DMD gene exon copy number variation detection method and application thereof
A gene copy number and detection method technology, applied in the field of molecular biology, can solve the problems of cumbersome detection methods and achieve superior performance, shorten detection time, and easy standardization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Primer Design
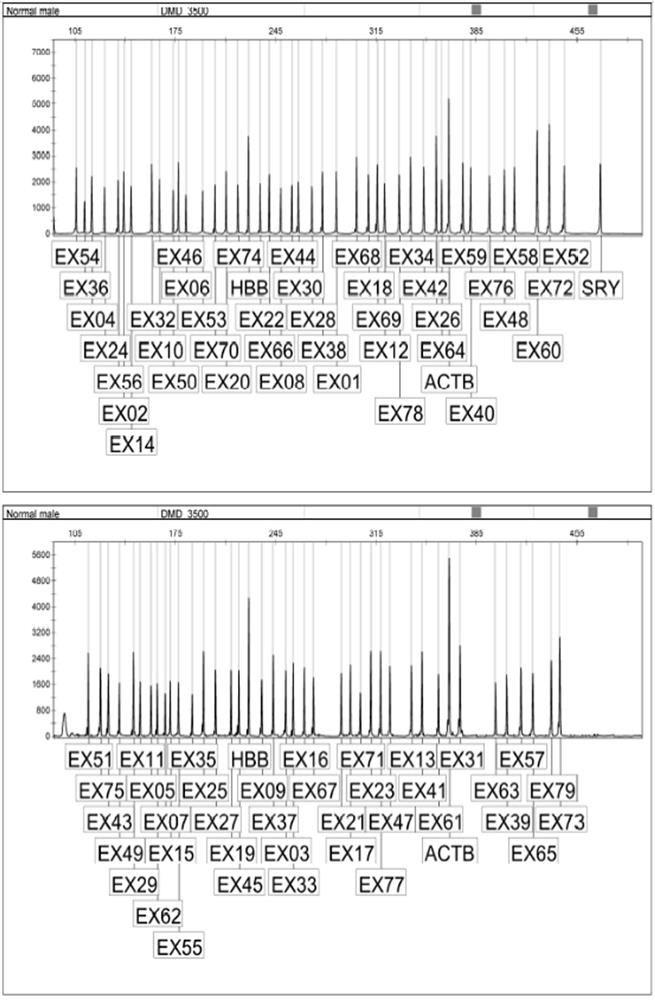
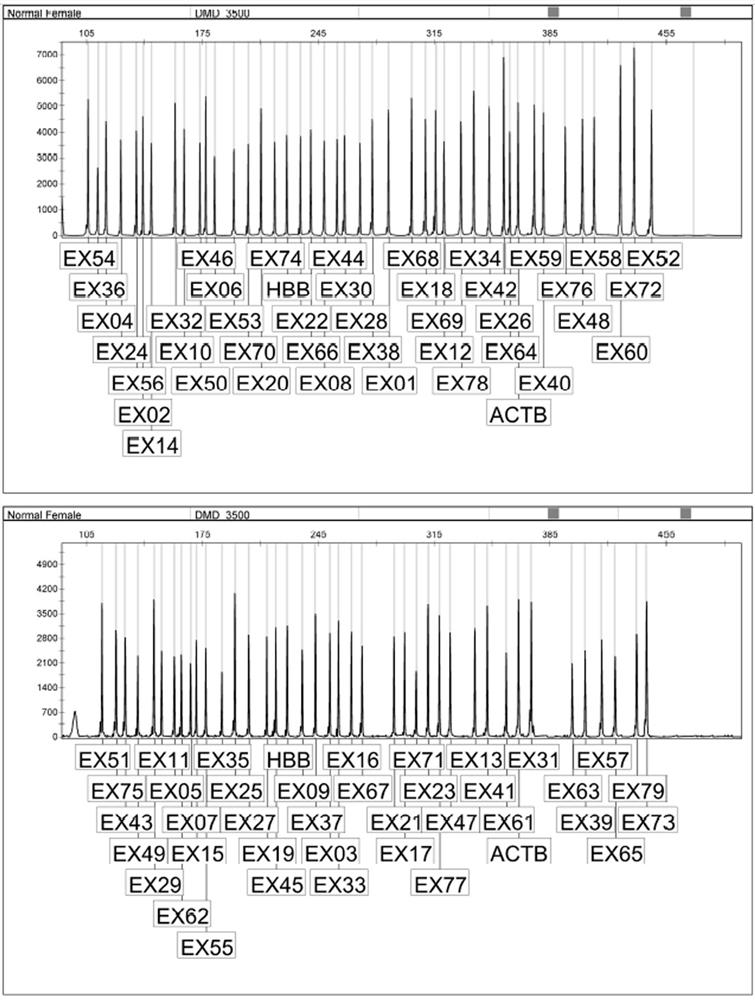
[0055] According to NCBI database, 79 exons of DMD gene, SRY, ACTB and HBB genes were searched, and specific primers were designed. Among them, the SRY gene was used as the interpretation of male and female samples, and two internal reference genes (ACTB and HBB) were used to monitor the PCR efficiency.
[0056] Preparation of DMD reaction solution and DMD primer mixture
[0057] Table 2 DMD reaction solution component distribution table
[0058] components final concentration per reaction 10X Taq Buffer (Thermo) 1.5X 25mM MgCl 2
0.5mM dATP / dTTP / dGTP / dCTP 0.5mM each betaine 2.0M
[0059] Table 3 DMD Primer Mix 1 Component Allocation Table
[0060]
[0061]
[0062] The 5' ends of SEQ ID NO.1 and SEQ ID NO.161 in Table 3 were modified with FAM fluorescent groups.
[0063] Table 4 DMD primer mixture 2 component distribution table
[0064] Primer name Final concentration (μM) P...
Embodiment 2
[0076] Embodiment 2 PCR amplification and result analysis
[0077] 1. Sample processing: Nucleic acid extractor (MagCore) and nucleic acid extraction kit (MagCore Genomic DNA WholeBlood Kit) extract human genomic DNA for subsequent PCR reaction, DNA concentration is 2.5ng / uL~60ng / uL, OD 260 nm / OD 280 The ratio of nm is between 1.6-2.0.
[0078] 2. Preparation of amplification reagents:
[0079] (1) Take out the DMD reaction solution, DMD primer mixture 1 and DMD primer mixture 2 from the kit, thaw at room temperature, mix them upside down, and centrifuge briefly with a microcentrifuge to make all the liquid settle to the bottom of the tube.
[0080] (2) Amplification reagent preparation: prepare the amplification reagent as shown in Table 5
[0081] Table 5 Amplification Reagent Preparation Table
[0082] Amplification reagent 1 Amplification reagent 2 per reaction volume DMD reaction solution DMD reaction solution 14.5μL DMD Primer Mix 1 DMD ...
Embodiment 3
[0101] Embodiment 3 Reagent Performance Verification
[0102] 1. Assess the diagnostic accuracy of the reagent of the present invention
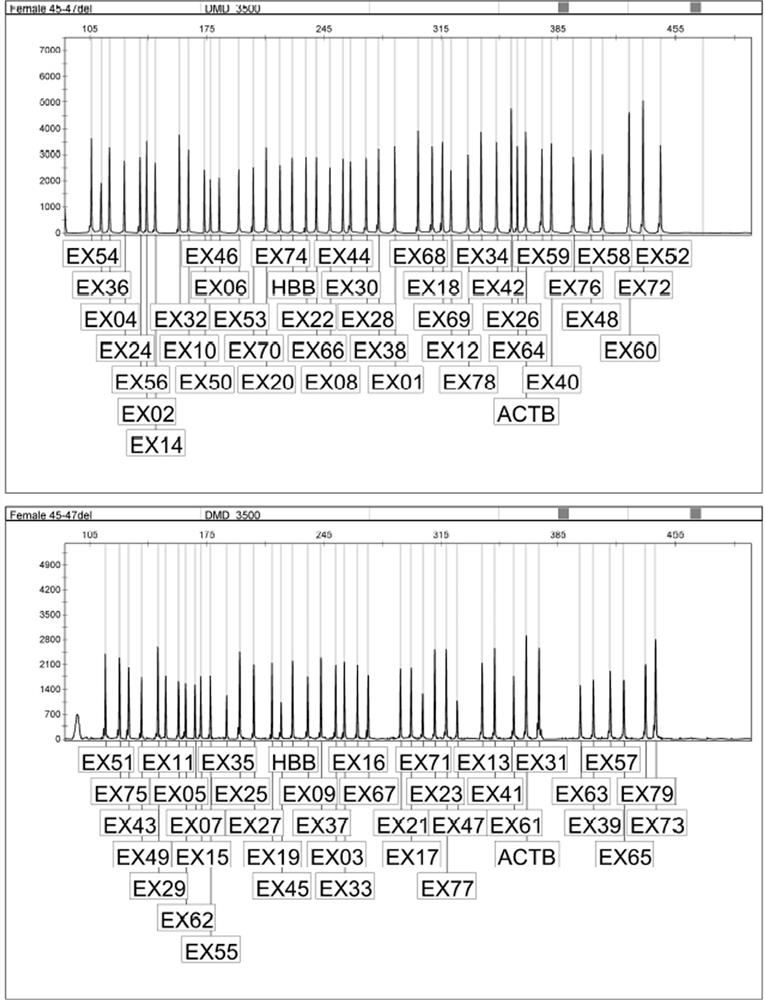
[0103] Detect 9 accuracy reference products, as shown in the following table, respectively detect high, medium and low concentrations, the sample concentrations are 60ng / uL, 25ng / uL, 15ng / uL respectively, each concentration is tested in triplicate, three batches of reagents are detected, DMD gene Exon copy number meets the requirements.
[0104] Table 6 Accuracy reference product DMD gene exon mutation type
[0105]
[0106] 2. Conformity rate of specific reference products
[0107] Detect 4 missing reference products, as shown in the following table, detect high, medium and low concentrations respectively, the sample concentrations are 60ng / uL, 25ng / uL, 15ng / uL, respectively, each concentration is tested in triplicate, three batches of reagents are tested, and the test results are specific The sexual compliance rate is 100%.
[0108]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com