Detection method and kit for spinal muscular atrophy related gene mutation
A spinal muscular atrophy and detection method technology is applied in the field of detection of spinal muscular atrophy-related gene mutations, which can solve the problems of long detection period, inability to judge diseases, and expensive instruments, and achieve rapid detection, ingenious design, and sample saving. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The design of embodiment 1 primer and probe
[0037] Design primers and probes according to the gene sequences of SMN, NAIP, GTF2H2 and the internal reference gene GAPDH to ensure that the concentration of upstream and downstream primers is consistent to generate double-stranded DNA through PCR reaction; the probe is complementary to the single-stranded target of PCR amplification during the annealing period of PCR reaction The DNA is hybridized and hydrolyzed by the 5' exonuclease activity of Taq DNA polymerase. After hydrolysis, the FAM fluorescein is away from the 3' quenching group, and the FAM fluorescein generates a fluorescent signal under the excitation light. The sequences of the designed primers and probes are shown in Table 1, and they were synthesized by China Invitrogen Company.
[0038] Table 1 Sequences of primers and probes
[0039]
[0040]
Embodiment 2
[0041] The extraction of the whole genome DNA of embodiment 2 samples to be tested
[0042] Whole blood DNA was extracted using TIANGEN "Whole Blood Genomic DNA Extraction Kit".
[0043] The final volume of extracted DNA is 100ul, the concentration is above 30ng / ul; OD260 / 280 is between 1.8-2.0.
Embodiment 3
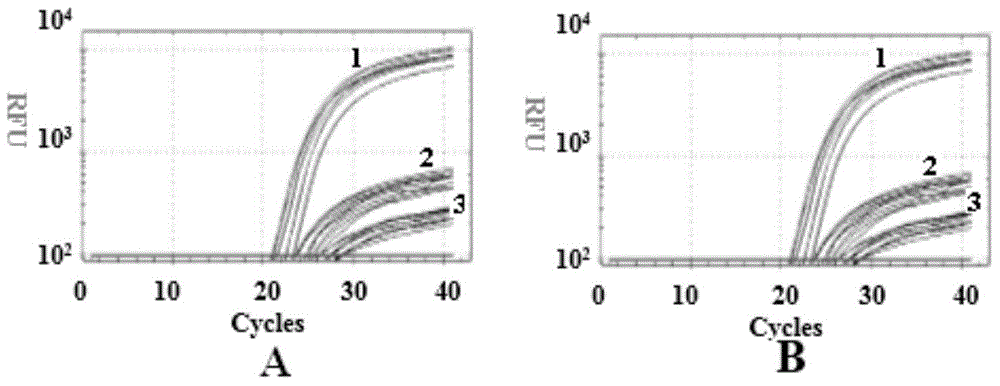
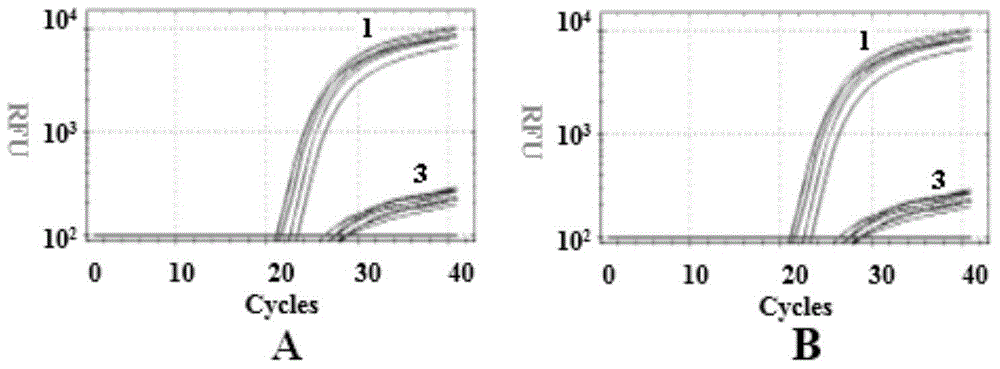
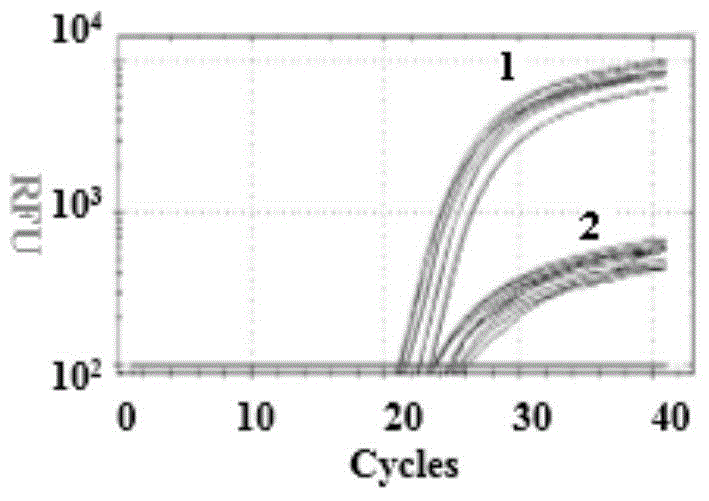
[0044] Example 3 Detection of 4 sites of the three related genes SMN, NAIP and GTF2H2 in spinal muscular atrophy
[0045] (1) Establishment of PCR reaction system
[0046] The primers and probes synthesized in Example 1 were taken, and the whole genome DNA extracted in Example 2 was used as a template, and PCR reaction system I and PCR reaction system II were established according to the data shown in Table 2.
[0047] Table 2 PCR reaction system
[0048]
[0049] In the above system, when performing PCR test, it is often necessary to add buffer, dNTP, DNase, primer, template, water and other reagents required for various PCR reactions to the PCR tube, and it is easy to operate if these reagents are not standardized. Contamination causes inaccurate PCR results. Therefore, in order to simplify the repeated addition of various reagents, buffer, dNTP, DNA Polymerase and other reagents are first mixed together, that is, prepared into a Master-mix and then used, which simplifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com