Spinal muscular atrophy detection kit and application thereof
A spinal muscular atrophy, detection kit technology, applied in the direction of microbial determination/inspection, biochemical equipment and methods, etc., can solve the problems of difficult clinical application, inability to detect carriers, and rising reagent costs, and achieve detection. The time required is short, the cost of inspection is reduced, and the effect of rapid inspection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Spinal muscular atrophy detection kit
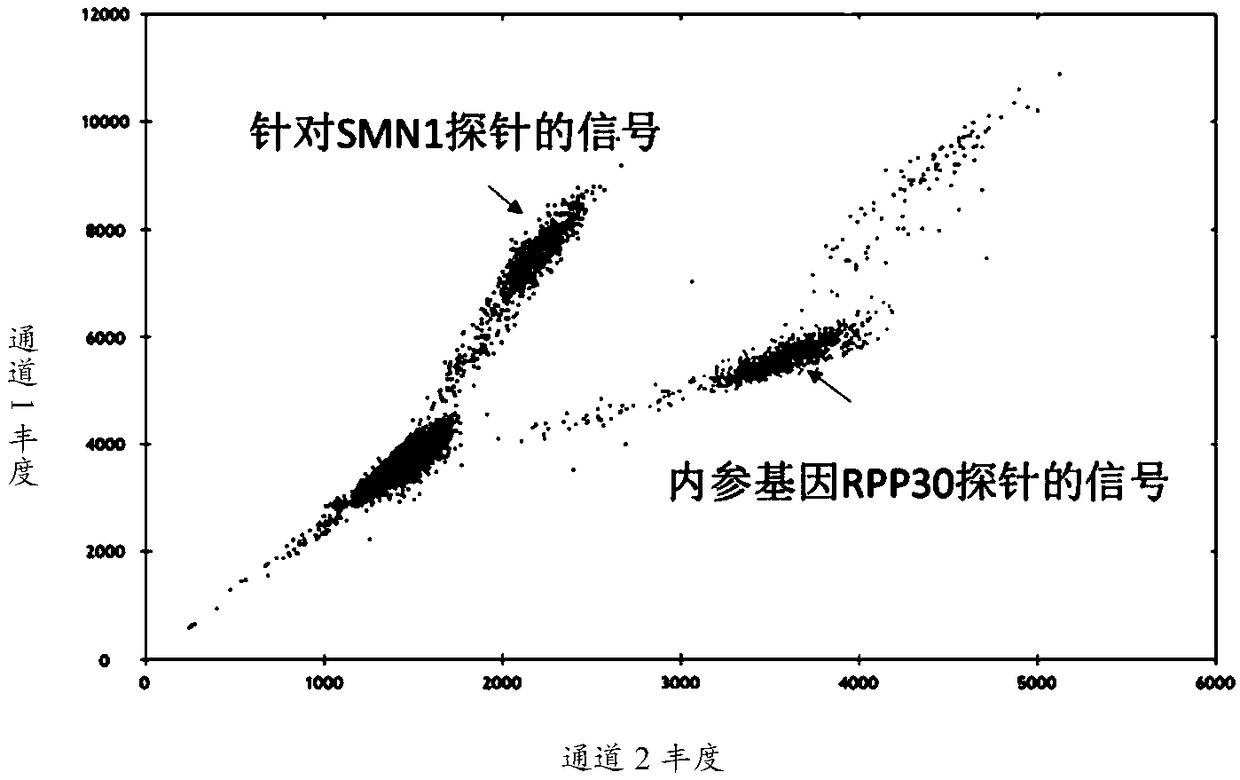
[0025] In order to overcome the defects of the method for quantitatively detecting the copy number of exon 7 (ex7) of the SMN1 gene mentioned above, the present invention provides a kit for absolute quantification of the copy number of the SMN1 gene ex7 based on a droplet digital PCR method. The primers, probe sequences, and digital PCR reaction system used in the method are as follows.
[0026] Table 1. Primers and probes for digital PCR detection of SMN1 gene ex7
[0027] name
Sequence (5'-3')
serial number
SMN1-F
AAATGTCTTGTGAAACAAAATGC
SEQ ID NO:1
SMN1-R
GAATGTGAGCACCTTCCTTCT
SEQ ID NO:2
SMN1-P
CCGCTTCATCGCAGTGGGCTACGTG
SEQ ID NO:3
[0028] In order to monitor whether the qPCR reaction system is working normally, the internal reference gene human RPP30 gene was added at the same time, and the primers and probes of the RPP30 gene are shown in Table 2...
Embodiment 2
[0033] Example 2. Application of Spinal Muscular Atrophy Detection Kit
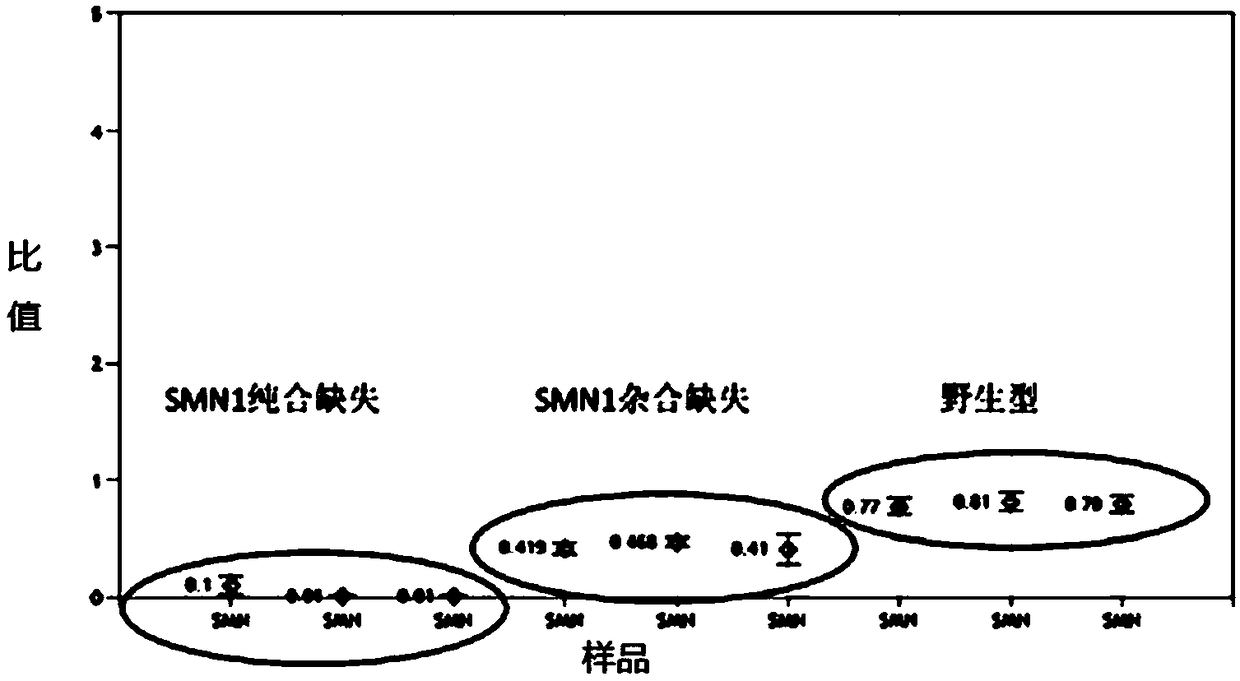
[0034] For clinical samples of peripheral whole blood, the sample type detected by the present invention, the extracted genomic DNA template is diluted to 20 ng / μl. After the genomic DNA was extracted from the blood sample, the ex7 deletion of SMN1 was detected by MLPA to find ex7 wild type, heterozygous type with 1 copy number deletion, and homozygous deletion type samples with 2 copy number deletions.
[0035] The microdroplet digital PCR reaction includes four steps: preparation system, microdroplet generation, amplification cycle and signal reading.
[0036] 1. Instruments and consumables: droplet generation instrument, PCR instrument T-100, oil droplet reader, PX1 heat sealer, droplet generation consumables (including DG8 cartridge, holder, rubber pad gasket), tin sealing film, 96 The orifice plates were all from Bo-Rad, USA.
[0037] 2. PCR reagents were purchased from Beijing Kangwei Century Comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com