Helicobacter pylori tetravalent virulence factor multi-epitope vaccine and preparation method thereof
A technology of Helicobacter pylori and virulence factor, which is applied in the field of biomedicine, can solve the problems of increasing the risk of gastrointestinal diseases of duodenal ulcer and gastric cancer, poor patient compliance, high price, etc. Avoid biological toxicity, easy to express effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
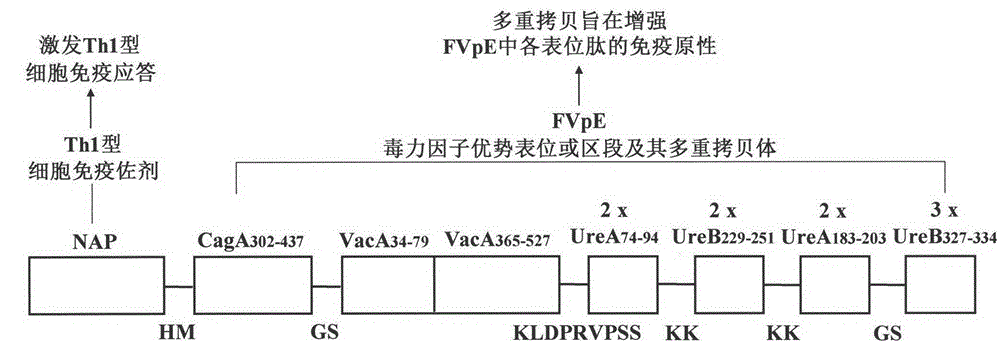
[0053] Example 1: Molecular structure design of Helicobacter pylori tetravalent virulence factor multi-epitope vaccine FVpE
[0054] According to "the body's immune protection mechanism against Hp" and "the immunological properties of key adhesion factor epitopes or segments such as Hp urease A and B double subunits, CagA and VacA", CagA was screened by bioinformatics 302-437 , VacA 34-79 , VacA 365-527 、UreA 74-94 , UreB 229-251 、UreA 183-203 , UreB 327-334 The isoantigen epitopes or segments and the Th1 cell immune adjuvant NAP are used in the construction of the tetravalent virulence factor multi-epitope vaccine FVpE of Helicobacter pylori. Then, through the construction theory of the epitope vaccine and the analysis of bioinformatics, the connection sequence, spacer sequence and antigen epitope copy number of the selected antigenic epitope or segment are analyzed and determined, and finally a scientifically reasonable vaccine is designed. Structure of the Helicobacte...
example 2
[0056] Example 2: Construction of recombinant expression plasmid pET-FVpE (containing fusion gene FVpE)
[0057] (1) Gene synthesis of adhesion factor polyepitope peptide FAdE nucleotide sequence
[0058] The pre-screened and designed CagA dominant antigen epitope segment (CagA E ), VacA dominant epitope segment complex (VacA E ), the amino acid sequence of the urease dominant epitope complex (UE) was converted into the corresponding nucleotide sequence according to the codon preference principle of Escherichia coli, and Zhongding Biotechnology Company was entrusted to carry out gene synthesis.
[0059] (2) Construction of recombinant expression vector pET-FVpE
[0060] The synthetic gene fragment (CagA E , VacA E and UE) and neutrophil activating protein NAP gene according to NAP-CagA E -VacA E -UE sequence is spliced into a fusion gene, which is the FVpE fusion gene; then, FVpE is cloned between the NcoI and XhoI sites of pET28a to obtain the recombinant expression v...
example 3
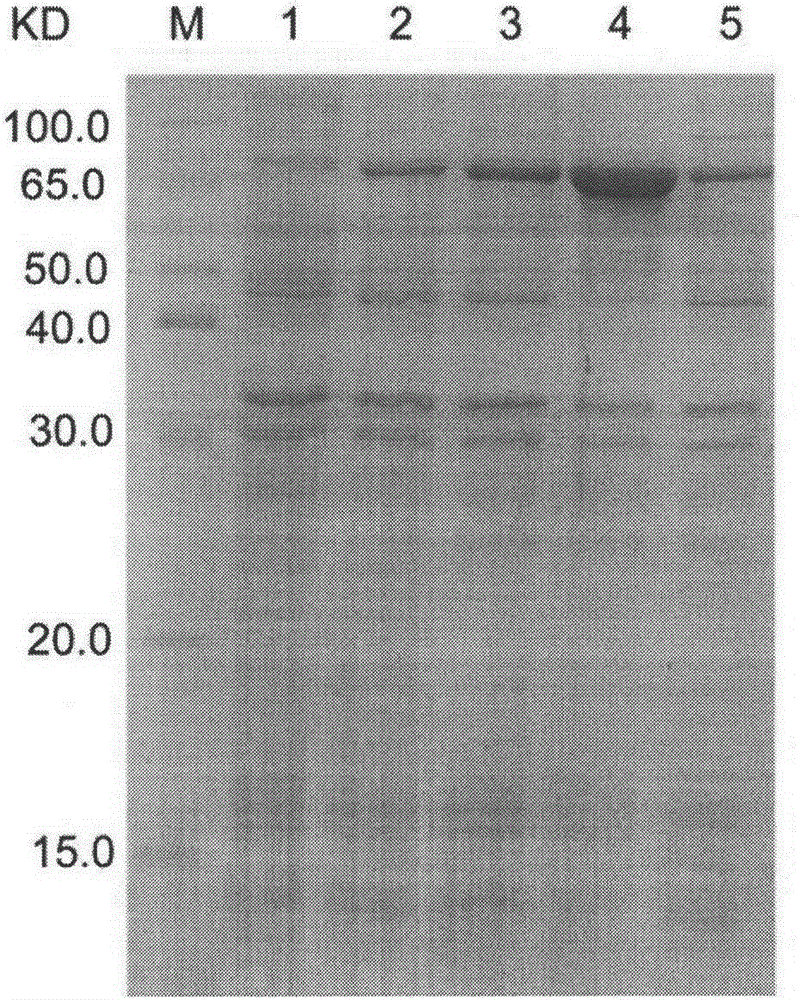
[0062] Example 3: Prokaryotic expression of recombinant protein FVpE
[0063] Transform the correct recombinant expression plasmid pET-FVpE into the ArcticExpress strain. On the pre-prepared LB plate containing 50 μg / ml Kan, inoculate the loop-streaked genetically engineered strain ArcticExpress / pET-FVpE, place it upside down in a 37°C incubator, and after cultivating overnight for 12-16 hours, pick a single colony and inoculate it on a plate containing 50 μg In a test tube of 3ml LB culture solution containing Kan / mlKan, shake overnight at 37°C at 220rpm; inoculate 1:100 the next day in 30ml LB culture solution containing 50μg / mlKan, shake at 37°C at 220rpm until the cell OD600 is 0.6-0.8 (about 2h ). Take out 1ml of the culture, centrifuge at 10000g for 2min at room temperature, discard the supernatant, and resuspend the bacterial pellet with 100μl of 1× loading buffer. Add IPTG to the remaining culture to a final concentration of 0.5 mM, shake at 220 rpm at 37°C for 4 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com