Detection kit for virulence gene of spinal muscular atrophy and application thereof
A spinal muscular atrophy, detection kit technology, applied in the direction of determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve the problems of indistinguishable, expensive, time-consuming, etc., and achieves simple operation, low detection cost, Detecting fast effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
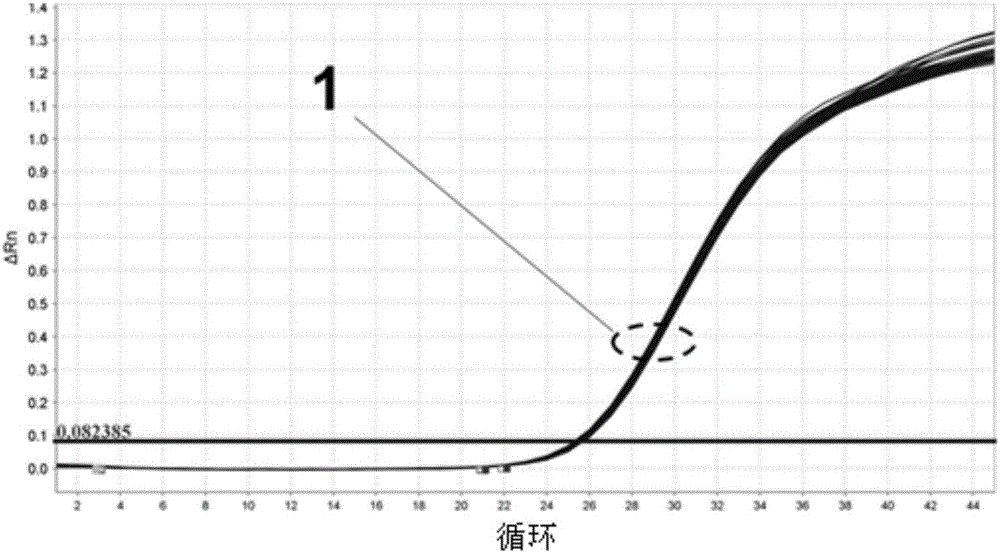
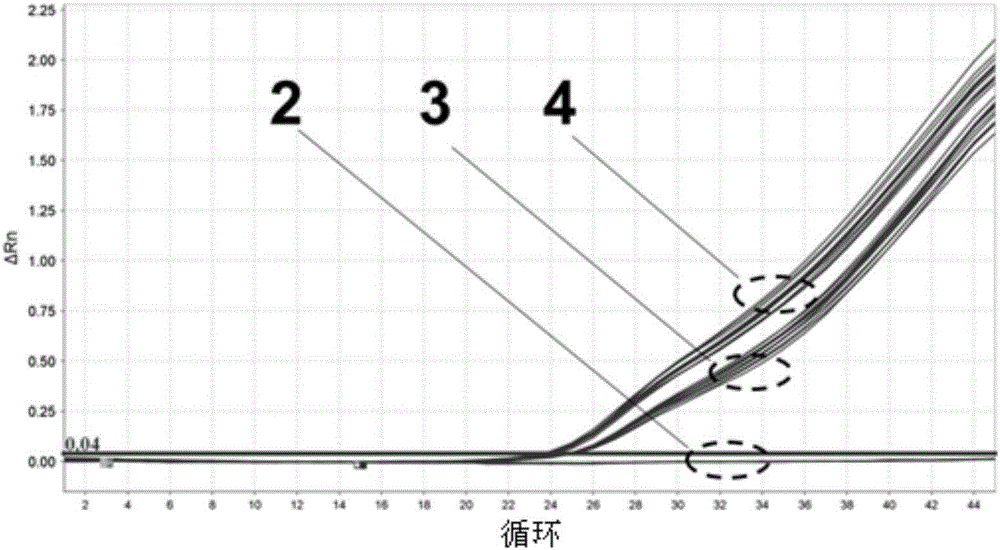
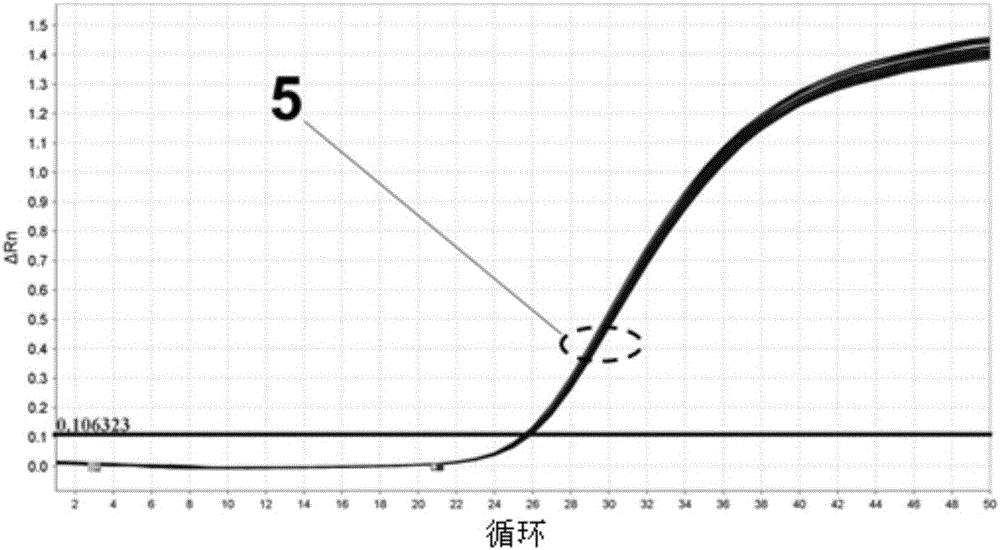
Image
Examples
Embodiment 1
[0046] Example 1 Spinal Muscular Atrophy Gene Detection Kit
[0047] The spinal muscular atrophy pathogenic gene detection kit of the present invention comprises:
[0048] Primer SMN-Ex7-F, its sequence is:
[0049] 5'-CCGCTATCTATATATAGCTATCTATG-3' (SEQ ID NO.1);
[0050] Primer SMN-Ex7-R, its sequence is:
[0051] 5'-CCTACATTAACCTTTCAACTTTT-3' (SEQ ID NO.2);
[0052] Probe SMN-Ex7-P, its sequence is:
[0053] 5'-FAM-CTTACAGGGTTTCAGACAAAAT-MGB-3' (SEQ ID NO. 3).
[0054] Further, the spinal muscular atrophy pathogenic gene detection kit of the present invention also includes:
[0055] Primer SMN-In7-F, its sequence is:
[0056] 5'-CCTATTTTCCTTACAGGGTTTC-3' (SEQ ID NO.4);
[0057] Primer SMN-In7-R, its sequence is:
[0058] 5'-TTGTGAAAGTATGTTTCTTCCACAT-3' (SEQ ID NO.5);
[0059] Probe SMN-In7-P, its sequence is:
[0060] 5'-FAM-CCTTTCAACTTTTTTAACATCT-MGB-3' (SEQ ID NO. 6).
[0061] Further, the spinal muscular atrophy pathogenic gene detection kit of the present inve...
Embodiment 2
[0071] Example 2 Application of Spinal Muscular Atrophy Gene Detection Kit
[0072] 1. Obtain the genomic DNA of the person to be tested
[0073] From the genomic DNA preserved in the sample resource bank of the genetic center, select 1050 samples (including 50 cases of homozygous deletion SMA patients, 500 cases of heterozygous deletion SMA carriers and 500 cases) that have been tested by MLPA method. normal people).
[0074] Blind analysis: use NanoDrop ND-2000 to measure the concentration of the above-mentioned genomic DNA, take the concentration at 20-150ng / μL, A 260 / A 280 Samples between 1.8 and 1.9 will be blindly numbered as the genomic DNA of the person to be tested for future use; for samples that do not meet the above requirements, they will not be included in this analysis and test.
[0075] 2. Accurate quantification of the genomic DNA of the subject to be tested
[0076] 1. Preparation of working solution (Qubit working solution)
[0077]The DNA-binding dye ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com