Method, primers and application for detecting SMN1 and SMN2 gene mutation
A sequencing primer, SMN-7-R technology, applied in the field of life sciences and biology, can solve the problems affecting the time of onset and disease severity of patients, and the lack of effective treatment options for SMA, so as to reduce the amount of samples and reagents used, and reduce the detection rate. The effect of low cost and detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The present invention will be further described below in conjunction with specific embodiments and accompanying drawings. It should be noted that the routine conditions and methods not described in the examples are generally used by experimenters in the field: for example, the fourth edition of the "Refined Molecular Biology Experiment Guide" edited by Osper and Kingston, Or follow the procedures and conditions recommended by the manufacturer.
[0056] A primer for detection of SMN1 and SMN2 gene c.835-44, c.840, INS7+100, INS7+215, *239 sites, the primer is designed for SMN1 and SMN2 gene c.835-44, c. Specific amplification primers designed for 840, INS7+100, INS7+215, *239 sites, including:
[0057] Primers for amplifying SMN1 and SMN2 gene c.835-44, c.840, INS7+100, INS7+215 sites, the base sequence of which is:
[0058] SMN-7-F: TGTAAAACGACGGCCAGTCAAGTGATCCCCCCTACCTCC
[0059] SMN-7-R: AACAGCTATGACCATGATTAGAACCAGAGGCTTGACGA
[0060] Primers for amplifying SMN1 a...
Embodiment 2
[0072] Operation process of blood / cell / tissue genomic DNA extraction kit (Tiangen Biology):
[0073] (1) Extract tissue DNA from blood:
[0074] 1) Take 300 μl of blood and add 900 μl of erythrocyte lysate, mix by inversion, and let stand at room temperature for 5 minutes, during which time, invert and mix several times. Centrifuge at 12,000rpm for 1min, suck off the supernatant, leave the white blood cell pellet, add 200μl buffer GA, shake until thoroughly mixed.
[0075] 2) Add 20 μl proteinase K solution and mix well.
[0076] 3) Add 200 μl of buffer GB, mix thoroughly by inversion, place at 70°C for 10 minutes, the solution should become clear, and briefly centrifuge to remove water droplets on the inner wall of the tube cap.
[0077] 4) Add 200 μl of absolute ethanol, vortex and mix well for 15 seconds. At this time, flocculent precipitates may appear. Briefly centrifuge to remove water droplets on the inner wall of the tube cap.
[0078] 5) Add the solution and floccu...
Embodiment 3
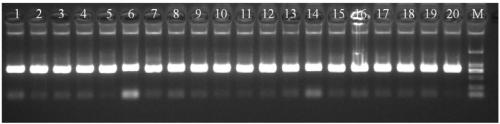
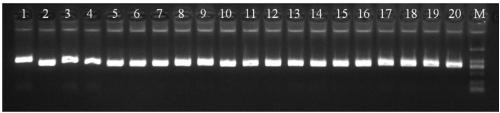
[0106]Take 20 clinical peripheral blood samples, extract the genome, prepare reagents and detect according to the method in Example 2. Add 2 μl of each sample to the detection system PCR reaction solution. At the same time, make positive, negative, and blank controls. The results of agarose gel electrophoresis of samples 1-20 are as follows figure 1 , figure 2 As shown, the results show that the amplification described in the present invention is effective and the bands are clear.
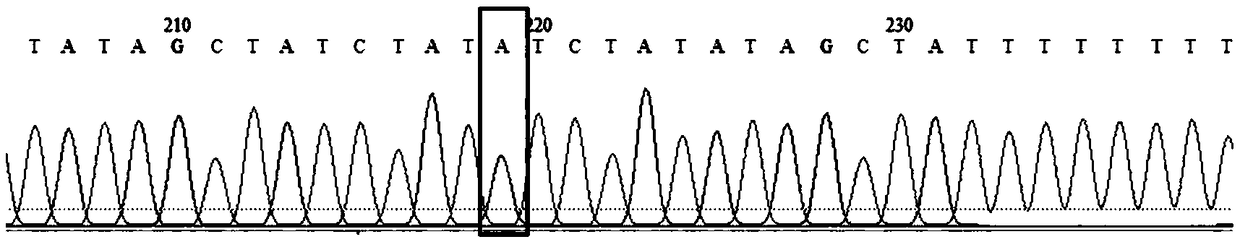
[0107] After sequencing, it was found that exons 7 and 8 of the SMN1 gene in sample 1 were homozygous deletions, such as image 3 , 5 , 7, 9, and 11. No homozygous deletion of the SMN1 gene occurred in the remaining samples. Taking sample 2 as an example, the sequencing results Figure 4 , 6 , 8, 10, 12 shown. In addition, based on the differences between the SMN1 gene and the SMN2 gene at these 5 sites, using the two pairs of primers of the present invention can also identify whether a ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com