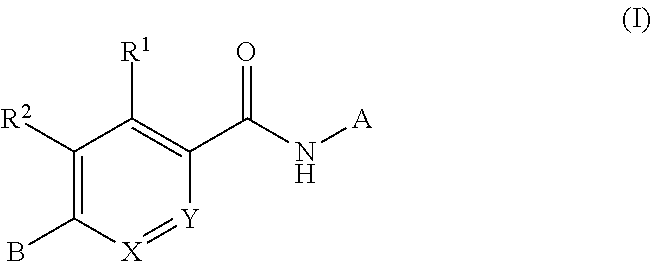
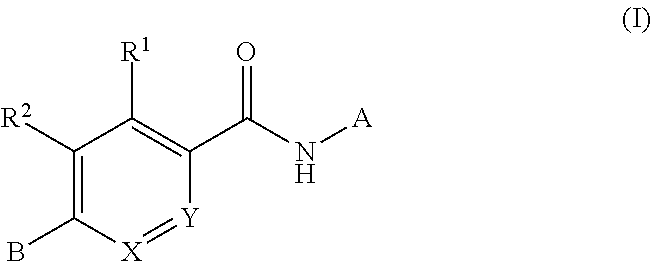
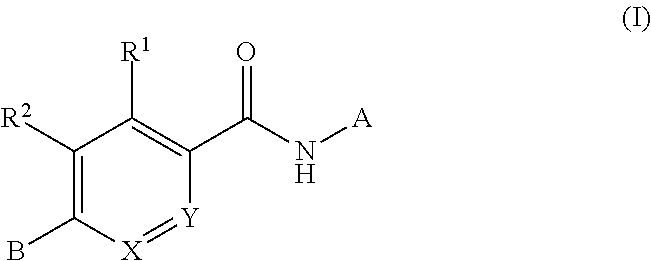
Compounds for treating spinal muscular atrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0534]The invention will be more fully understood by reference to the following examples. They should however not be construed as limiting the scope of the invention.
Preparation of Intermediates
example a.1
Preparation of N-(6,8-dimethylimidazo[1,2-a]pyrazin-2-yl)-4-fluorobenzamide
[0535]
[0536]To a mixture of 6,8-dimethylimidazo[1,2-a]pyrazin-2-amine trihydrochloride (Example B.1) (134 mg, 494 μmol) and N-Ethyldiisopropylamine (320 mg, 420 μl, 2.47 mmol) in dioxane (2.0 ml) was added dropwise a solution of 4-fluorobenzoyl chloride (80 mg, 60.3 μl, 494 μmol) in dioxane (0.5 ml) at room temperature. The mixture was stirred for 1 hour. The solvent was removed in vacuo. The solid was taken in water and the suspension was stirred for 15 minutes. The solid was filtered and dried to provide 125 mg (89%) of the title compound as an off-white solid. MS (m / e): 285.4 (M+H+)
[0537]In analogy to Example A. 1, Examples A.2 to A.7 of the following table were prepared from the acylchloride and amine derivatives:
ExampleNo.StructureSystematic NameStarting materialsA.2N-(6,8- dimethylimidazo[1,2- a]pyrazin-2-yl)-2,4- difluorobenzamide6,8-dimethylimidazo[1,2- a]pyrazin-2-amine trihydrochloride (Example B.1)...
example a.8
Preparation of 6-Chloro-N-(2-methyl-8-trifluoromethyl-imidazo[1,2-a]pyridin-6-yl)-nicotinamide
[0538]
[0539]To a solution of 6-chloronicotinic acid (332 mg, 2.11 mmol) in DMF (4 ml) under argon at room temperature, were added HATU (O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate) (1.2 g, 3.16 mmol) and N,N-diisopropylethylamine (1.4 ml, 8.42 mmol). After 5 minutes stirring, 2-methyl-8-(trifluoromethyl)imidazo[1,2-a]pyridin-6-amine hydrochloride (Example B.2) (530 mg, 2.11 mmol) was added. The mixture was stirred at room temperature for two days. The solvent was removed in vacuo. The residue was taken in aqueous bicarbonate. The aqueous layer was extracted 3 times with ethyl acetate. The combined extracts were dried over sodium sulfate, filtered and concentrated in vacuo. The crude mixture was purified with flash column chromatography on silica eluting with a gradient formed from dichloromethane and methanol (0 to 5%) to provide the title compound.
[0540]In a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com