Antigens and immunoassays for diagnosing Chagas' disease
an immunoassay and antibody technology, applied in the field of antibodies and immunoassays for diagnosing chagas' disease, can solve the problem of not being able to detect enough sensitive single tests, and achieve the effect of high sensitive and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
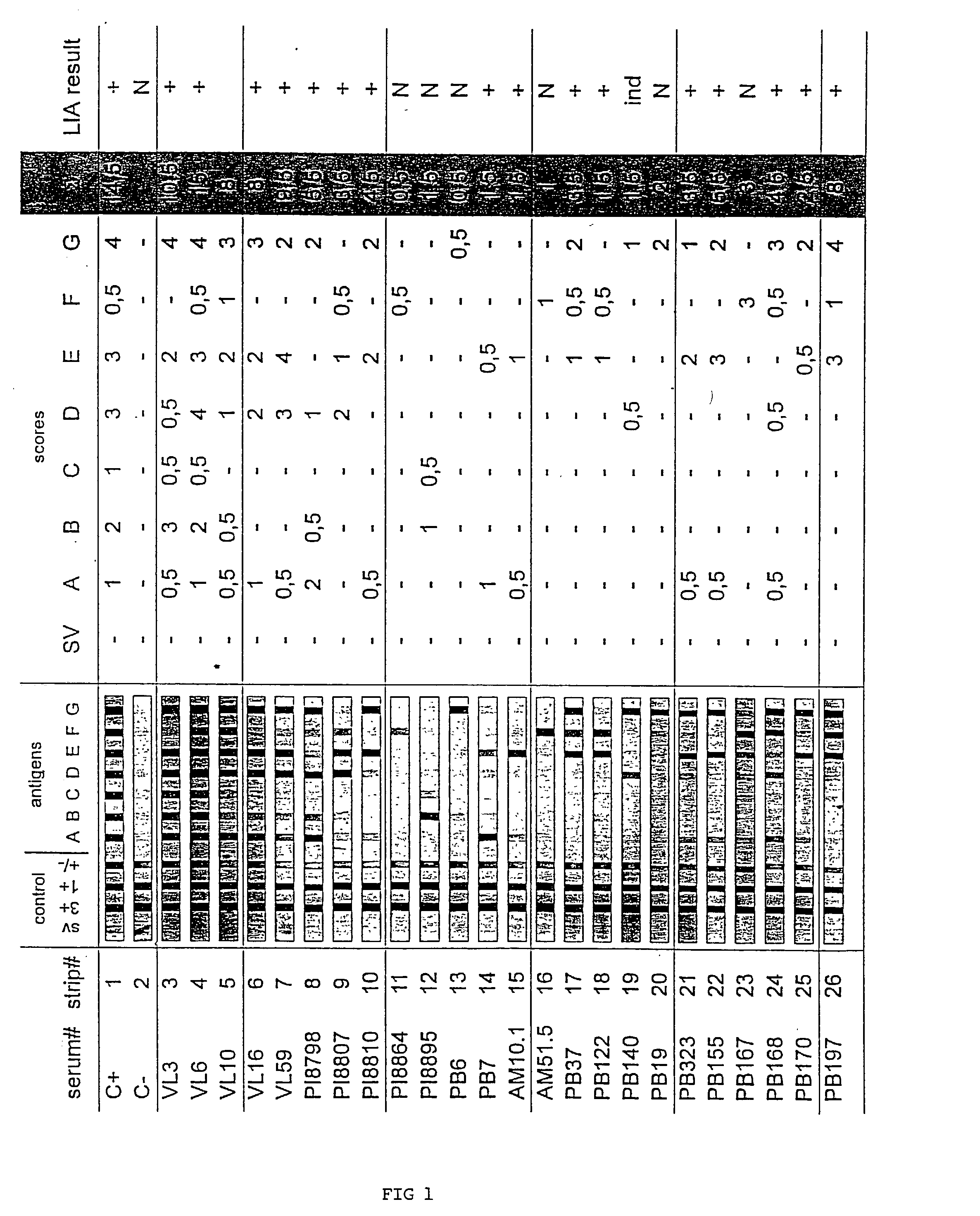
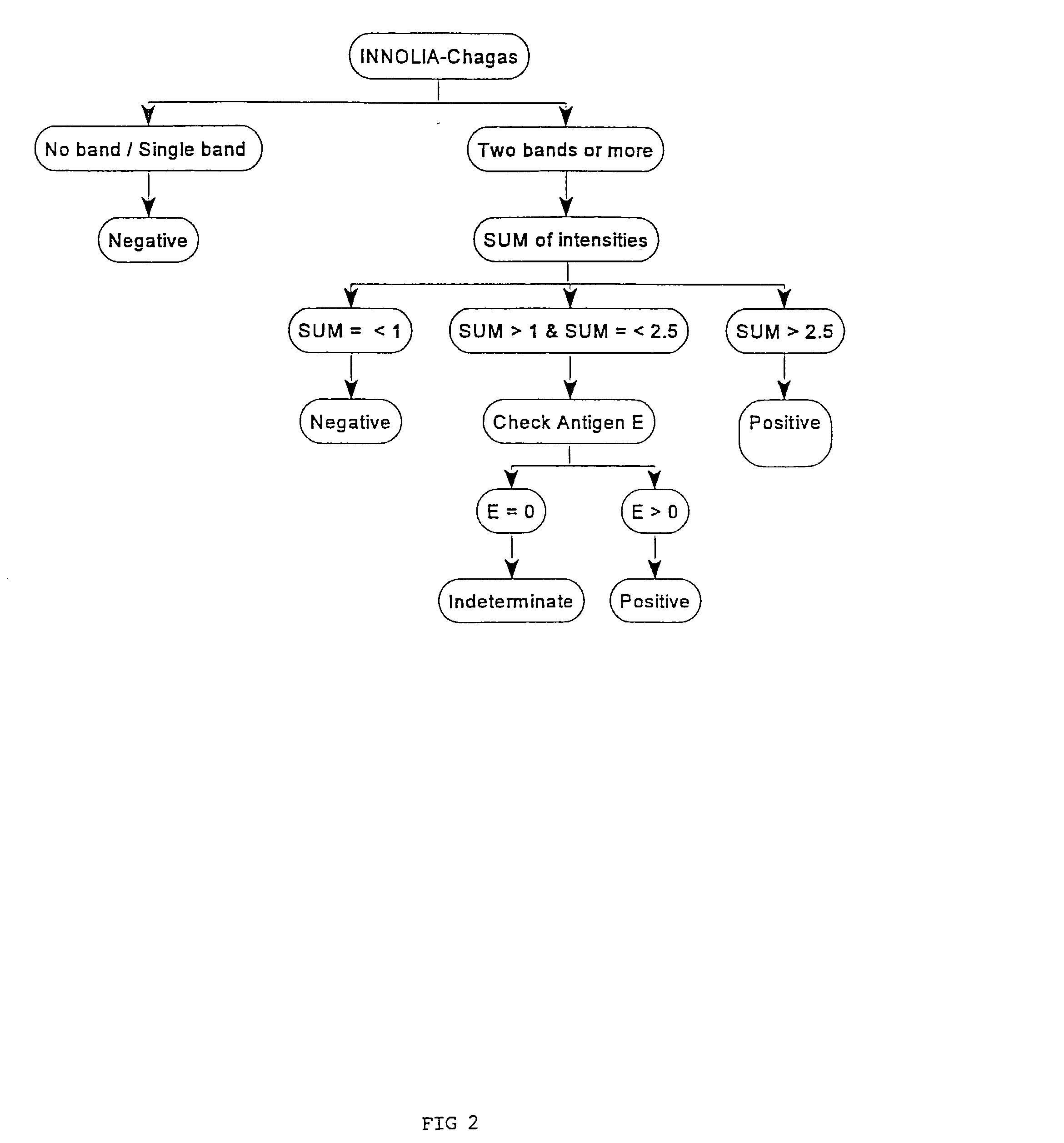
[0034] Evaluating a Recombinant and Peptide Antigen Line Immunoassay for Chagas' Disease: the INNO-LIA Chagas Antibody (Ab) Assay.
[0035] a. Materials and Methods
[0036] Study Population
[0037] The 1062 sera employed in this retrospective study were obtained from patients and healthy residents of four Brazilian regions endemic for Chagas' disease: 261 sera were from the state of Minas Gerais (municipality Virgem da Lapa) where the cardiac and digestive forms of the disease are frequent; 465 and 253 sera were obtained in the hinterlands of Paraiba and Piaui, respectively, where the indeterminate form of the disease is common; and 83 sera were from the Amazon (municipality of Barcellos) where Chagas' disease is emerging. Most of the blood samples originated from patients who have been participating in long-term follow-up studies for 2-20 years. Serologic analysis was performed using several immunological methods (see below). In addition, the presence of the parasite could be demonstrated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reactivity | aaaaa | aaaaa |
| secondary structure | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com