4-aminopyridine as a therapeutic agent for spinal muscular atrophy
A spinal muscular atrophy, pyridine technology, applied in the direction of muscular system diseases, organic active ingredients, neuromuscular system diseases, etc., can solve problems such as no SMA drug treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
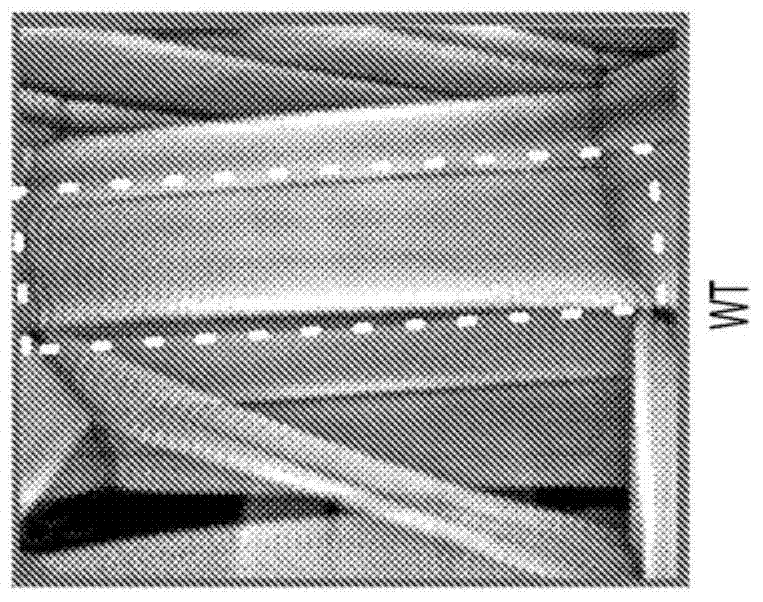
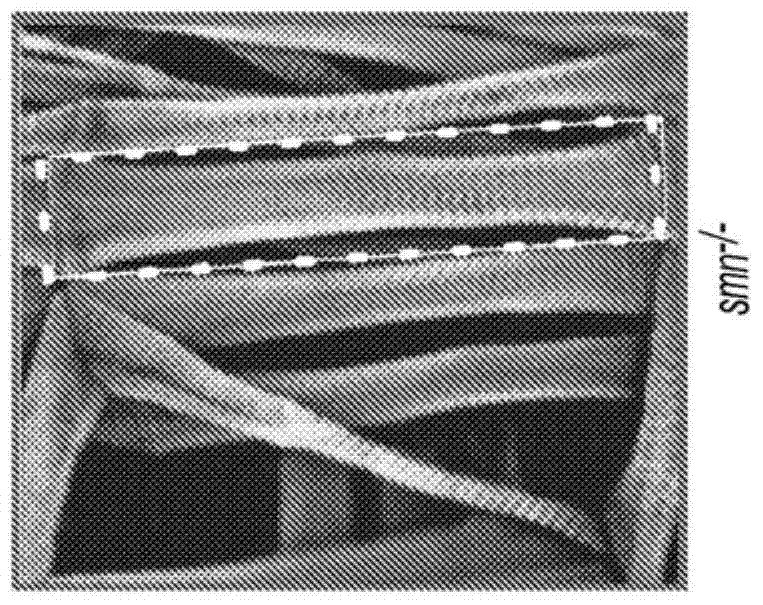
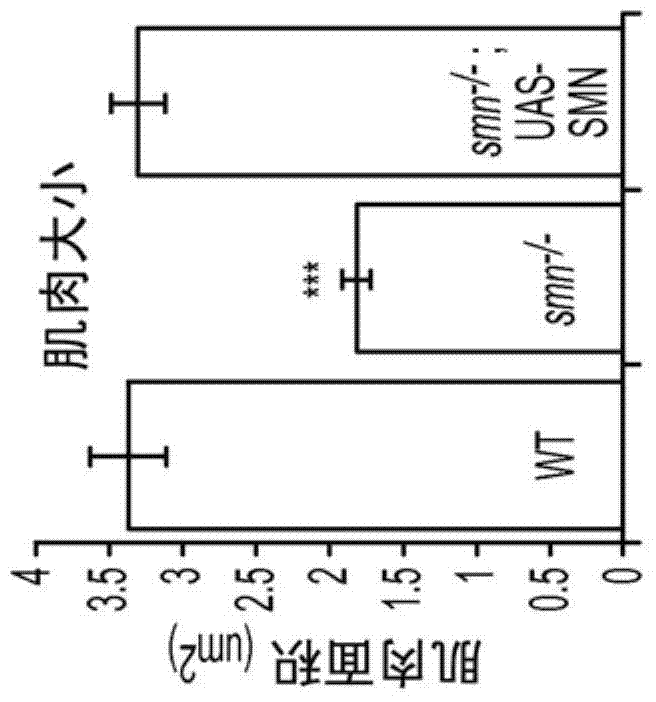
[0034] The results in Example 1 confirm the Drosophila model and also show that Drosophila smn mutants have increased NMJ-evoked neurotransmitter release with concomitant defects in muscle growth, movement and motor rhythmicity (Figures 1 and 2) .
Embodiment 3
[0035] The results in Example 3 show that, in contrast to muscle recovery of SMN, pan-neuronal recovery of SMN fully rescues the muscle surface area of smn mutants to control levels ( Figure 2B , D, E), and also completely recovered their locomotion velocity, rhythmic motor output, and NMJ eEPSP amplitude ( Figure 2F -H). These results show that the defect in muscle growth in smn mutant larvae is due to an involuntary requirement for normal SMN levels in the nervous system rather than to the muscle fibers themselves.
[0036] The results in Example 3 show that SMN is required in cholinergic neurons but not in glutamatergic motor neurons, and that SMN is required in both proprioceptive and central cholinergic neurons. Transgenic SMN expression levels in central cholinergic neurons completely rescued the muscle growth, locomotor, and rhythmic activity defects of smn mutants ( Figure 3E -G). Furthermore, SMN expression in cholinergic neurons also completely rescued the eE...
Embodiment 4
[0037] Example 4 Based on the hypothesis that motor circuits are functionally defective in smn mutants, experiments were designed to test whether increasing the excitability of central cholinergic neurons in these animals could increase motor network activity and alter smn mutant phenotypes. The results of various experiments showed that pharmacological inhibition of K+ channels (using the FDA-approved broad-spectrum K+ channel antagonist 4-AP) satisfactorily benefited the smn mutant phenotype, results consistent with important results for SMN depletion. Motor circuits are consistent with defective excitability generated by their interneuron or sensory neuron input.
[0038] Discussion of results
[0039] The results presented here show that restoration of the SMN in at least two groups of motor circuit neurons (bd and type I md sensory neurons) resulted in a complete rescue of the larval phenotype. The bd and type I md sensory neurons are important components of the propri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com