Recombinant adeno-associated viruses carrying designed SMN1 gene expression cassettes and application
A technology of gene and virus vector, applied in the application field of treating spinal muscular atrophy, can solve the problems of safety risk, high-efficiency expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
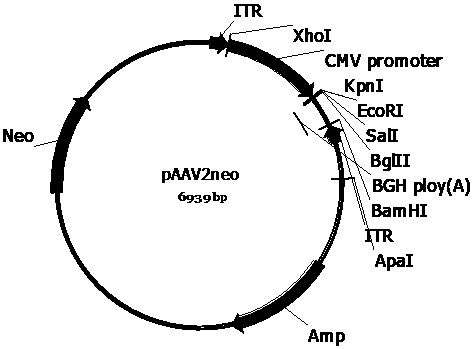
[0094] Example 1 Plasmid vector construction
[0095] (1) Promoter design and synthesis
[0096] Reference (Niwa H, et al. Gene. 1991;108:193-200.), with the CAG promoter (promoted by CMV enhancer and chicken beta-actin) in the mammalian expression vector pCAGGS vector (GenBank: LT727518.1) Based on the composition of the promoter), considering the length of the sequence, the partial sequence of the chicken beta-actin promoter in the CAG promoter was deleted to obtain a truncated CAG promoter, which was named CA promoter. Next, a partial sequence of the 5' untranslated region of human SMN1 gene mRNA (GenBank: NM_001297715.1) was introduced at the 3' end of the CA promoter sequence, and it was named CAS promoter. The intron sequence from position 449 to position 532 in the human RNA polymerase II 14.5kDa subunit gene (GenBank: Z23102.1) was introduced into the 3' end of the CA promoter sequence, and it was named CAT promoter. The intron sequence from position 62804 to positio...
Embodiment 2
[0114] Example 2 Preparation and assay of recombinant AAV virus
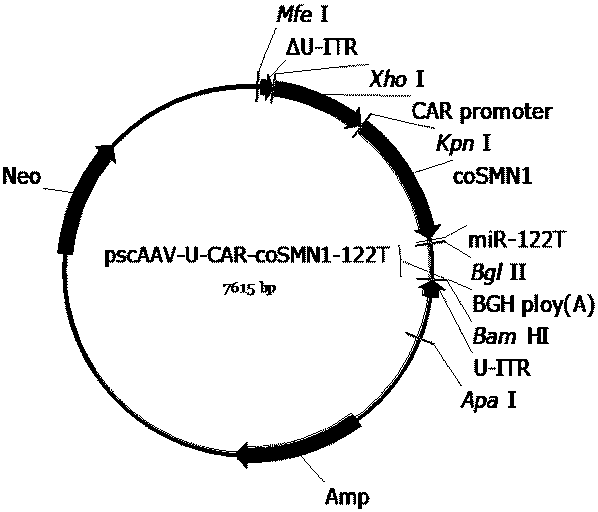
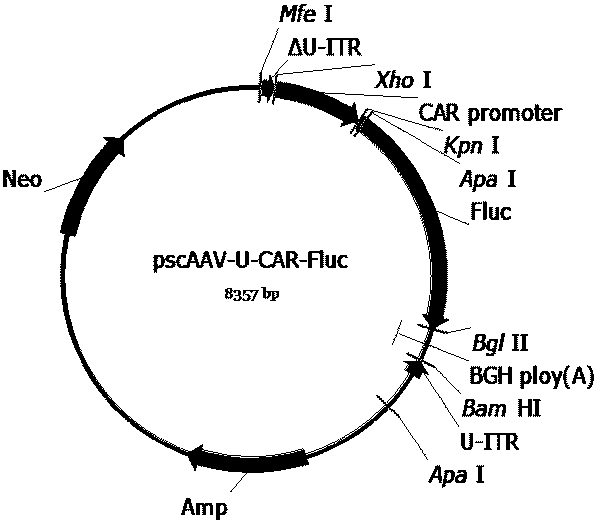
[0115] References (Xiao X, et al . J Virol. 1998;72(3):2224-2232.), the three-plasmid packaging system was used to package the recombinant AAV virus, and the cesium chloride density gradient centrifugation method was used to separate, purify and package the AAV virus. Briefly, AAV vector plasmids (pAAV2neo-CA-Fluc, pAAV2neo-CAT-Fluc, pAAV2neo-CAP-Fluc, pAAV2neo-CAS-Fluc, pAAV2neo-CAR-Fluc, pscAAV-CA-SMN1, pscAAV-CA-coSMN1, pscAAV -CAR-SMN1, pscAAV-CAR-coSMN1, pscAAV-U-CAR-coSMN1, pscAAV-CAR-SMN1-122T, pscAAV-CAR-coSMN1-122T or pscAAV-U-CAR-coSMN1-122T), helper plasmid (pHelper ) and AAV Rep and Cap protein expression plasmids (pAAV-DJ, pAAV-R2C5, pAAV-R2C9 or pAAV-R2C10) were mixed according to the molar ratio of 1:1:1, and the calcium phosphate method was used to transfect HEK293 cells. After 48 hours of transfection, the cells and culture supernatant were harvested, and the recombinant AAV virus was isolated...
Embodiment 3
[0122] Example 3 In vivo and in vitro evaluation experiments of promoters
[0123] (1) In vitro evaluation experiment
[0124] Since the AAVDJ vector has high transduction activity to various cells in vitro (Grimm D, et al. JVirol. 2008; 82: 5887-5911.), we will contain the Fluc gene expression cassette regulated by different promoters ( pAAV2neo-CA-Fluc, pAAV2neo-CAT-Fluc, pAAV2neo-CAP-Fluc, pAAV2neo-CAS-Fluc, pAAV2neo-CAR-Fluc) were packaged into AAVDJ virus. HEK293 cells and GM03813 cell lines were selected for in vitro evaluation of the designed promoters. HEK293 cells were derived from human embryonic kidney cells. The GM03813 cell line was purchased from Coriell Cell Repository in the United States, and was derived from a fibroblast cell line from a patient with SMA type I (Coovert DD, et al. Hum Mol Genet.1997; 6: 1205-1214.). The expression activities of the designed promoters in the cells derived from SMA patients and non-SMA patients were evaluated respectively fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com