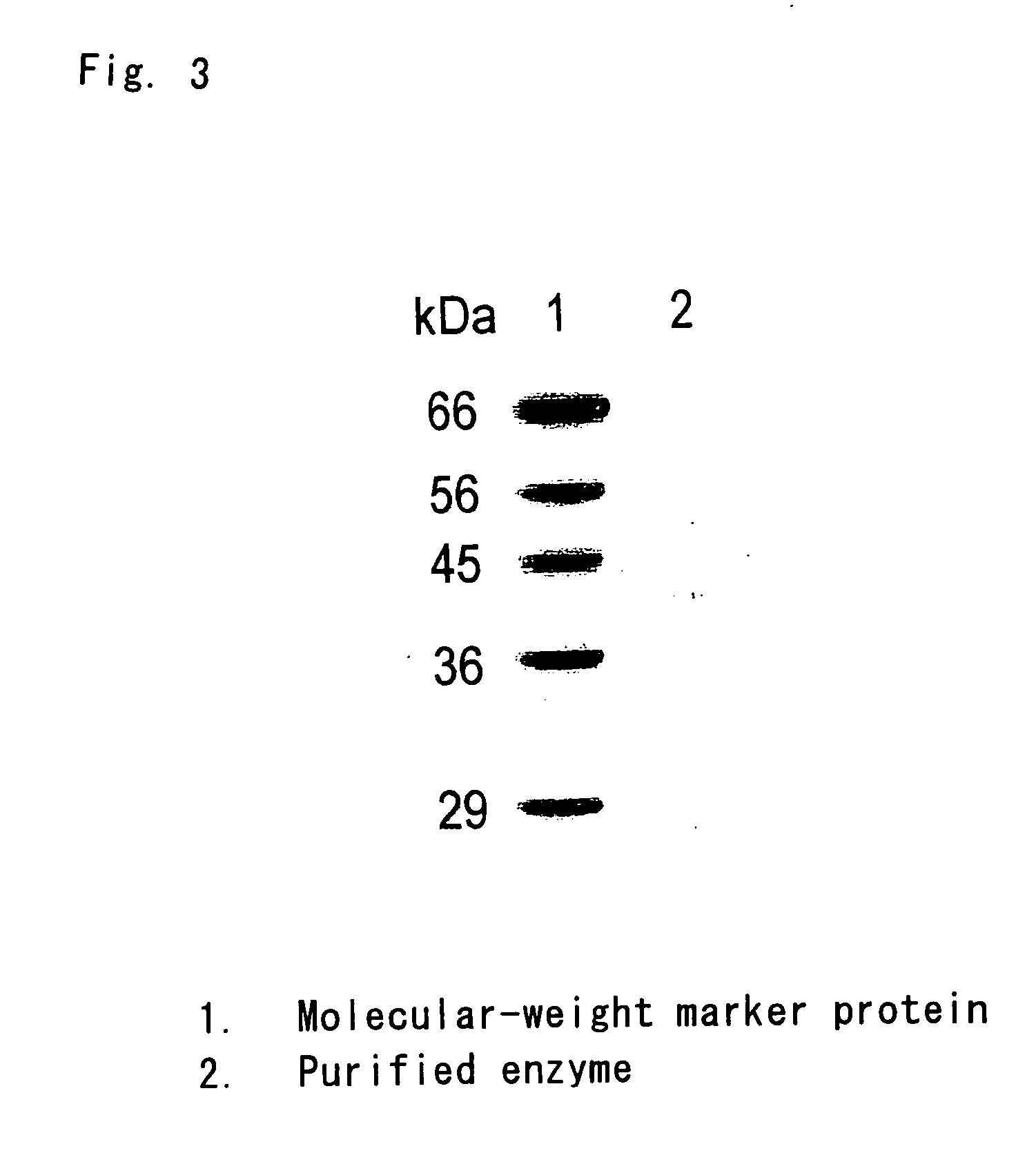
InactiveUS20060275329A1Promote decompositionHighly specific and simple methodCompound screeningApoptosis detectionFlavoproteinTrypanocidal Drugs
It is intended to provide a method of diagnosing infection with Chagas disease by screening a trypanocidal drugs for Trypanosoma cruzi which is the pathogen of Chagas disease. Using a flavin protein TcOYE specific to Trypanosoma cruzi, a trypanocidal drugs effective against Trypanosoma cruzi is screened. Using the gene sequence of TcOYE and an antibody therefor, infection with Trypanosoma cruzi is diagnosed.
Owner:OSAKA BIOSCI INST +1
Multicomponent or monocomponent vaccine to be used against Chagas disease, pharmaceutical compositions containing them, procedure for the obtention of immunogen of said vaccines, and nucleic acid used in said procedure
InactiveUS8900598B2Stimulate immune responseEliminates itAntibacterial agentsPeptide/protein ingredientsChagas diseaseTrypanosomiasis
A vaccine against the Chagas disease, capable of stimulating the immune response against the trans-sialidase virulence factor of the Trypanosoma cruzi parasite, which is a multicomponent vaccine comprising: (a) an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of thereof and (b) one or more polynucleotides including the regions codifying one or more immunogenic polypeptides, or a monocomponent vaccine comprising at least one component selected among an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of them and a group of polynucleotides including the regions codifying one or more immunogenic polypeptides derived from Trypanosoma cruzi and pharmaceutical compositions containing said multicomponent and monocomponent vaccines, the procedures for obtaining the immunogen portion of said vaccines and the nucleic acid used in the procedure.
Owner:DE BAEREMAECKER BARROS CARLOS
Multicomponent or monocomponent vaccine to be used against chagas disease, pharmaceutical compositions containing them, procedure for the obtention of immunogen of said vaccines, and nucleic acid used in said procedure
InactiveUS20100297186A1Stimulate immune responseReduces clinical consequenceAntibacterial agentsProtozoa antigen ingredientsChagas diseaseTrypanosomiasis
A vaccine against the Chagas disease, capable of stimulating the immune response against the trans-sialidase virulence factor of the Trypanosoma Cruzi parasite, which is a multicomponent vaccine comprising: (a) an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of thereof and (b) one or more polynucleotides including the regions that codifying one or more immunogenic polypeptides, or a monocomponent comprising at least one component selected among an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of them and a group of polynucleotides including the regions codifying one or more immunogenic polypeptides derived from Trypanosoma Cruzi and pharmaceutical compositions containing said multicomponent and monocomponent vaccines, the procedures for obtaining the immunogen portion of said vaccines and the nucleic acid used in the procedure.
Owner:DE BAEREMAECKER BARROS CARLOS
Trypanosoma cruzi proteome compositions and methods
Molecular targets are identified in T. cruzi suitable for use in diagnosis of Chagas disease, drug development, and vaccines, including live vaccines.
Owner:GEORGIA RESERACH FOUND INC UNIV OF
Serum biomarkers for Chagas disease
InactiveUS20050260691A1Microbiological testing/measurementBiological material analysisMedicineChagas disease
Owner:MCGILL UNIV
Protease inhibitors
A peptidyl nitrile of the general formula (I) or a pharmaceutically acceptable salt or prodrug thereof, is capable of selectively inhibiting dipeptidyl-peptidase (DPP-I), also known as cathepsin C. A compound of the invention is useful as an active substance for the treatment of inflammation, type 2 diabetes, asthma, severe influenza, respiratory syncytial virus infection, CD8 T cell inhibition, inflammatory bowel diseases, psoriasis, atopic dermatitis, Papillon Lefevre syndrome, Haim Munk syndrome, gun disease, periodontitis, rheumatoid arthritis, Huntington's disease, Chagas' disease, Alzheimer's disease, sepsis or for application in target cell apoptosis.
Owner:PROZYMEX
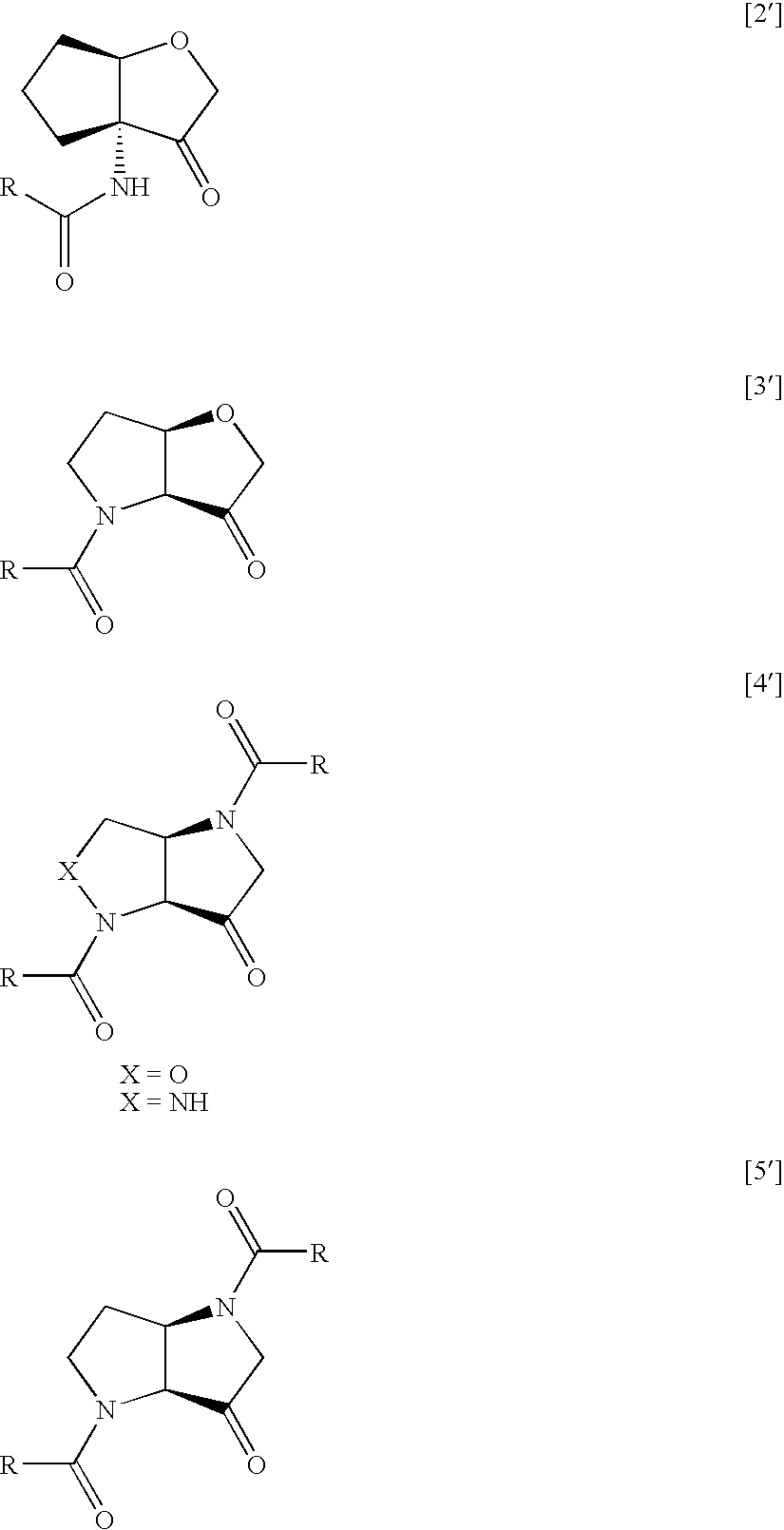
Tetrahydrofuro(3,2-B) pyrrol-3-one derivatives as inhibitors of cysteine proteinases
InactiveUS7799791B2Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryDiseaseChagas disease
A compound of formula (I), or a pharmaceutically acceptable salt, hydrate, complex or pro-drug thereof,wherein: one of R1 and R2 is H, and the other is selected from OR6, SR6, NR6R7, N3, Me, Et, CF3, SOR8 and SO2R8; orR1 and R2 are both H;one of R3 and R4 is H, and the other is selected from tert-butylmethyl, iso-propylmethyl, sec-butyl, tert-butyl, cyclopentyl and cyclohexyl; orR3 and R4 are joined together with the adjacent backbone carbon atom to form a spiro-C5-C6 cycloalkyl group;R6 and R7 are each independently selected from H, C1-8-alkyl and C3-8-cycloalkyl; orR6 and R7 are linked to form a cyclic group together with the nitrogen to which they are attached;R8 is C1-8-alkyl or C3-8-cycloalkyl;R9 is a para-substituted 6-membered monocyclic aryl or heteroaryl ring which includes up to five heteroatoms.The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:AMURA THERAPEUTICS
Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors
InactiveUS7846935B2Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryDiseaseGingival disease
Owner:AMURA THERAPEUTICS
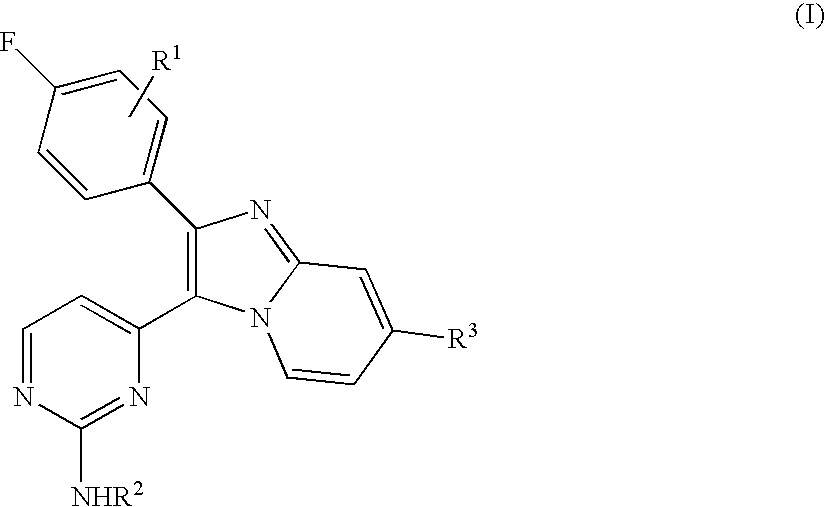
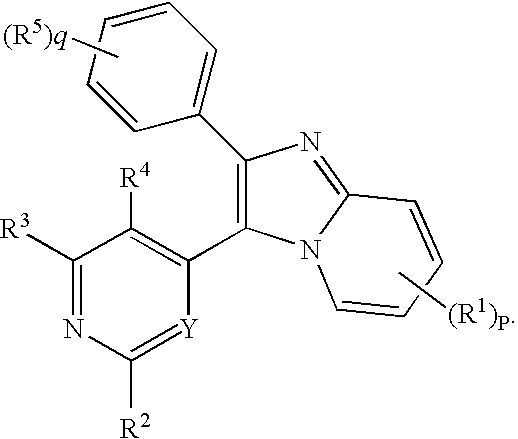
Antiprotozoal imidazopyridine compounds
Owner:MERIAL INC
Antiprotozoal Compounds
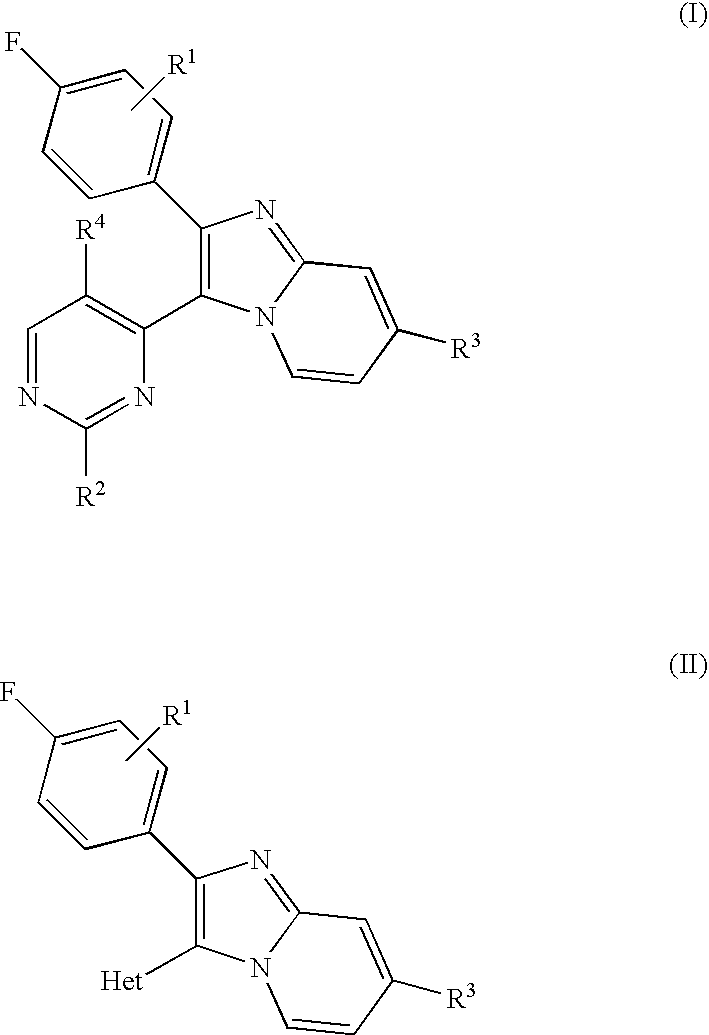
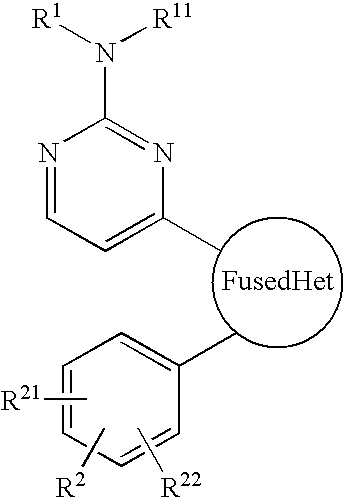
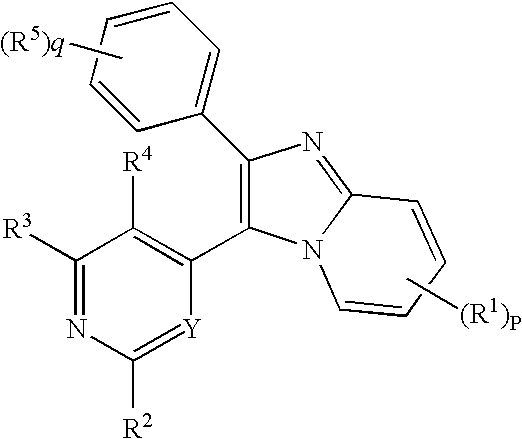
The invention is directed to a compound of formula I, as defined herein, or a pharmaceutically acceptable salt thereof; a pharmaceutical composition containing a compound of formula I, a method of treatment of a disorder or condition that may be treated by administration of the compound, the method comprising administering to a mammal in need of such treatment a compound of formula I as described above, and a method of treatment of a disorder or condition selected from the group consisting of Human African Trypanosomiasis (HAT), Chagas disease, Leishmaniasis and malaria, the method comprising administering to a mammal, including a human, in need of such treatment a compound of formula I as described above.
Owner:MEDISYNERGICS
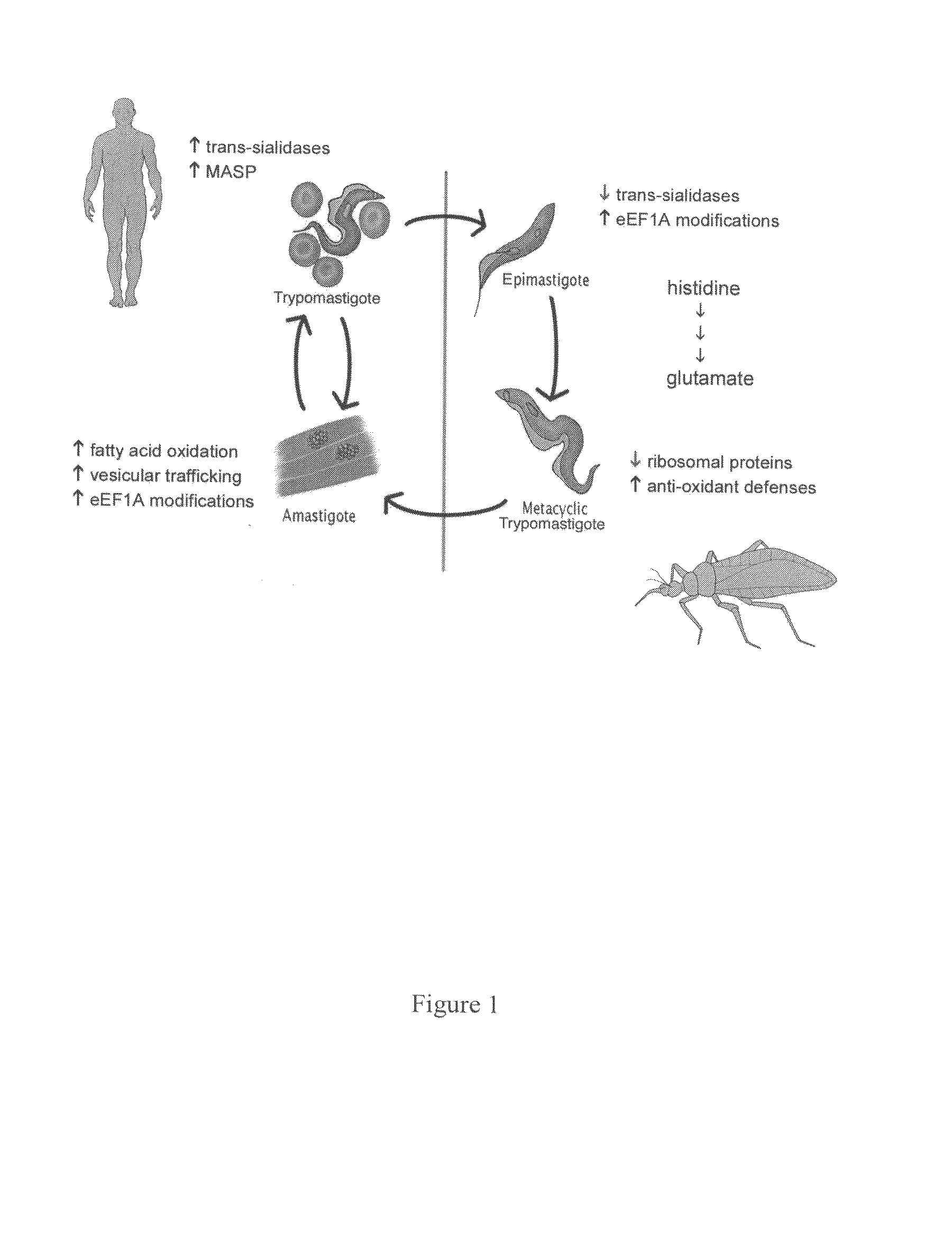
Diagnosis, Prevention and Treatment of Disorders Characterized by Undesirable Cell Proliferation
InactiveUS20140287432A1Reduce presenceIncrease the number ofMicrobiological testing/measurementDisease diagnosisDiseaseVulnerable plaque
The present invention relates to methods for the diagnosis, prevention and treatment of heart disease or heart failure in a subject. The present invention also relates to methods for the diagnosis, prevention and treatment of atherosclerosis with vulnerable plaque in a subject. Furthermore, the present invention relates to methods for the diagnosis, prevention and treatment of cardiomyopathies resulting from Chagas disease.
Owner:HIGUCHI MARIA DE LOURDES
Treatment of chagas disease
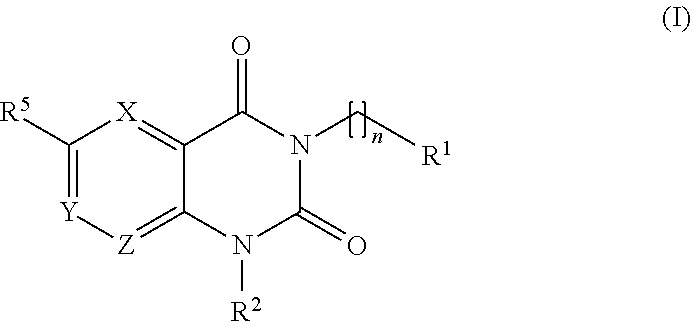
The invention provides compounds of the formula: wherein L1 and L2 are independently selected from O and S; R1 is C3-C6 straight or branched alkyl, C3-C7cycloalkyl, C5-C7cycloalkenyl, adamantly, phenyl or saturated heterocyclyl, any of which being optionally substituted; R2 is H, methyl or ethyl; R5 is NRxCORy, NRxRy, CH2COCH3, CH2C≡N, or a 5- or 6-membered heteroaryl group which is optionally substituted; X, Y and Z are independently N or CH; Rx is independently H or C1-C4alkyl; Ry is independently H, CrC4alkyl, phenyl or benzyl, either of which is optionally substituted; n is 0-3; salts, hydrates and N-oxides, wherein the optional substituents are further defined in the claims. The compounds have utility in the prophylaxis or treatment of trypanosomal diseases, such as T. cruzi (Chagas disease).
Owner:UNIVERSITY OF DUNDEE +3
Antiprotozoal amidine compounds
InactiveUS9169237B1Effective treatmentTreatment can be effected and facilitatedOrganic chemistryHeterocyclic compound active ingredientsLeishmaniasisMedicine
The invention is directed to a compound of formula I, as defined herein, or a pharmaceutically acceptable salt thereof; a pharmaceutical composition containing a compound of formula I, a method of treatment of a disorder or condition that may be treated by administration of the compound, the method comprising administering to a mammal in need of such treatment a compound of formula I as described above, and a method of treatment of a disorder or condition selected from the group consisting of Human African Trypanosomiasis (HAT), Chagas disease, malaria and Leishmaniasis, the method comprising administering to a mammal, including a human, in need of such treatment a compound of formula I as described above.
Owner:MEDI SYNERGICS
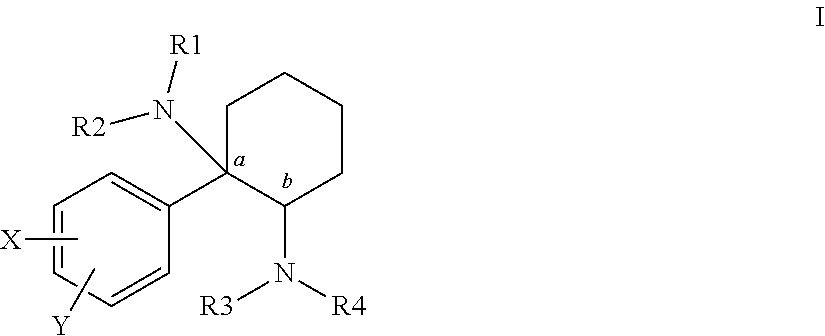
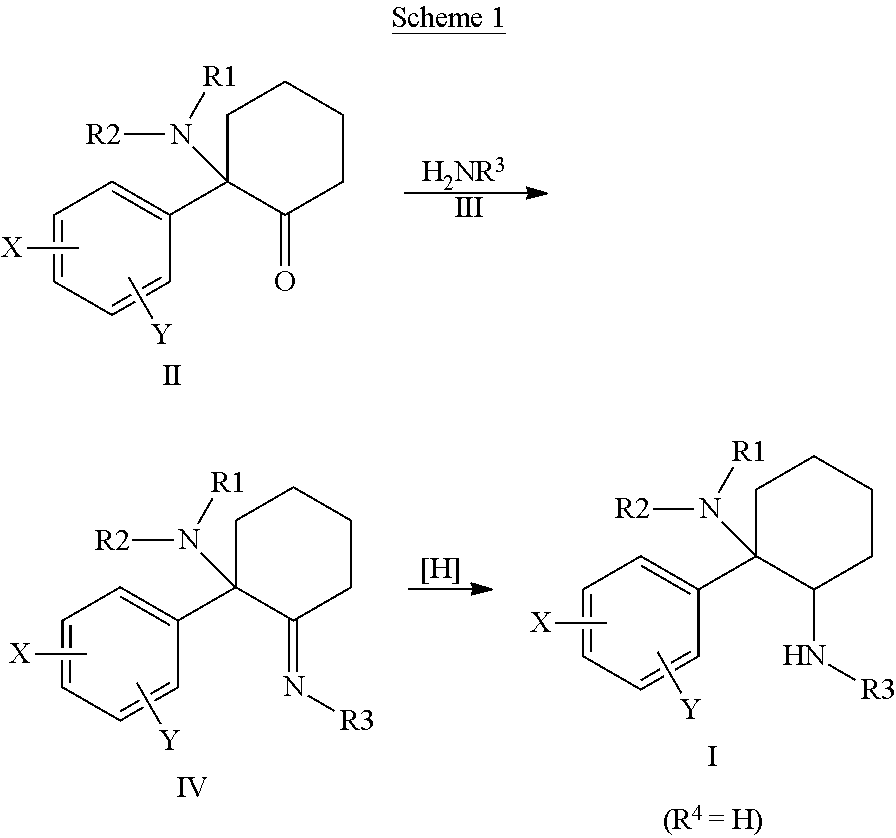
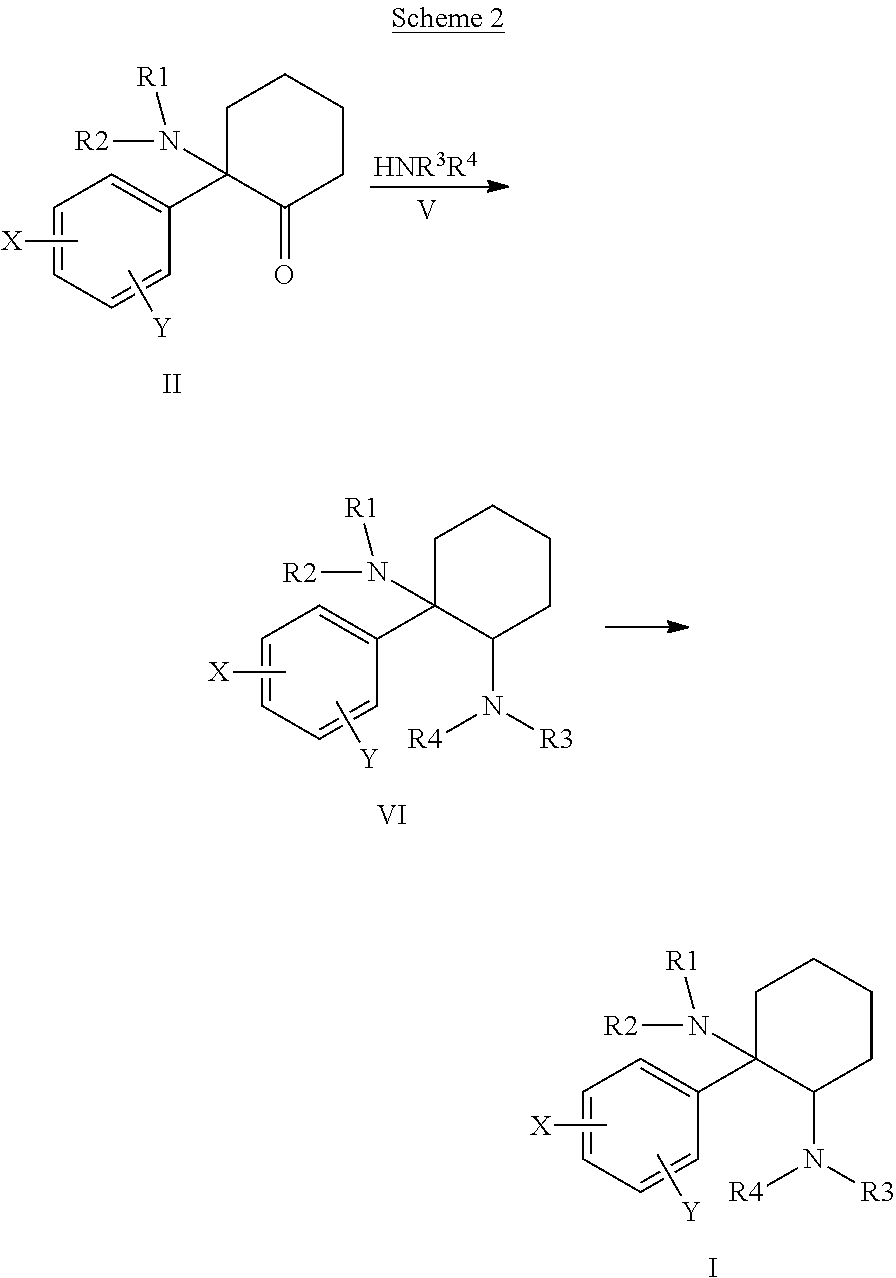
Cycloalkyl-Diamines
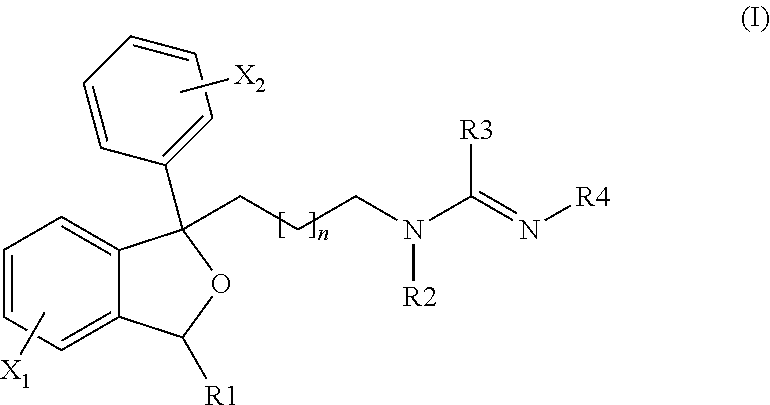
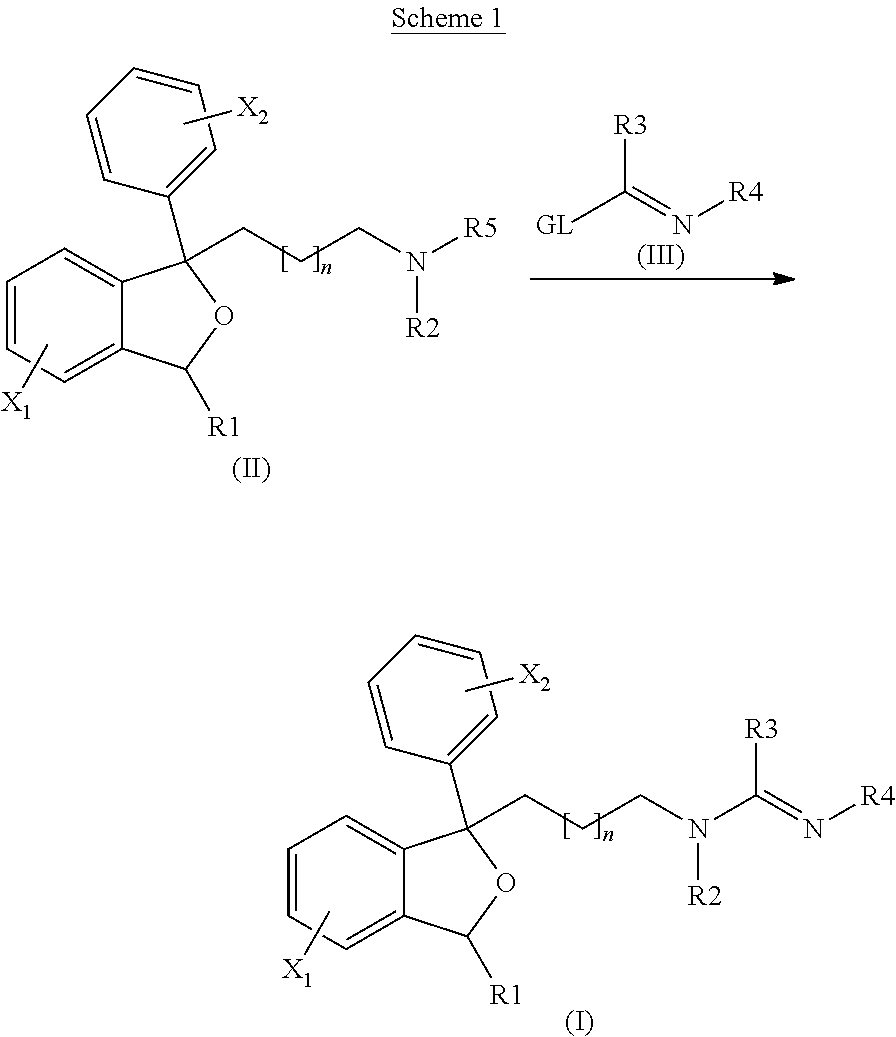
The invention is directed to a compound of formula I, as defined herein, or a pharmaceutically acceptable salt thereof; a pharmaceutical composition containing a compound of formula I, a method of treatment of a disorder or condition that may be treated by administration of the compound, the method comprising administering to a mammal, especially a human, in need of such treatment a compound of formula I as described above, and a method of treatment of a disorder or condition selected from the group consisting Human African Trypanosomiasis (HAT), Chagas Disease, Malaria, Leishmaniasis, and other infectious diseases transmitted to humans and animals by exposure to parasites, the method comprising administering to a human or mammal in need of such treatment a compound of formula I as described above.
Owner:MEDISYNERGICS
Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors
InactiveUS7803803B2Improve efficacyDesirable pharmacokinetic propertyAntibacterial agentsBiocideDiseaseCathepsin K
The present invention relates to compounds of formula (I), and pharmaceutically acceptable salts thereof,wherein:X is CH or N;one of R1 and R2 is H, and the other is selected from OR6, SR6, NR6R7, N3, Me, Et, CF3, SOR8 and SO2R8;R3 is selected from tert-butylmethyl, iso-propylmethyl, sec-butyl, tert-butyl, cyclopentyl and cyclohexyl;R4 is optionally substituted C1-8 alkyl or optionally substituted C3-8 cycloalkyl;R6 and R7 are each independently selected from H, C1-8-alkyl and C3-8-cycloalkyl, or R6 and R7 are linked to form a cyclic group together with the nitrogen to which they are attached; andR8 is C1-8-alkyl or C3-8-cycloalkyl.The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:AMURA THERAPEUTICS
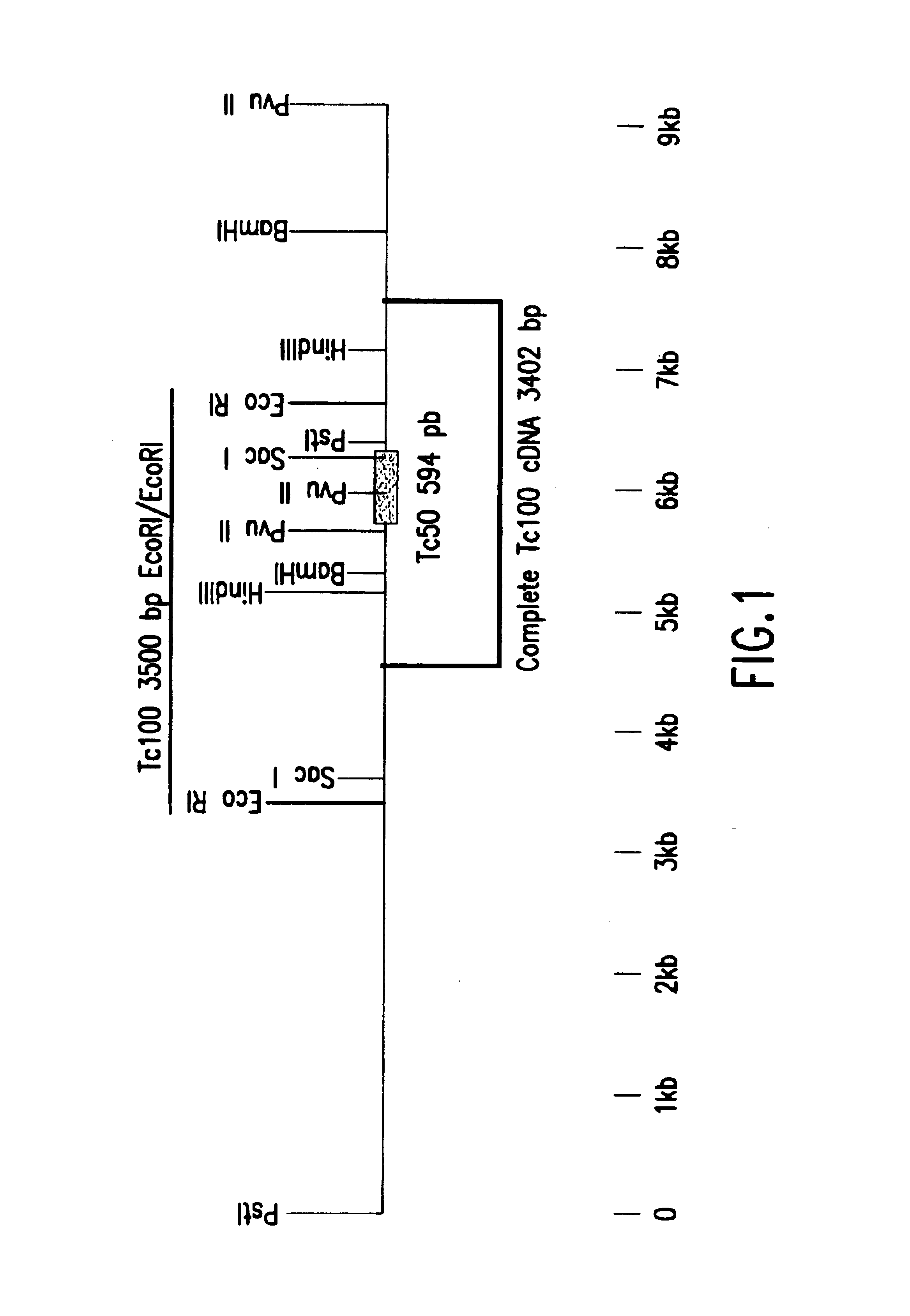
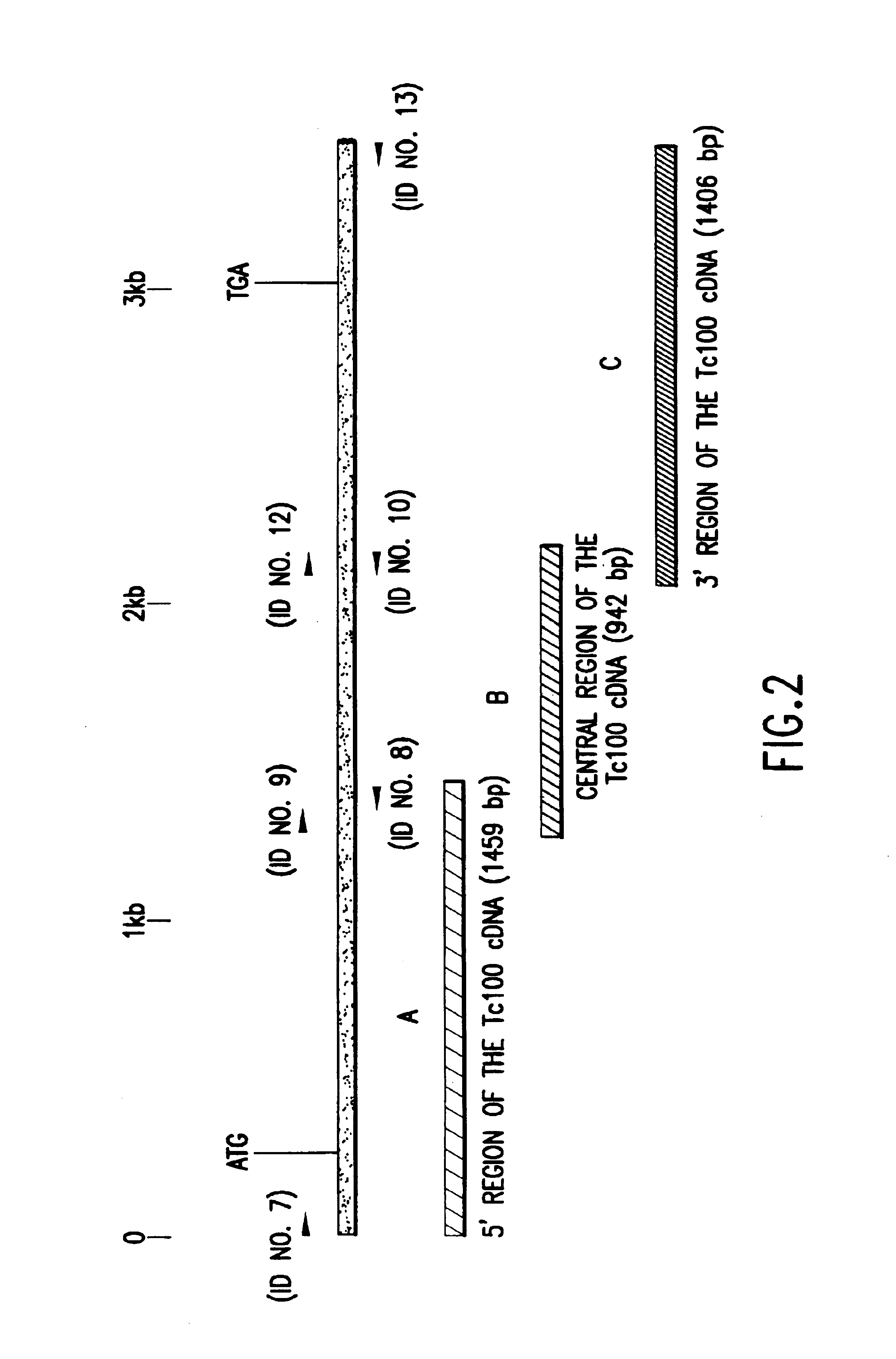
Trypanosoma cruzi antigen, gene encoding therefor and methods of detecting and treating chagas disease
InactiveUS6933110B1Improve presentationImprove stabilitySugar derivativesMicrobiological testing/measurementAntigenTrypanosoma antigen
The nucleotide sequence of Tc100, a gene encoding PTc100, a new Trypanosoma antigen, and the amino acid sequence of PTc100 are described. Tc100 and PTc100, or fragments thereof, modified or otherwise, can be used directly or indirectly for the detection of Trypanosoma cruzi, or for the monitoring of the infection generated by Trypanosoma cruzi, in man or in animals.
Owner:BIOMERIEUX SA
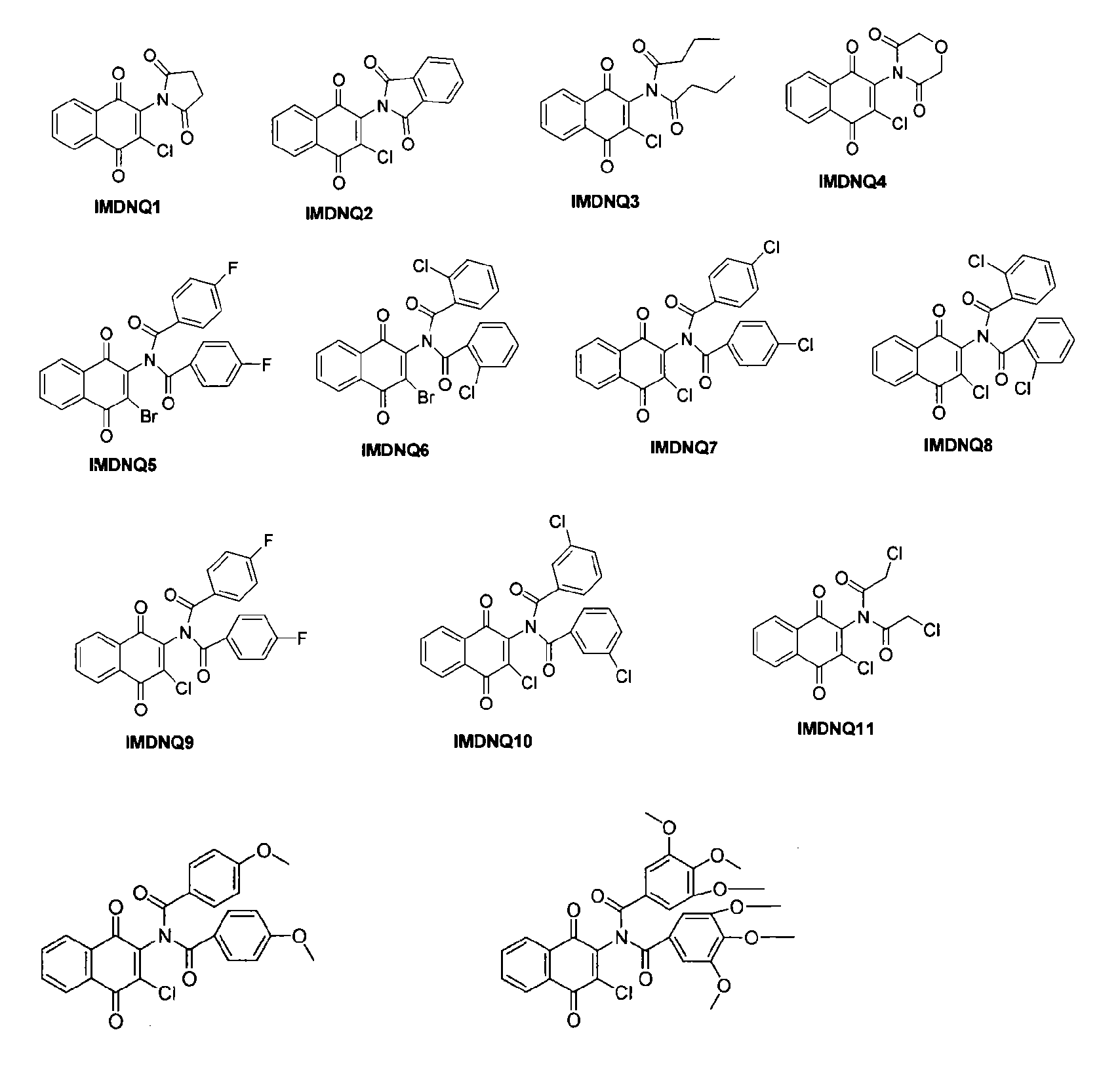
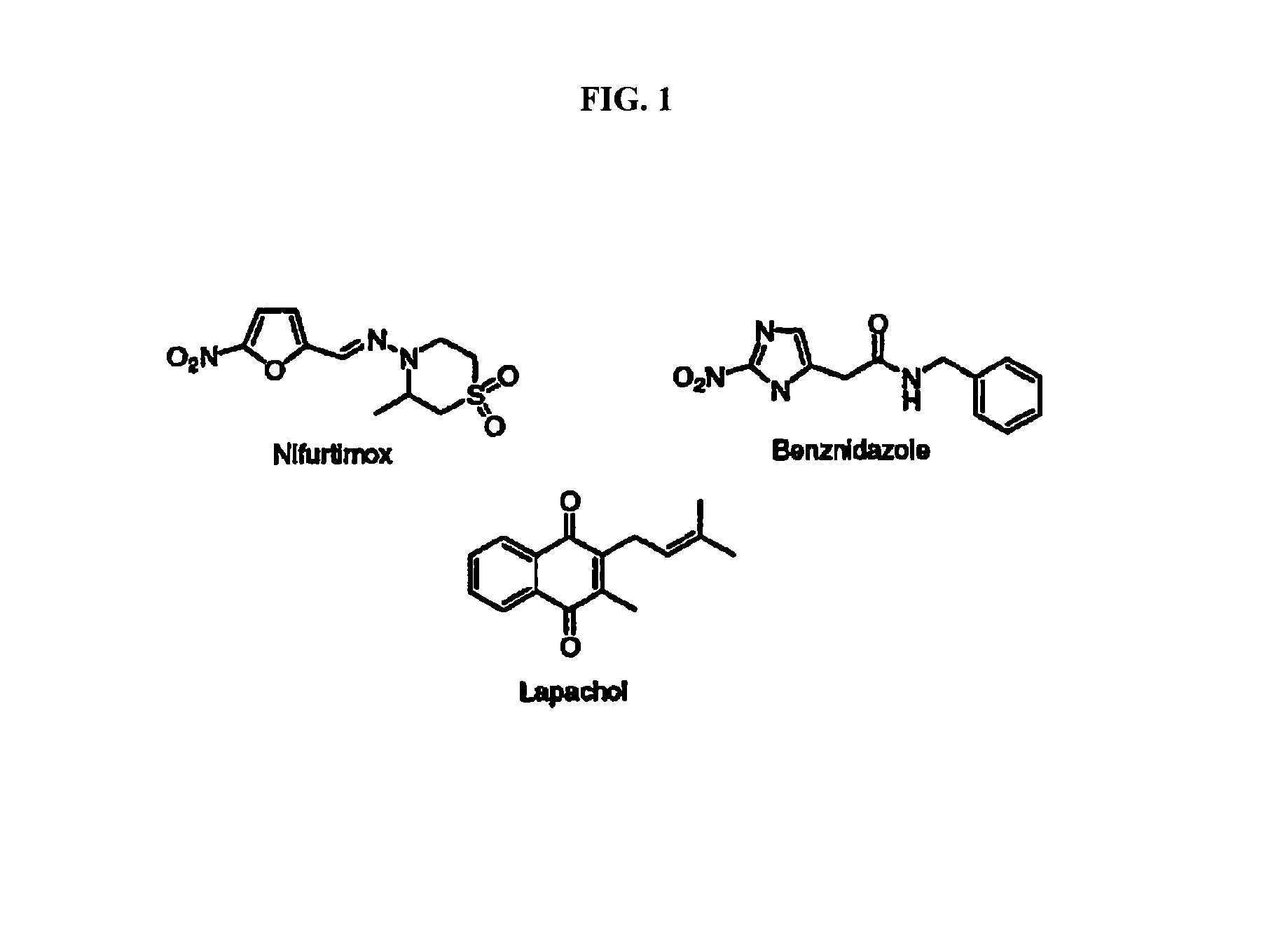
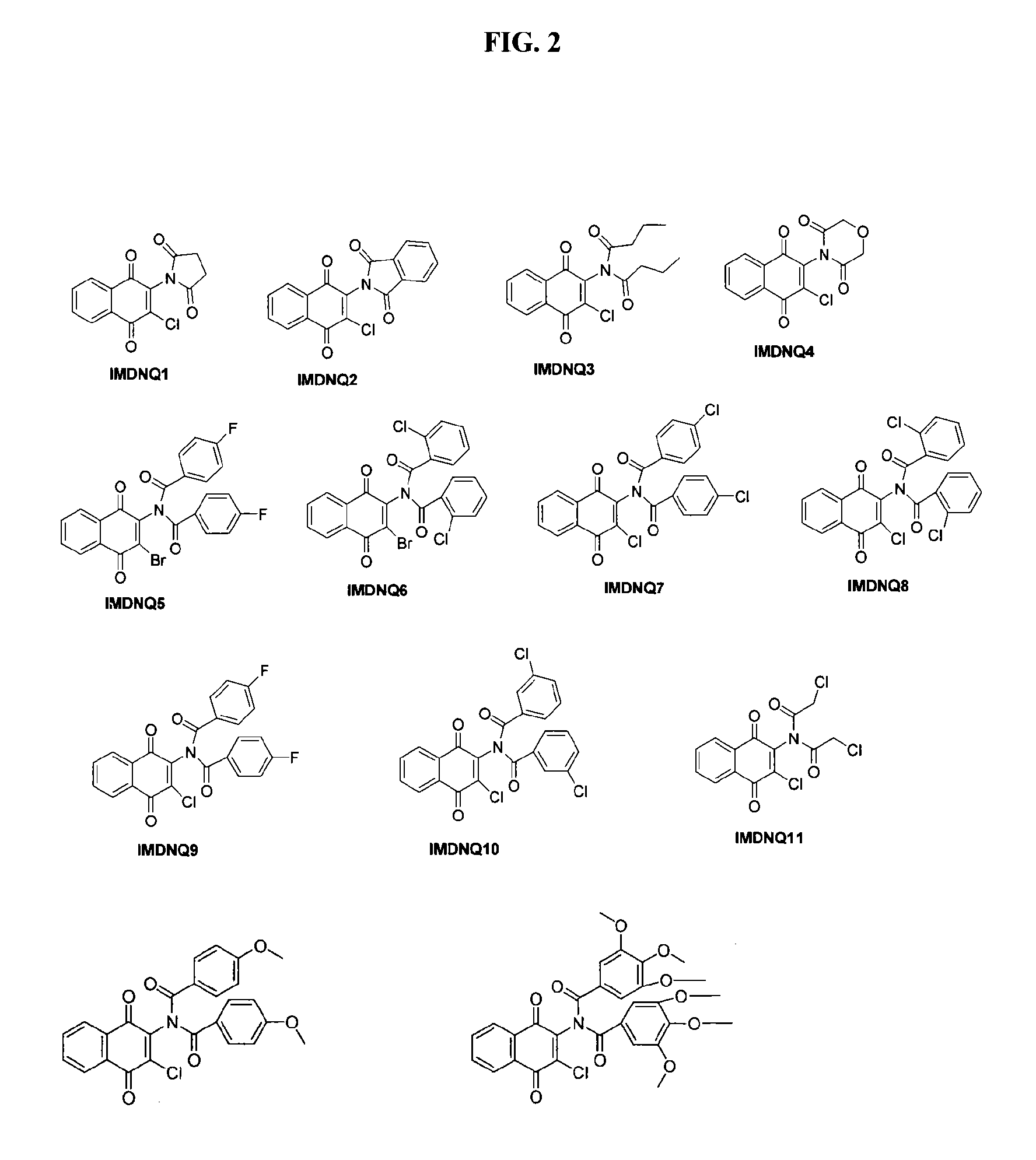
Method for inhibiting Trypanosoma cruzi
Methods are provided to inhibit proliferation of Trypanosoma cruzi with imido-substituted 1,4-naphthoquinones, including novel compounds. Administering an imido-substituted 1,4-naphthoquinone can be used to provide prophylaxis or treatment to a patient in need of treatment against Chagas disease.
Owner:HOWARD UNIVERSITY
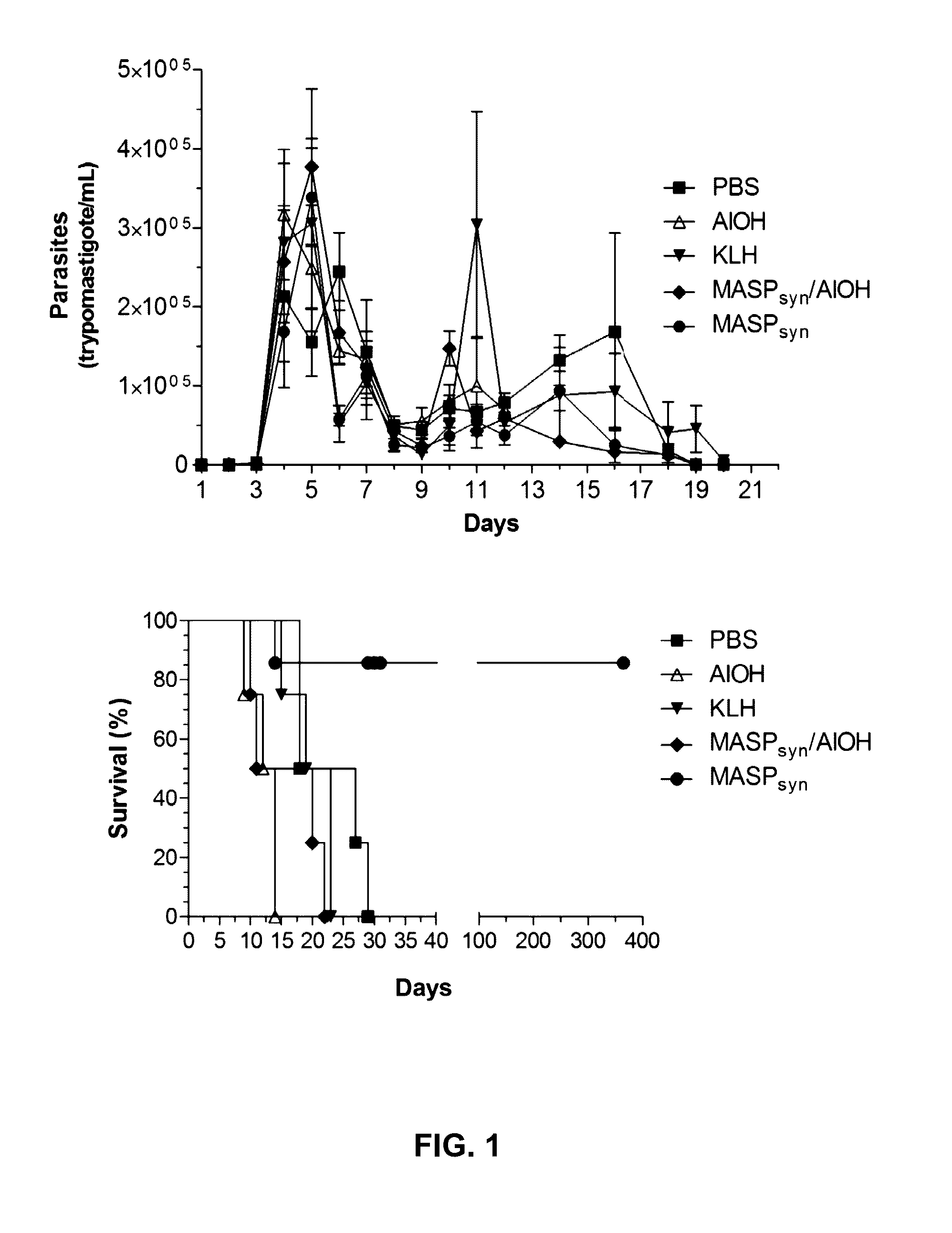
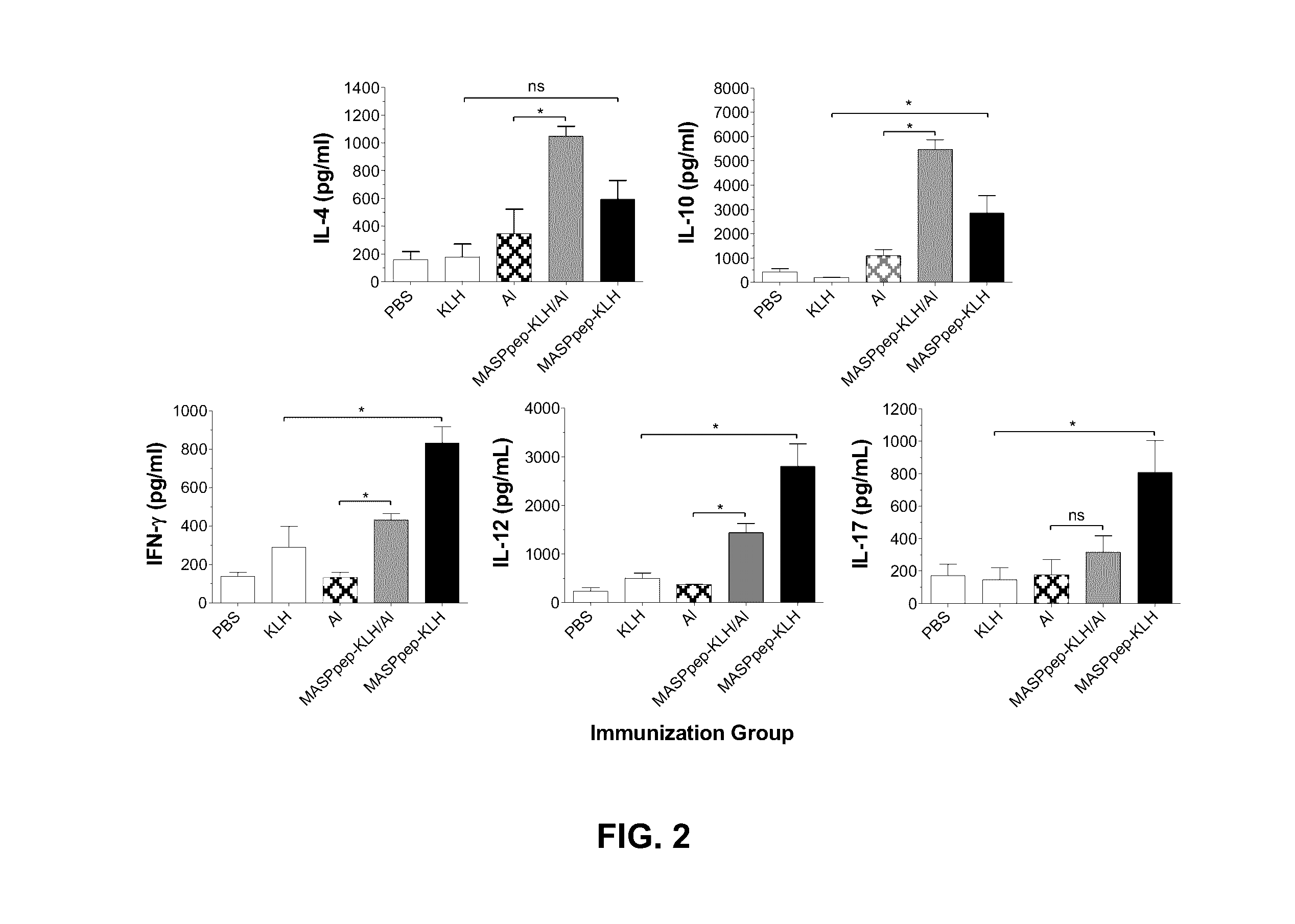
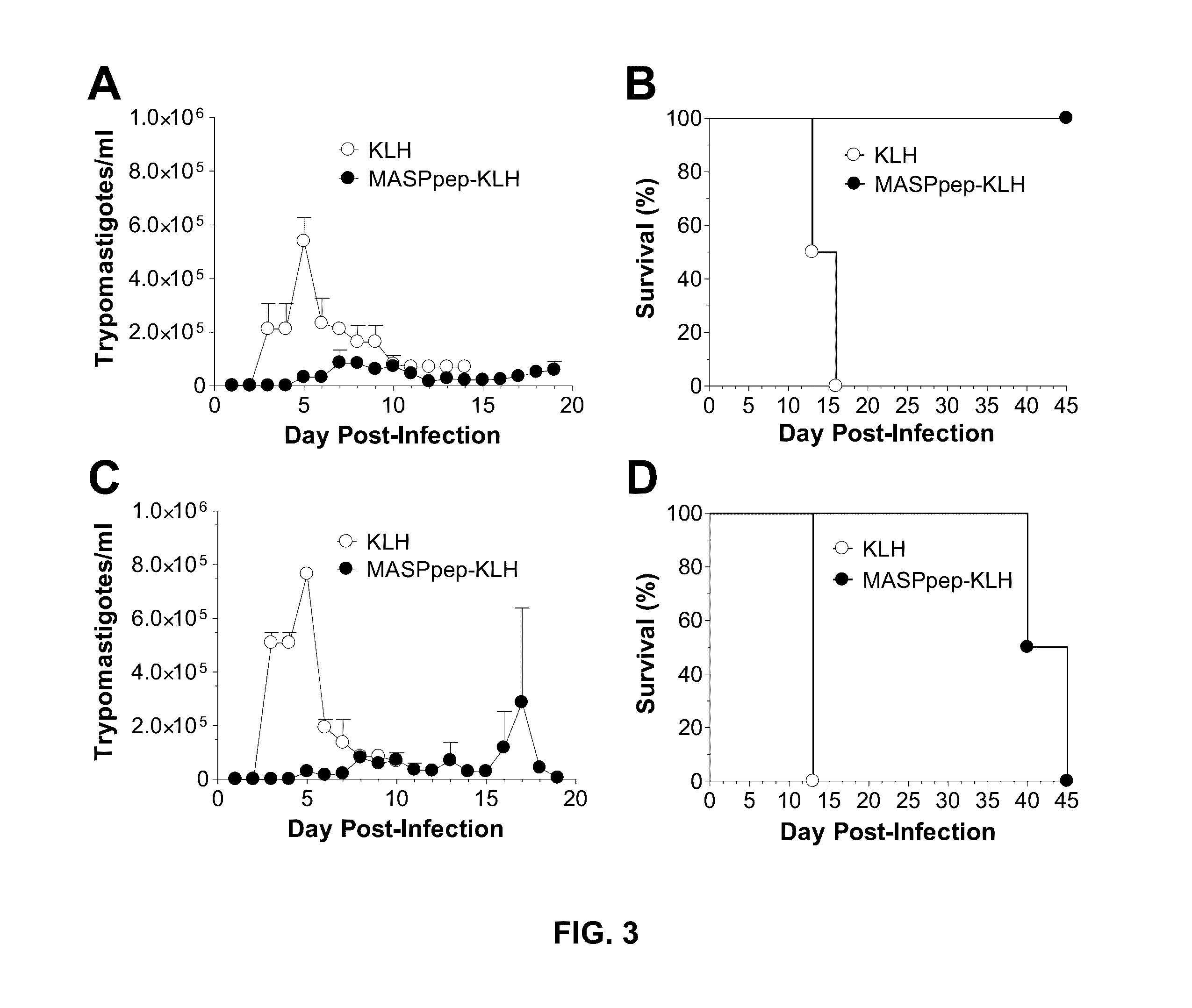
Mucin-associated surface protein as vaccine against Chagas disease
Use of synthetic peptides derived from Trypanosoma cruzi antigens and their use in vaccination against trypomastigote infection and Chagas disease. T. cruzi uses several surface proteins to invade the host. In their role of protection, the surface protients ensure the targeting and invasion of specific cells or tissues. A conserved region in the family of mucin-associated surface proteins (MASP) was used to analyze the expression of MASP at different points of invasion and proved to be important for host cell invasion, thus suggesting MASP as a candidate for vaccine development. A synthetic peptide, MASPsyn, was studied and showed efficacy in stimulating antibody and cytokine production necessary for resistance against the parasite.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Diagnosis, prevention and treatment of disorders characterized by undesirable cell proliferation
InactiveUS8551940B2Increase the number ofReduce in quantityPeptide/protein ingredientsMicrobiological testing/measurementDiseaseChagas disease
The present invention relates to compositions and methods for the reduction of atherosclerotic plaques and the decrease in the level of total serum cholesterol, triglycerides, serum LDL cholesterol, and serum HDL cholesterol. The present invention also relates to methods for the diagnosis, prevention and treatment of atherosclerosis and mycoplasma associated diseases, cardiotoxicity related to cancer treatment, and Chagas disease related cardiomyopathies.
Owner:DE LOURDES HIGUCHI MARIA
New pradimicin derivatives for the treatment of diseases caused by kinetoplastids
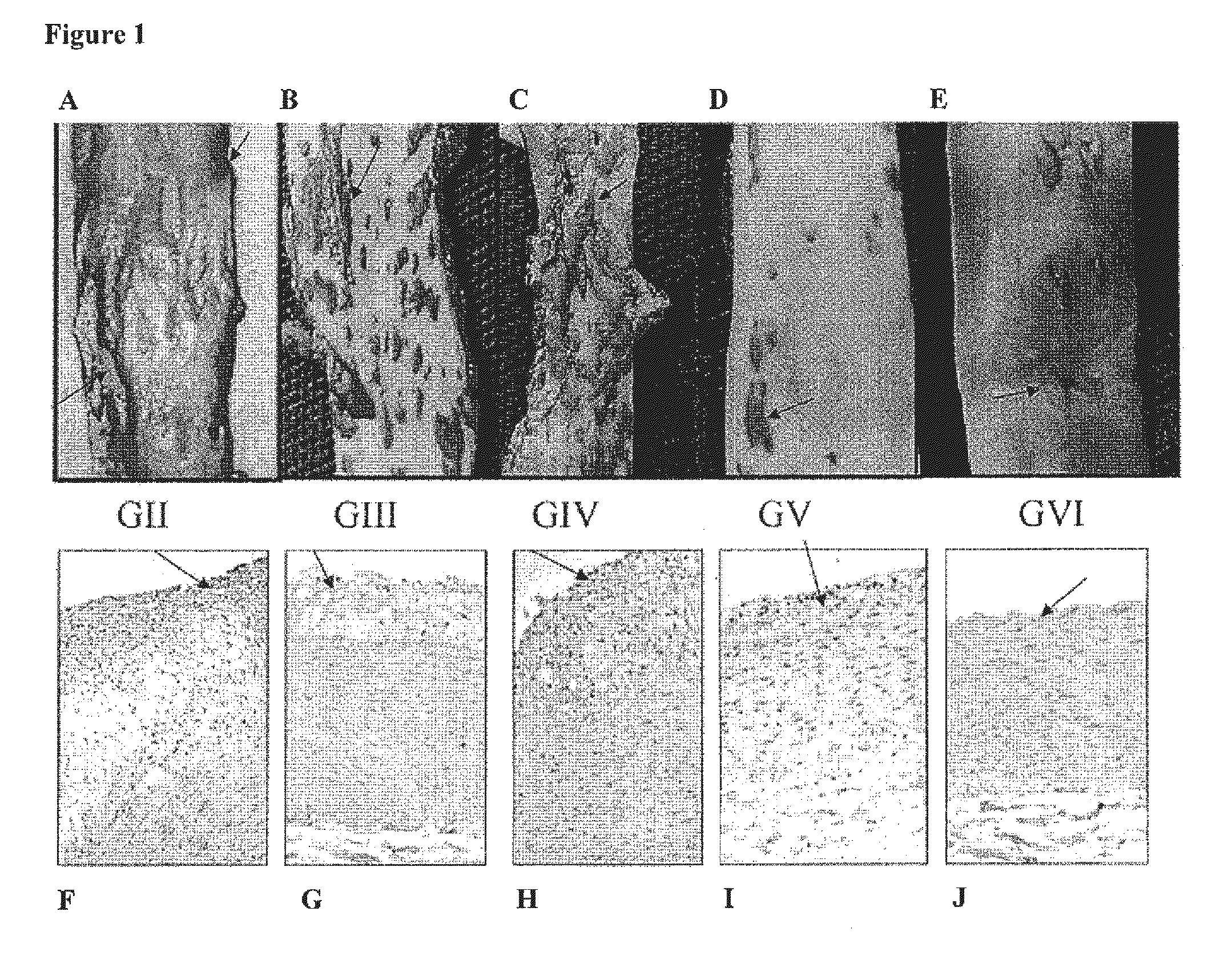
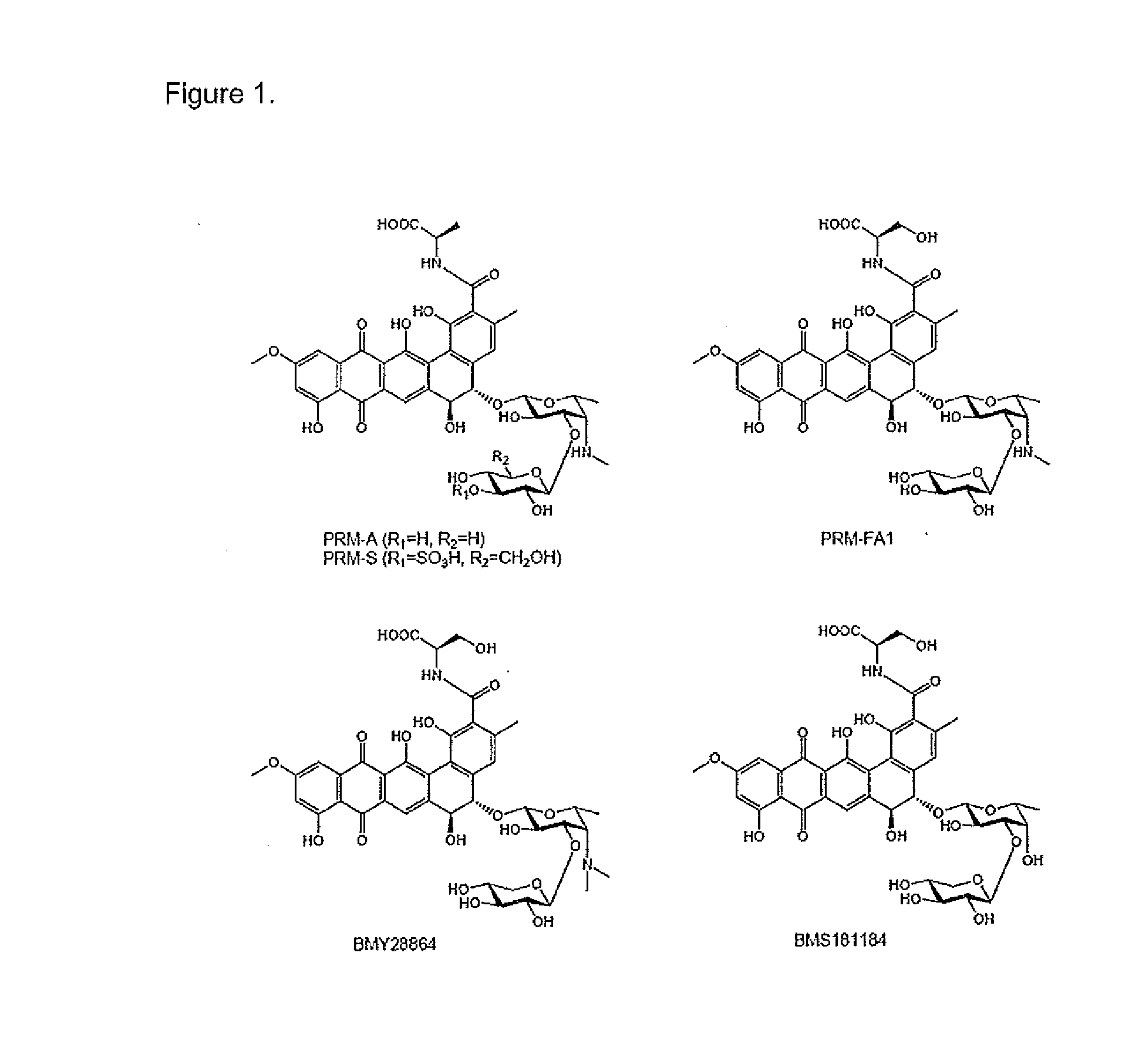
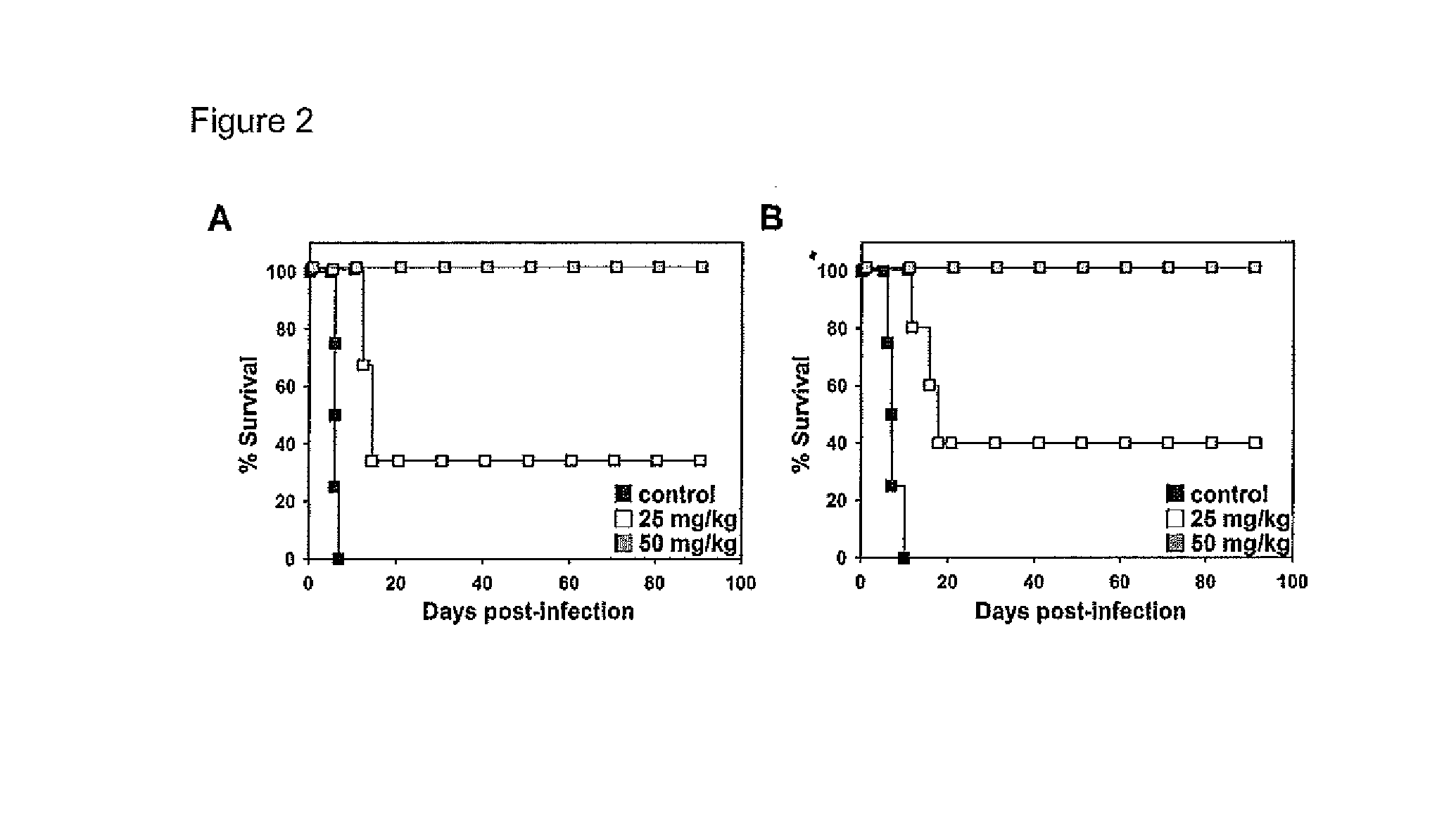
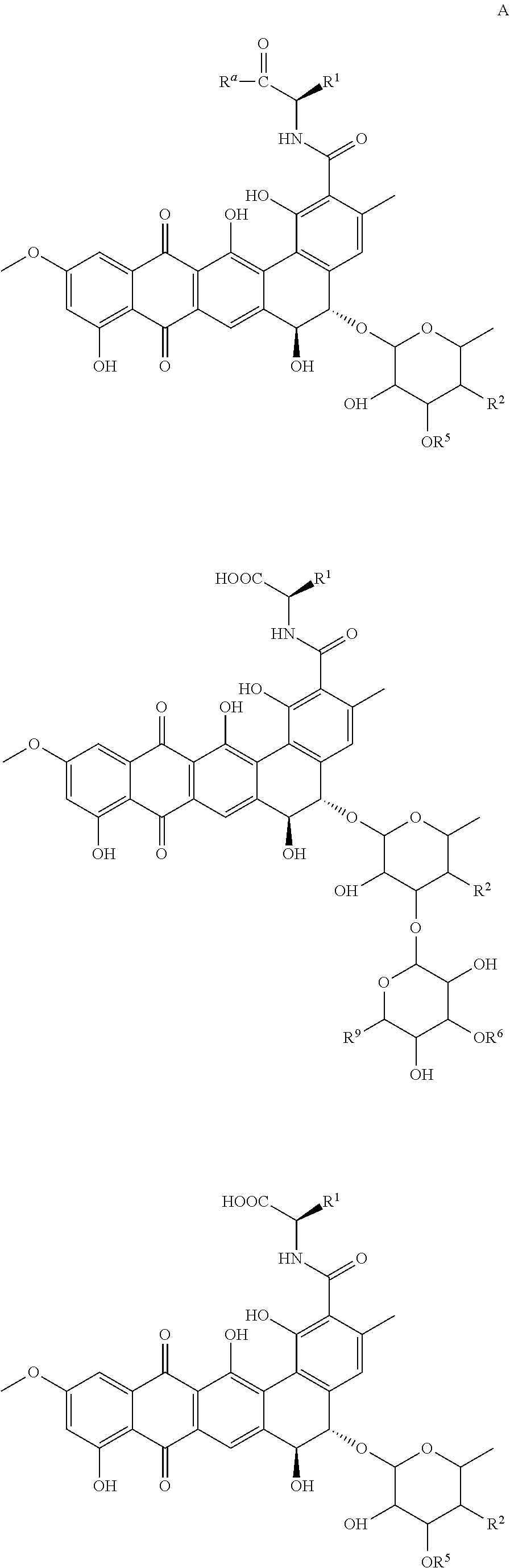
ActiveUS20160243140A1Reduce usageUseful in treatmentOrganic active ingredientsAntiparasitic agentsDiseaseChagas disease
The present invention relates to a class of novel pradimicins and analogues and derivatives thereof, including the compounds of formula A, I and 111, and / or a pharmaceutical acceptable addition salt thereof and / or a stereoisomer thereof and / or a solvate thereof and their use to treat or prevent kinetoplastid infections and their use to manufacture a medicine to treat or prevent kinetoplastid infections, particularly infections with trypanosoma and leishmania, such as Trypanosoma brucei, Trypanosoma cruzi and Leischmania donovani. wherein Ra, R1, R2, R3, R4, R5, R6, R7, R8 and R9 are as defined in the claim 1 or as described in detail in the description of the invention. The present invention also relates to pharmaceutical compositions of said compounds and the use of said pharmaceutical compositions to treat or prevent kinetoplastid infections. The present invention further relates active ingredients, more specifically as medicaments for the treatment of kinetoplastid infections and pathologic conditions such as, but not limited to Trypanosomiasis, such as African trypanosomiasis, sleeping sickness, Chagas disease and leishmaniasis.
Owner:KATHOLIEKE UNIV LEUVEN +1
Antiprotozoal imidazopyridine compounds
Compounds described by the Formula (I) or (II): or pharmaceutically acceptable salts, or N-oxides thereof. The compounds are useful for the treatment and prevention of protozoal diseases in mammals and birds. A method for controlling coccidiosis in poultry comprises administering an effective amount of the compound alone, or in combination with one or more anticoccidieal agent(s). A composition for controlling coccidiosis in poultry comprises the compound alone, or in combination with one or more anticoccidial agent(s). Methods for the treatment and prevention of mammalian protozoal diseases, such as, for example, toxoplasmosis, malaria. African typanosomiasis, Chagas disease, and opportunistic infections comprise administering the compound alone, or in combination with one or more antiprotozoal agent(s).
Owner:MERCK SHARP & DOHME LLC
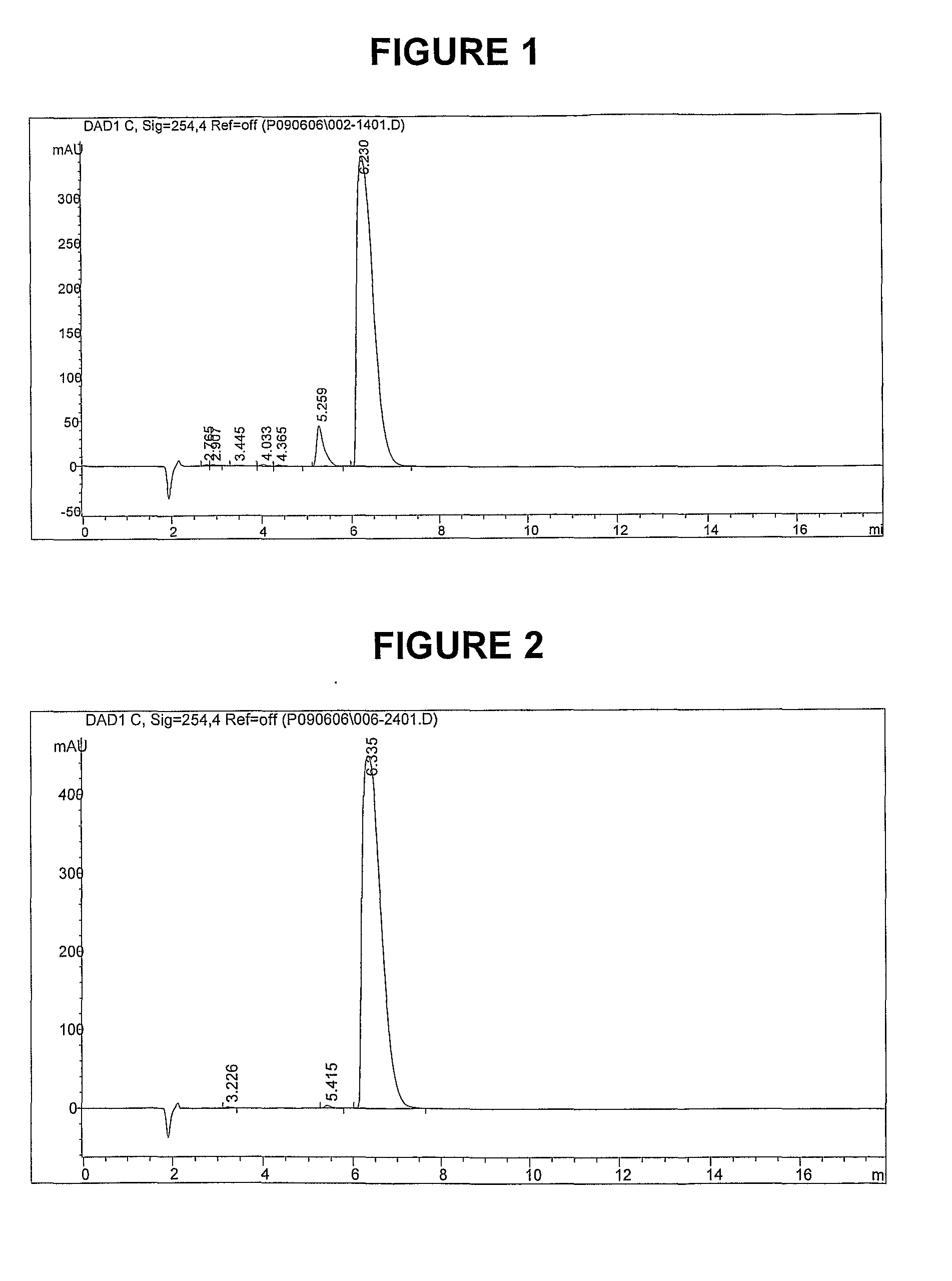
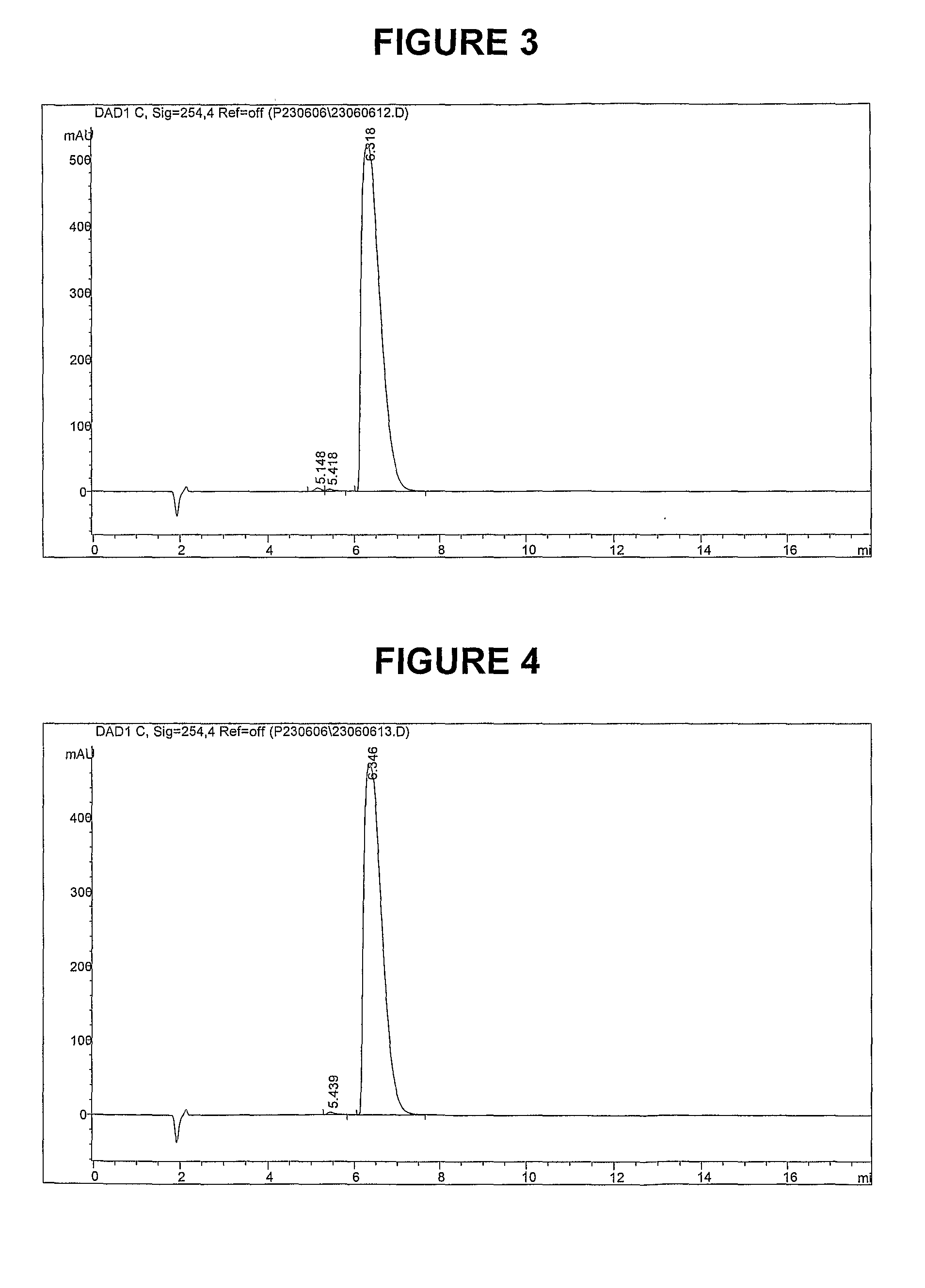
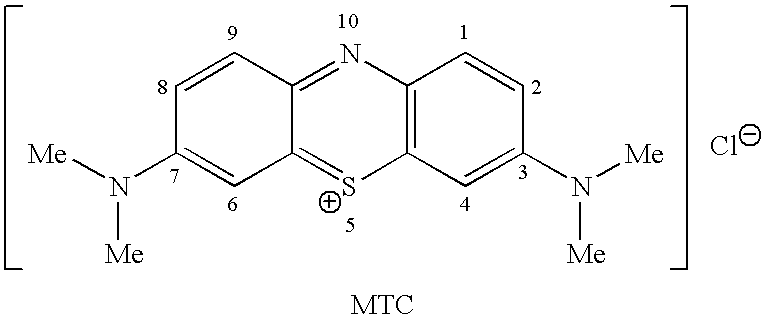
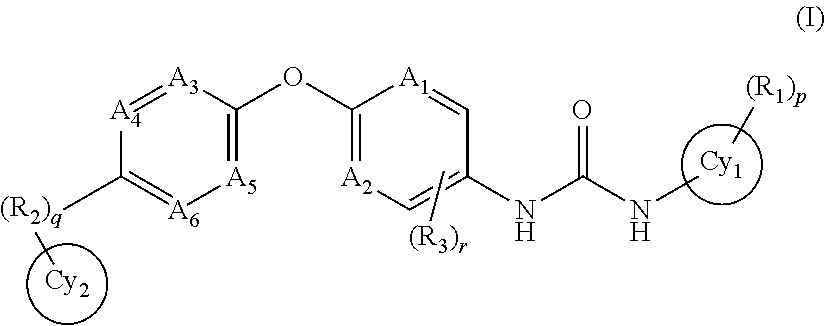
Methods of synthesis and/or purification of diaminophenothiazinium compounds
ActiveUS20090259040A1Antibacterial agentsOrganic active ingredientsDementia with Lewy bodiesChemical synthesis
This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesis and / or purification of certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methylthioninium Chloride (MTC) (also known as Methylene Blue). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment, prophylaxis, and diagnosis, etc., for example, a tauopathy; a disease of tau protein aggregation; Alzheimer's disease (AD); Pick's disease; Progressive Supranuclear Palsy (PSP); fronto temporal dementia (FTD); parkinsonism linked to chromosome 17 (FTDP-17); disinhibition-dementia-parkinsonism-amyotrophy complex (DDPAC); pallido-ponto-nigral degeneration (PPND); Guam-ALS syndrome; pallido-nigro-luysian degeneration (PNLD); cortico-basal degeneration (CBD); mild cognitive impairment (MCI); skin cancer; melanoma; methemoglobinemia; a viral infection; a bacterial infection; a protozoal infection; a parasitic infection; malaria; visceral leishmaniasis; African sleeping sickness; toxoplasmosis; giardiasis; Chagas' disease; Hepatitis C virus (HCV) infection; human immunodeficiency virus (HIV) infection; West Nile virus (WNV) infection; a synucleinopathy; Parkinson's disease (PD); dementia with Lewy bodies (DLB); multiple system atrophy (MSA); drug-induced parkinsonism; and pure autonomic failure (PAF).
Owner:WISTA LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors](https://images-eureka.patsnap.com/patent_img/028210cc-fd6a-4a6c-a658-85011196fcf8/US20100010009A1-20100114-C00001.png)
![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors](https://images-eureka.patsnap.com/patent_img/028210cc-fd6a-4a6c-a658-85011196fcf8/US20100010009A1-20100114-C00002.png)
![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors](https://images-eureka.patsnap.com/patent_img/028210cc-fd6a-4a6c-a658-85011196fcf8/US20100010009A1-20100114-C00003.png)

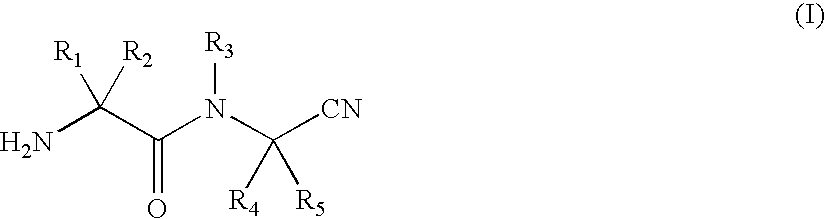
![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka.patsnap.com/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00001.png)
![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka.patsnap.com/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00002.png)
![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka.patsnap.com/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00003.png)
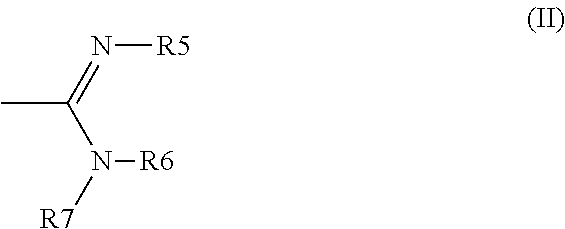


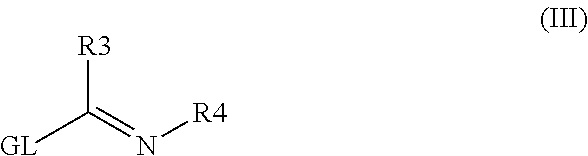
![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka.patsnap.com/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00001.png)
![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka.patsnap.com/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00002.png)
![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka.patsnap.com/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00003.png)