Patents
Literature
55 results about "O antigen synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
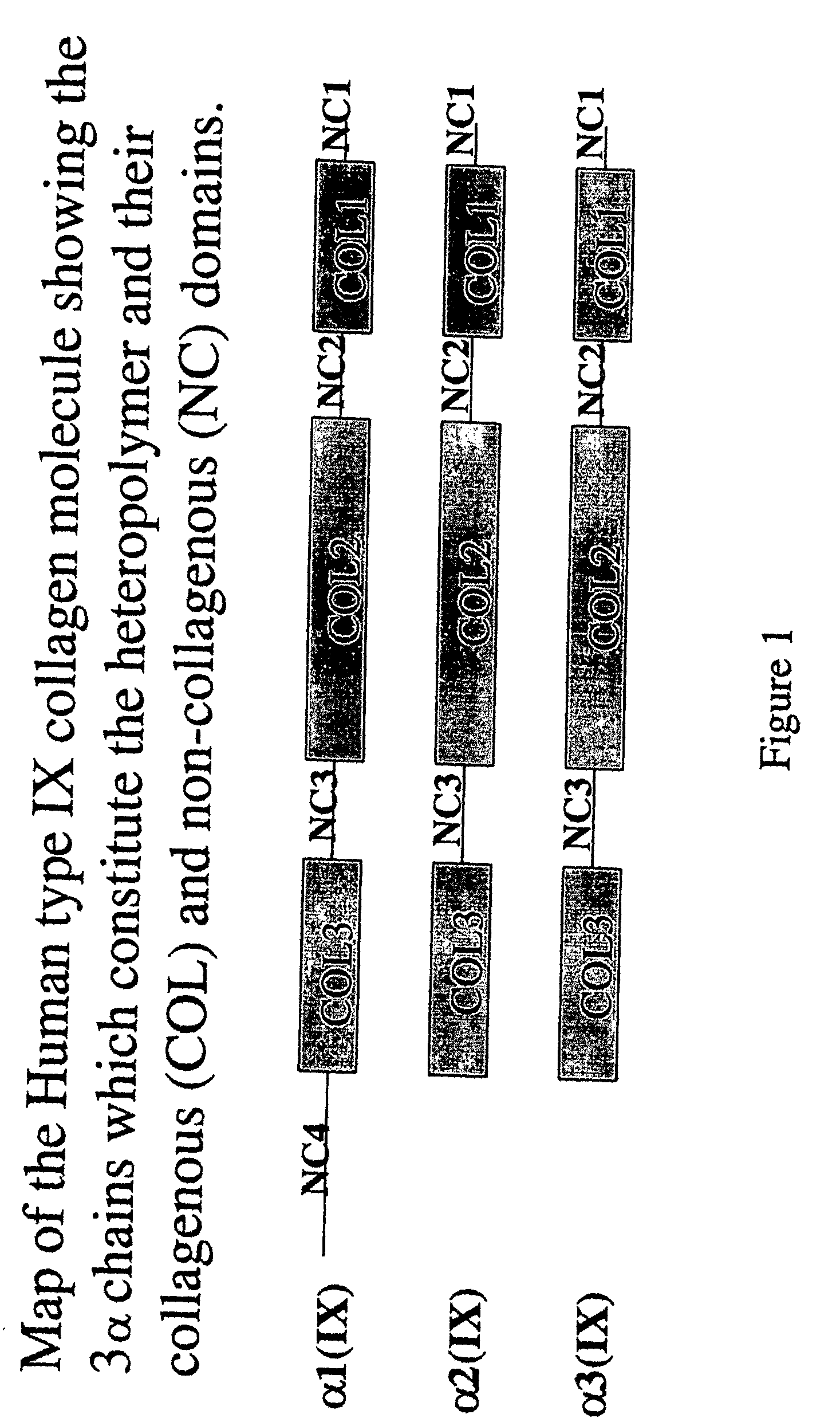
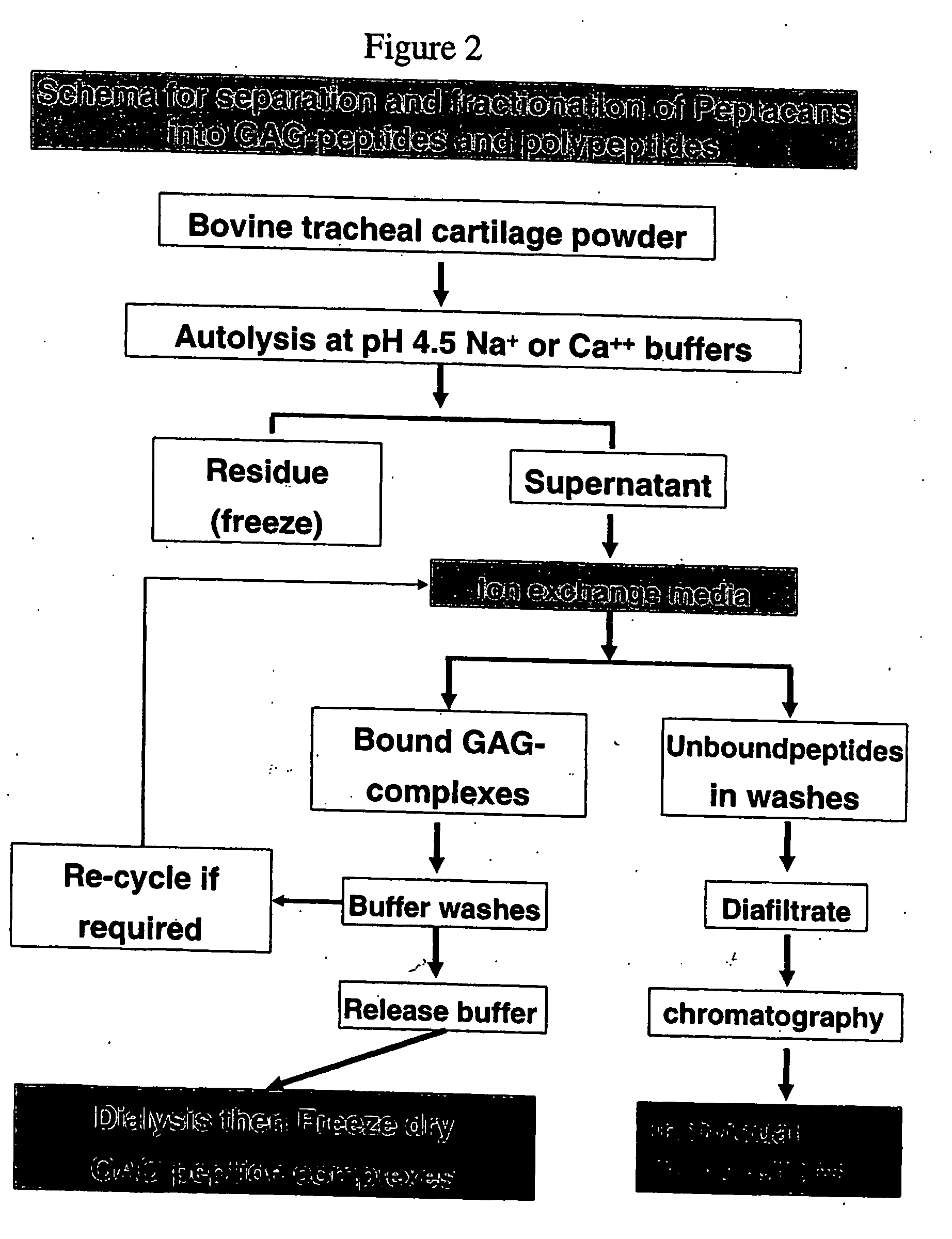
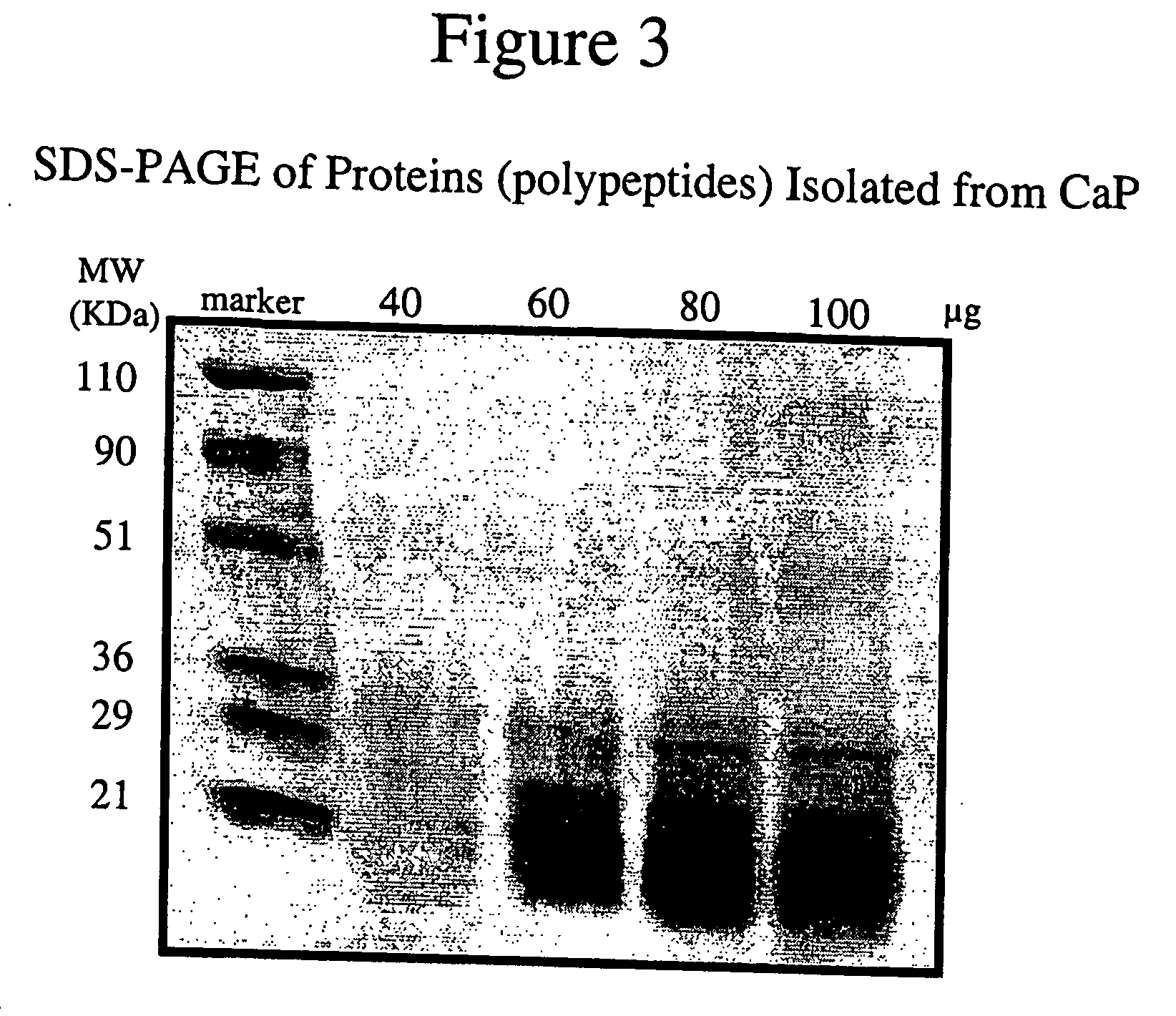
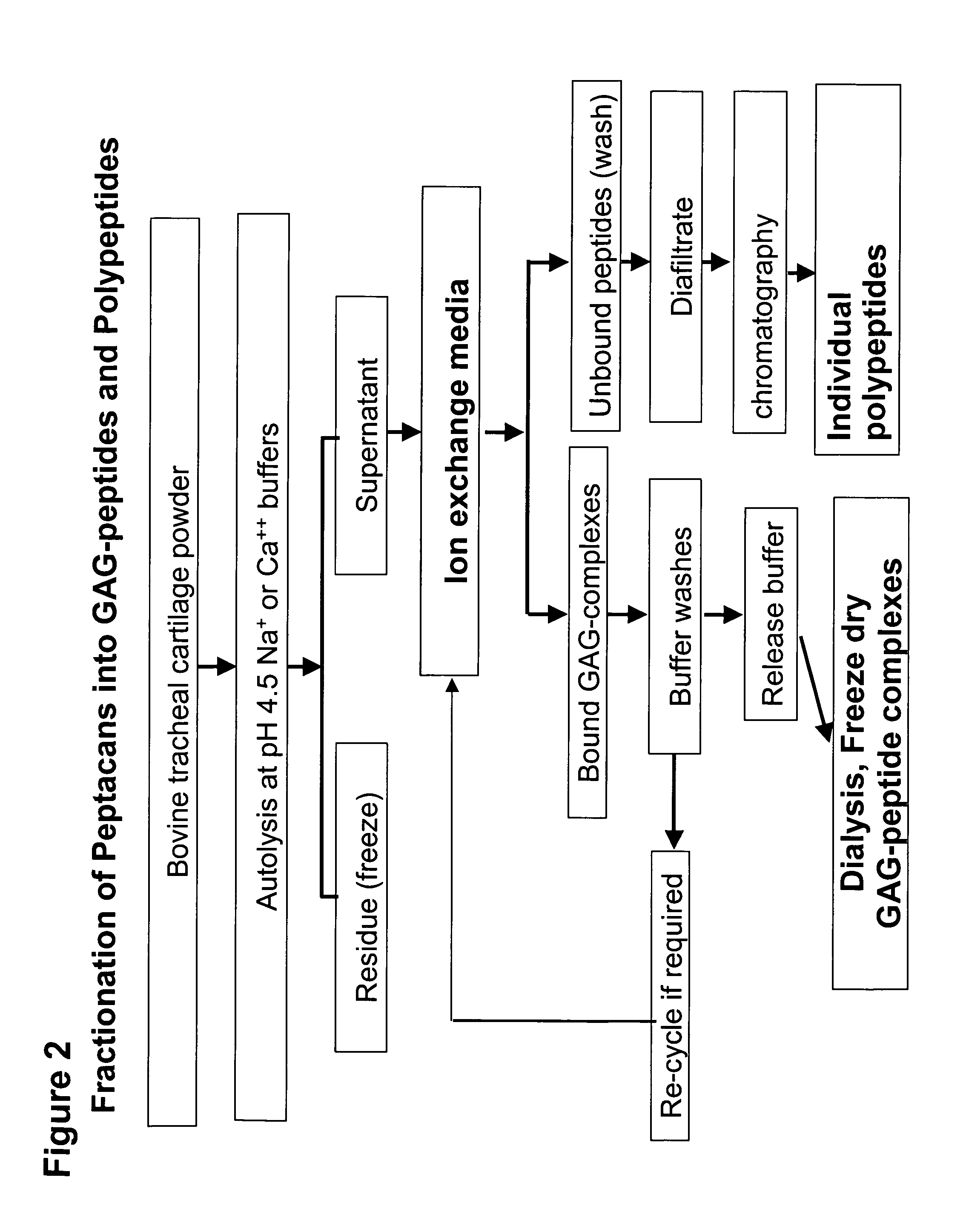
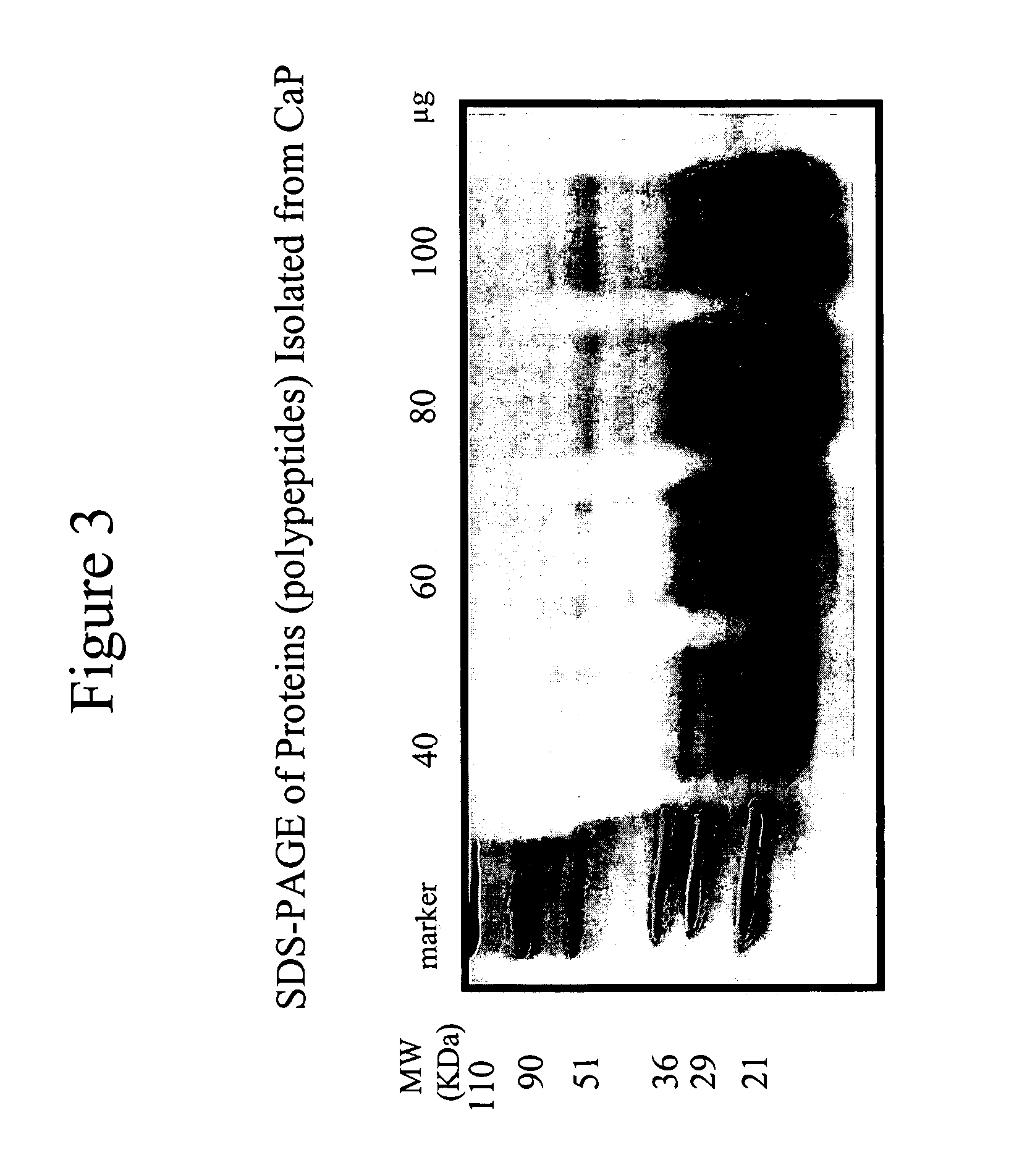
Connective tissue derived polypeptides
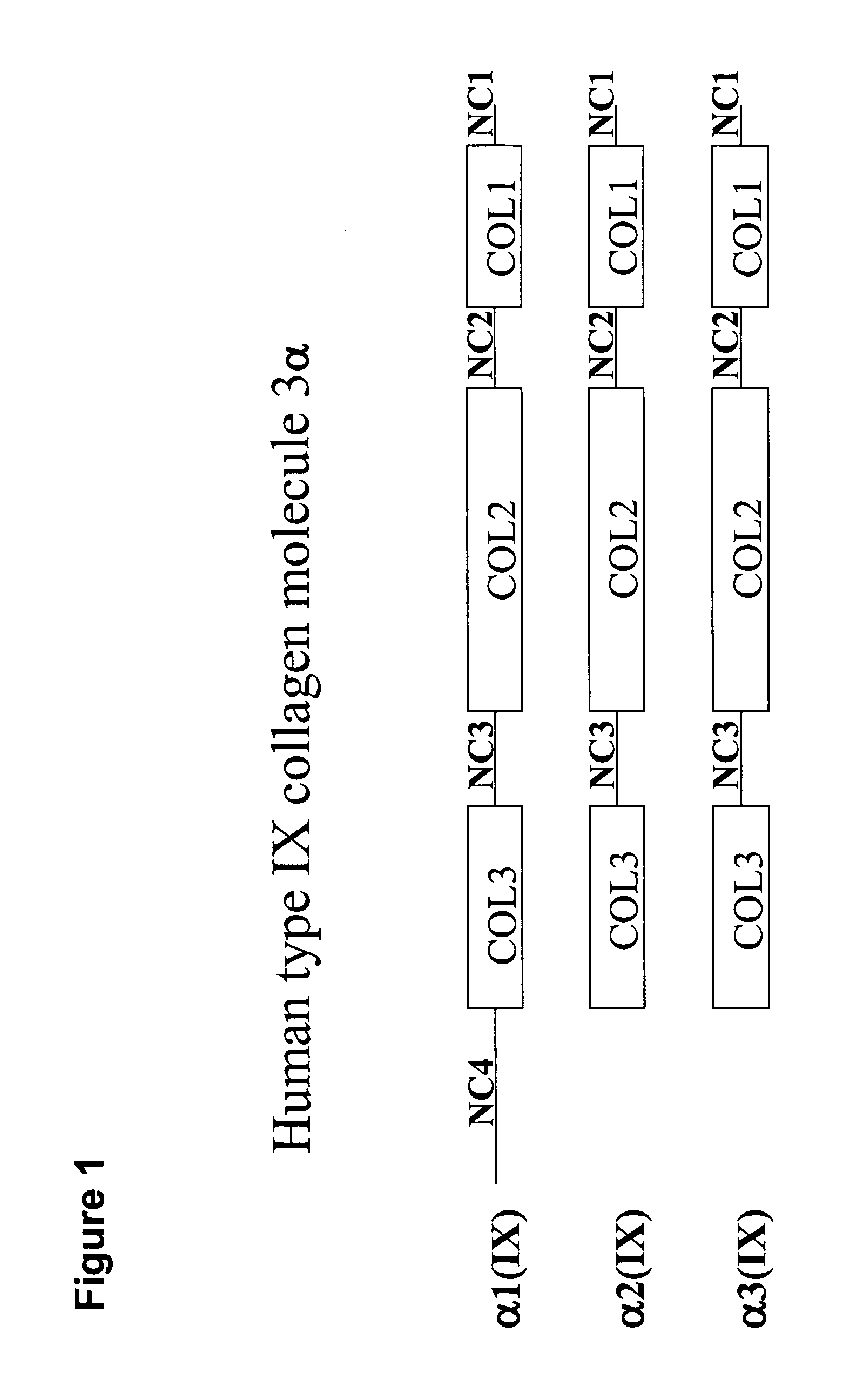
InactiveUS20070105763A1Preventing symptomReduce severityPeptide/protein ingredientsAntipyreticAntigenCollagen Type IX
The present invention relates to compositions comprising one or more connective tissue derived polypeptides having a molecular weight of less than 30,000 Da that are capable of tolerising individuals to antigenic components of cartilage and prevent the appearance of and / or treat symptoms of arthritis and other musculoskeletal degenerative conditions. The present invention provides methods for recovering polypeptides having a molecular weight of less than 30,000 Da from connective tissue and having anti-arthritic or anti-inflammatory activity. The present invention further relates to compositions comprising a polypeptide containing an NC4 domain of collagen type IX alpha 1 chain or fragment thereof, having a molecular weight of less than 30,000 Da, where the polypeptide is capable of tolerising individuals to antigenic components of cartilage, preventing the appearance of arthritic symptoms, and / or treating the symptoms of arthritis.
Owner:PROTEOBIOACTIVES PTY LTD
Compositions for immunising against staphylococcus aureus
ActiveUS20120093850A1Reduce and eliminate haemolytic activityAntibacterial agentsHydrolasesMicrobiologyStaphyloccocus aureus
Staphylococcus aureus Hla polypeptides having various deletions, insertions, and / or mutations which are useful for immunisation are provided herein. Also provided herein are Hla heptamers which are non-haemolytic. Additionally, an effective Staphylococcus aureus vaccine may require several antigenic components, and so various combinations of S. aureus antigens, including Hla polypeptides having deletions, insertions, and / or mutations, are identified for use in immunisation. These polypeptides may optionally be used in combination with S. aureus saccharides.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
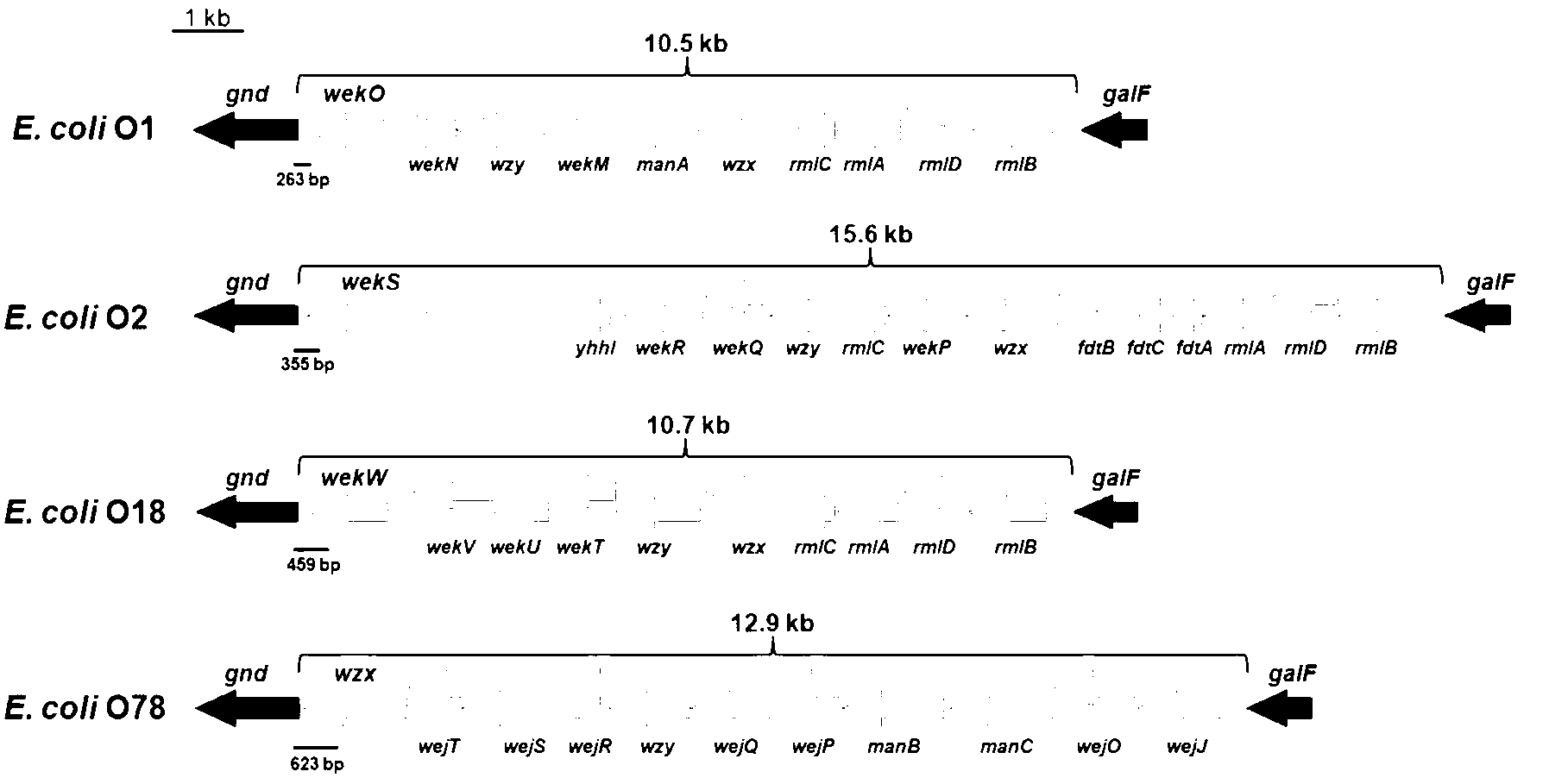
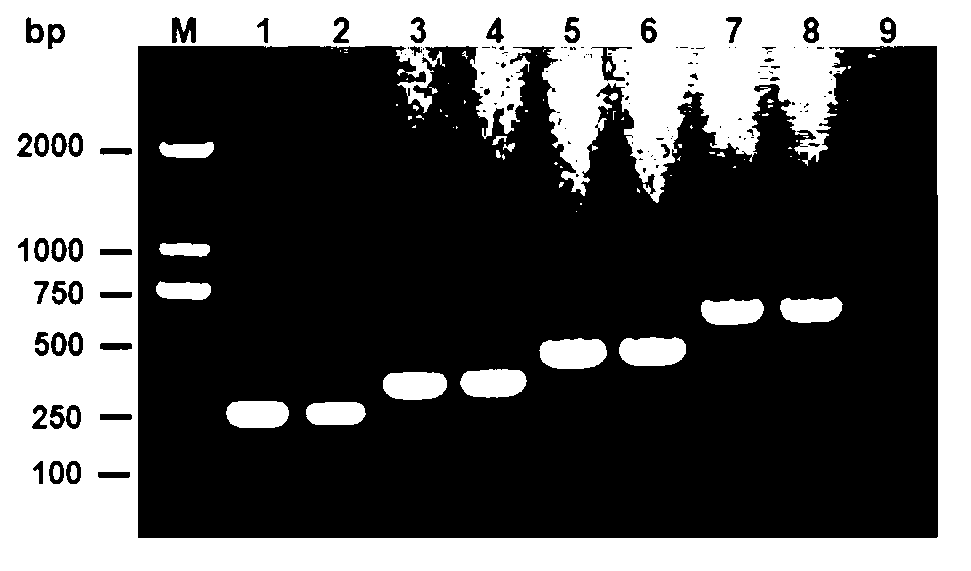
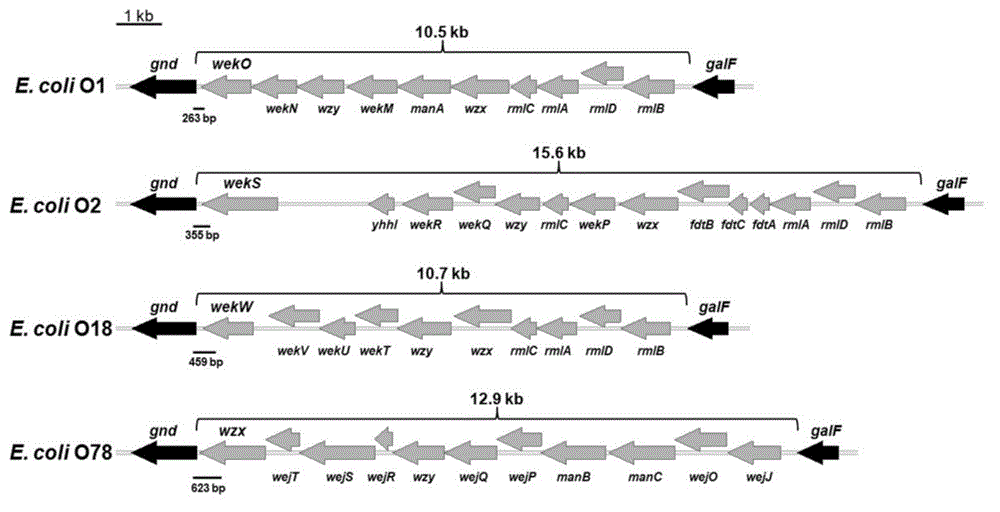
Escherichia coli O1, O2, O18 and O78 serotype detection kit and detection method thereof
ActiveCN103305627ACross reactionReduce sensitivityMicrobiological testing/measurementMicroorganism based processesESCHERICHIA COLI ANTIGENEscherichia coli serotype
The invention belongs to the technical field of biology detection and relates to an escherichia coli O1, O2, O18 and O78 serotype detection kit and a detection method thereof. Forward primers of a primer group of the kit are highly conservative escherichia coli O antigen combined relative gene sequences, and backward primers are four serotype specific primers designed on the basis of four O1, O2, O18 and O78 serotype escherichia coli O antigen synthesis relative genes. The detection kit containing the primer group and the detection method of the detection kit have the advantages of being fast, sensitive, specific, low in cost, easy to operate and capable of well overcoming the shortcomings in the current escherichia coli O1, O2, O18 and O78 serotype detection method, can meet the requirements for the current escherichia coli O1, O2, O18 and O78 serotype detection, can be popularized and used easily in a wide range and have wide market prospect and large economic benefit.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Compositions for immunising against Staphylococcus aureus
ActiveUS8632783B2Reduce and eliminate haemolytic activityAntibacterial agentsBacterial antigen ingredientsAntigenStaphylococcus aureus
Staphylococcus aureus Hla polypeptides having various deletions, insertions, and / or mutations which are useful for immunization are provided herein. Also provided herein are Hla heptamers which are non-haemolytic. Additionally, an effective Staphylococcus aureus vaccine may require several antigenic components, and so various combinations of S. aureus antigens, including Hla polypeptides having deletions, insertions, and / or mutations, are identified for use in immunization. These polypeptides may optionally be used in combination with S. aureus saccharides.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel Compositions and Uses Thereof
InactiveUS20100040589A1Efficient presentation of antigenReduce and prevent likelihoodAntibacterial agentsBiocideVaccinationAdjuvant
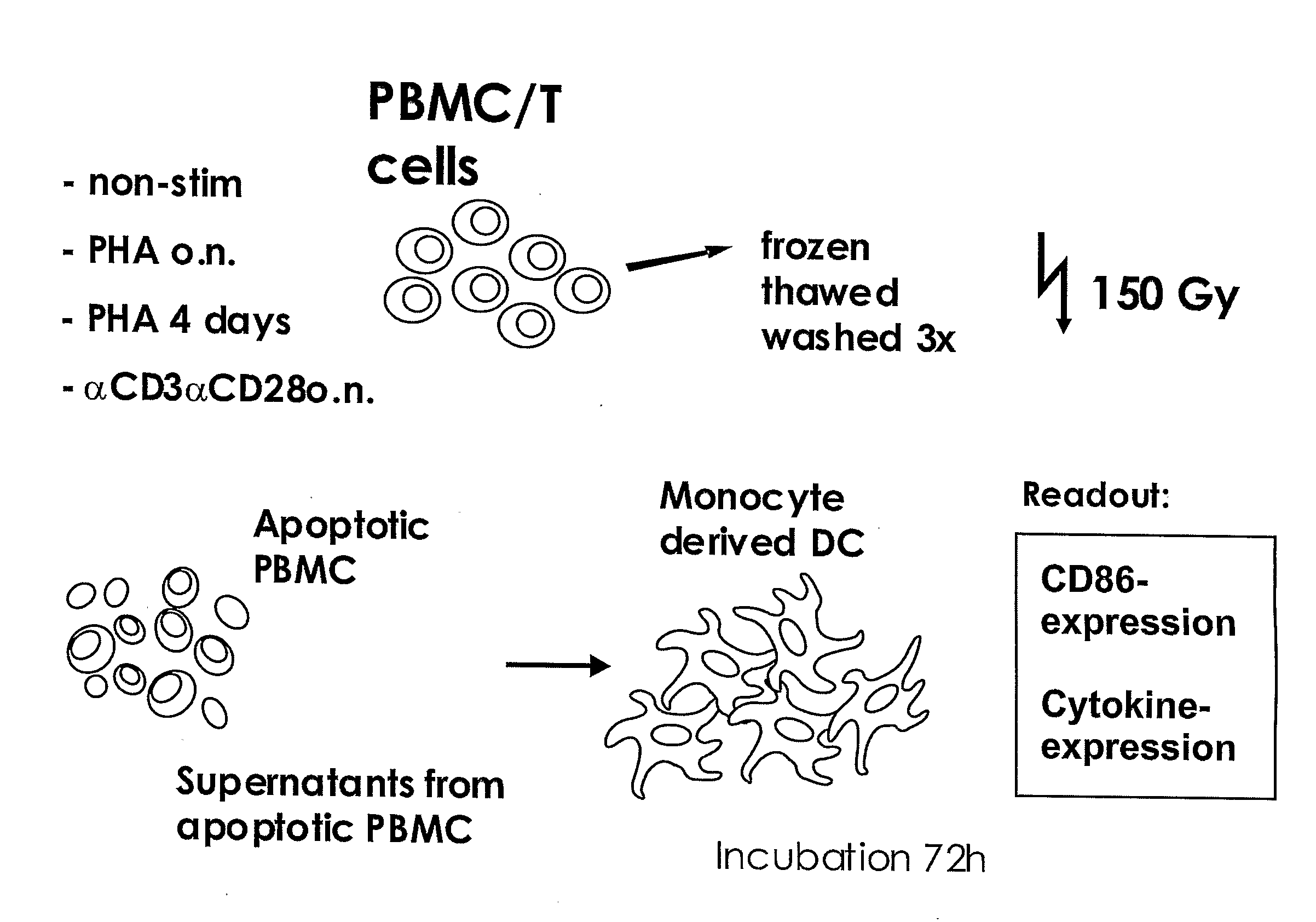
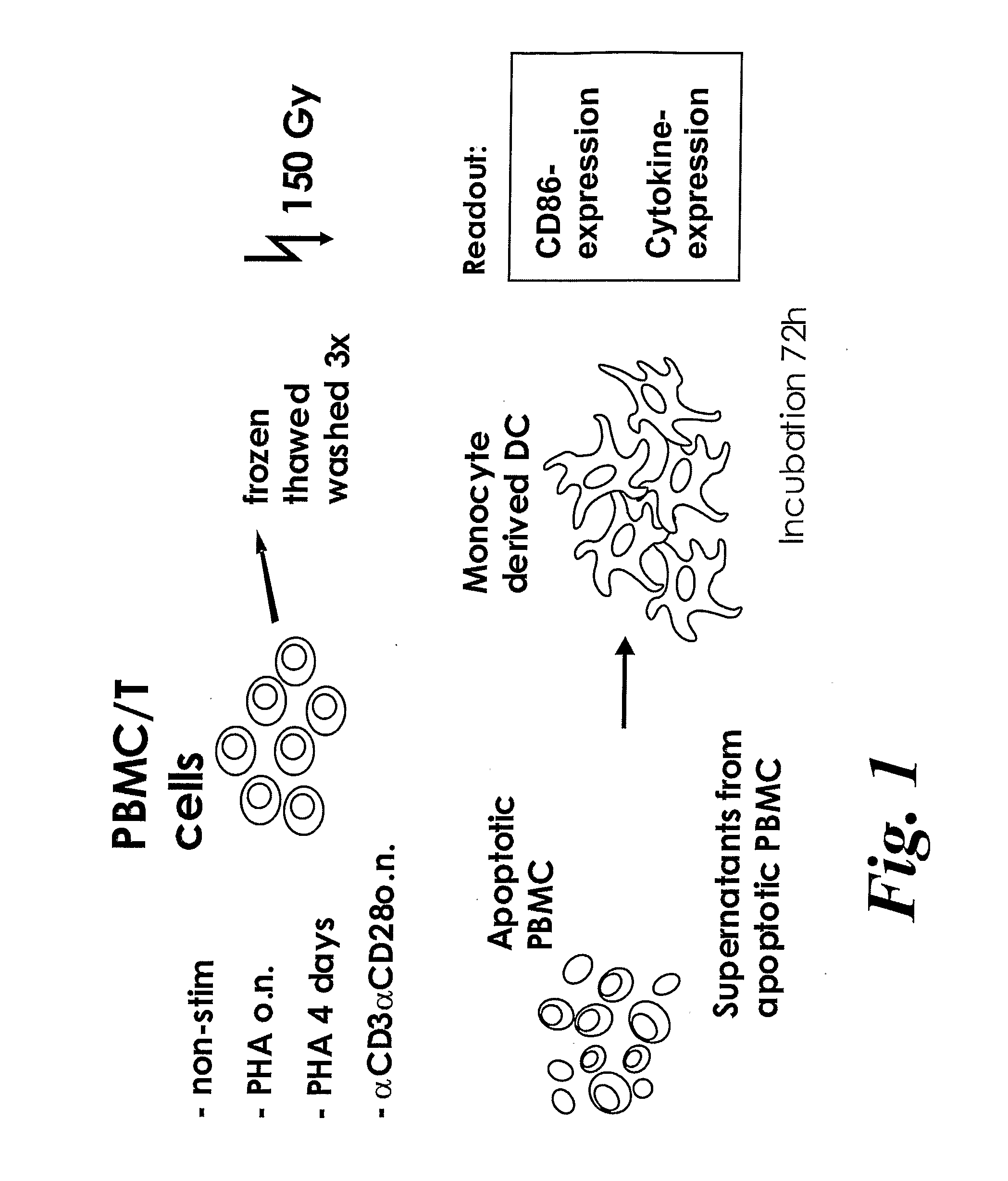
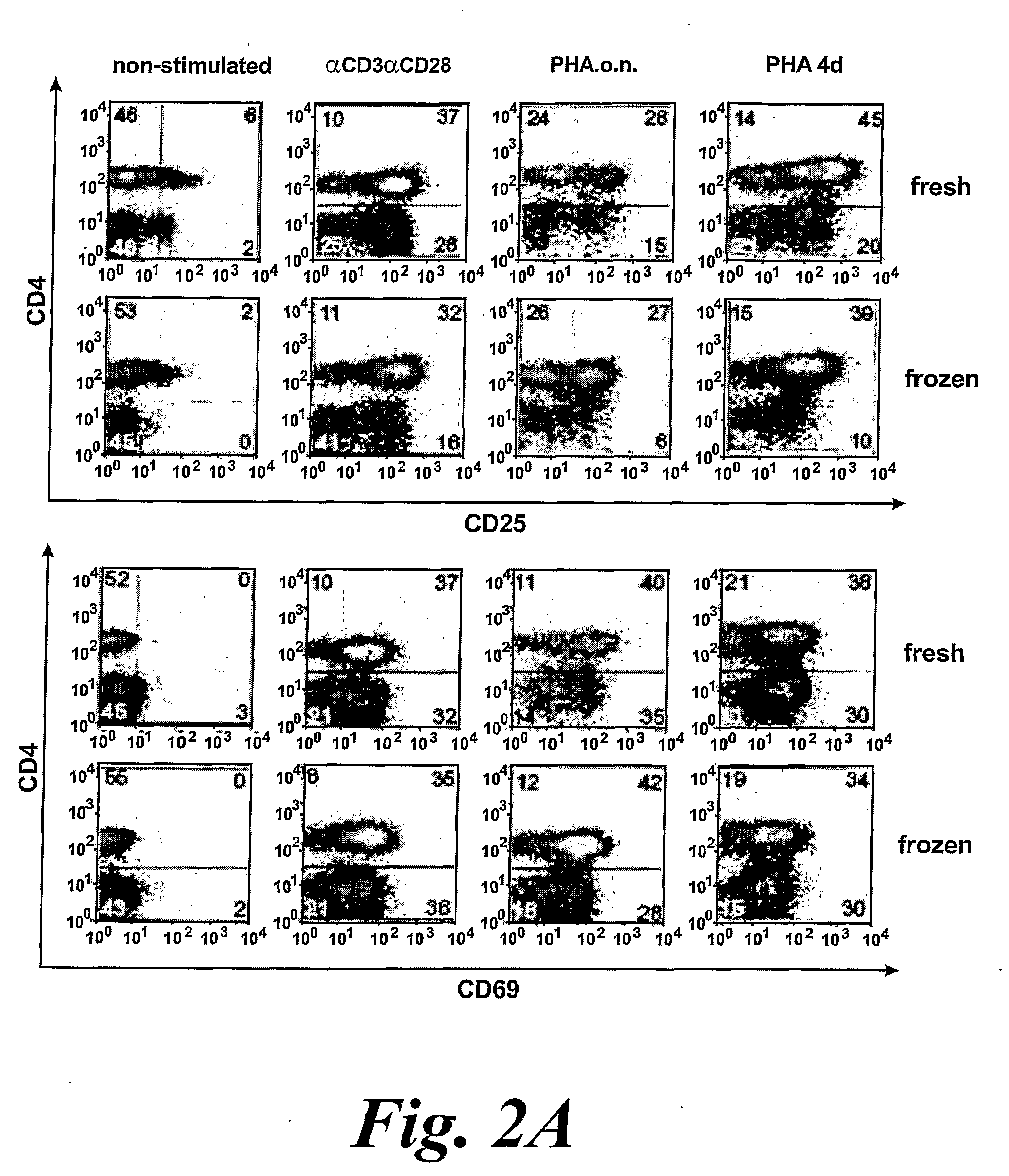
The present invention provides a cellular vaccine for therapeutic or prophylactic treatment of a pathological condition, the vaccine comprising or consisting of a population of CD 4+ T cells modified such that they contain an antigenic component, and / or a nucleic acid molecule encoding an antigenic component thereof, wherein the T cells are (a) activated, or capable of being activated, and (b) apoptotic, or capable or being made apoptotic. The invention further provides an adjuvant composition for use in a method of vaccination, the composition comprising or consisting of a population of T cells, wherein the T cells are (a) activated, or capable of being activated, and (b) apoptotic, or capable or being made apoptotic. In addition, the invention provides a composition having microbicide activity, or capable thereof upon exposure to antigen-presenting cells, the composition comprising or consisting of a population of T cells, wherein the T cells are (a) activated, or capable of being activated, and (b) apoptotic, or capable or being made apoptotic. Also provided by the present invention are methods for making and using the vaccines and compositions described herein.
Owner:AVARIS
Influenza vaccines extemporaneously adsorbed to aluminium adjuvants
InactiveUS7959931B2SsRNA viruses negative-senseViral antigen ingredientsFlu immunizationInfluenza vaccine
Antigen and adjuvant components of an adjuvanted influenza vaccine are not mixed during manufacture, but are provided as separate components for extemporaneous mixing at the time of use, for example as a kit comprising (i) an antigen component, comprising an influenza virus antigen; and (ii) an adjuvant component, comprising an aluminium salt.
Owner:NOVARTIS AG
Vaccine vector prepared based on anionic polymer and derivatives thereof
ActiveCN111658780AWill not cause denaturationMaintain conformationPowder deliveryInorganic non-active ingredientsEngineeringTGE VACCINE
The invention provides a vaccine vector prepared based on an anionic polymer and derivatives thereof, and provides a preparation method of the vaccine vector. Nanoparticles are compounded with aluminum salt through a series of anionic polymers and derivatives thereof to form aluminum hydroxide; and different antigen components are added in the preparation process to encapsulate the antigen. The prepared vaccine can be efficiently taken by antigen presenting cells, transferred to lymph nodes and induce antigen-specific immune response, and has a wide vaccine application prospect.
Owner:SICHUAN UNIV
Vaccine composition and method of using the same
InactiveUS7192588B2Great accuracy of dose and ease of useEliminate needBiocideBacterial antigen ingredientsAntigenDisease
The present invention relates to a stable compacted, compressed or hard tableted injectable composition, including a vaccine composition comprising at least one freeze dried antigenic component and a dissolution aid. A package containing the above injectable composition and method to facilitate immunizing a subject against a disease comprising the steps of first dissolving the compacted, compressed or hard tableted vaccine composition in a package with a diluent to form a vaccine solution, and administering the resulting vaccine solution in an amount effective for immunizing is also provided.
Owner:ABIC BIOLOGICAL LAB
Compositions and methods for generating antibodies
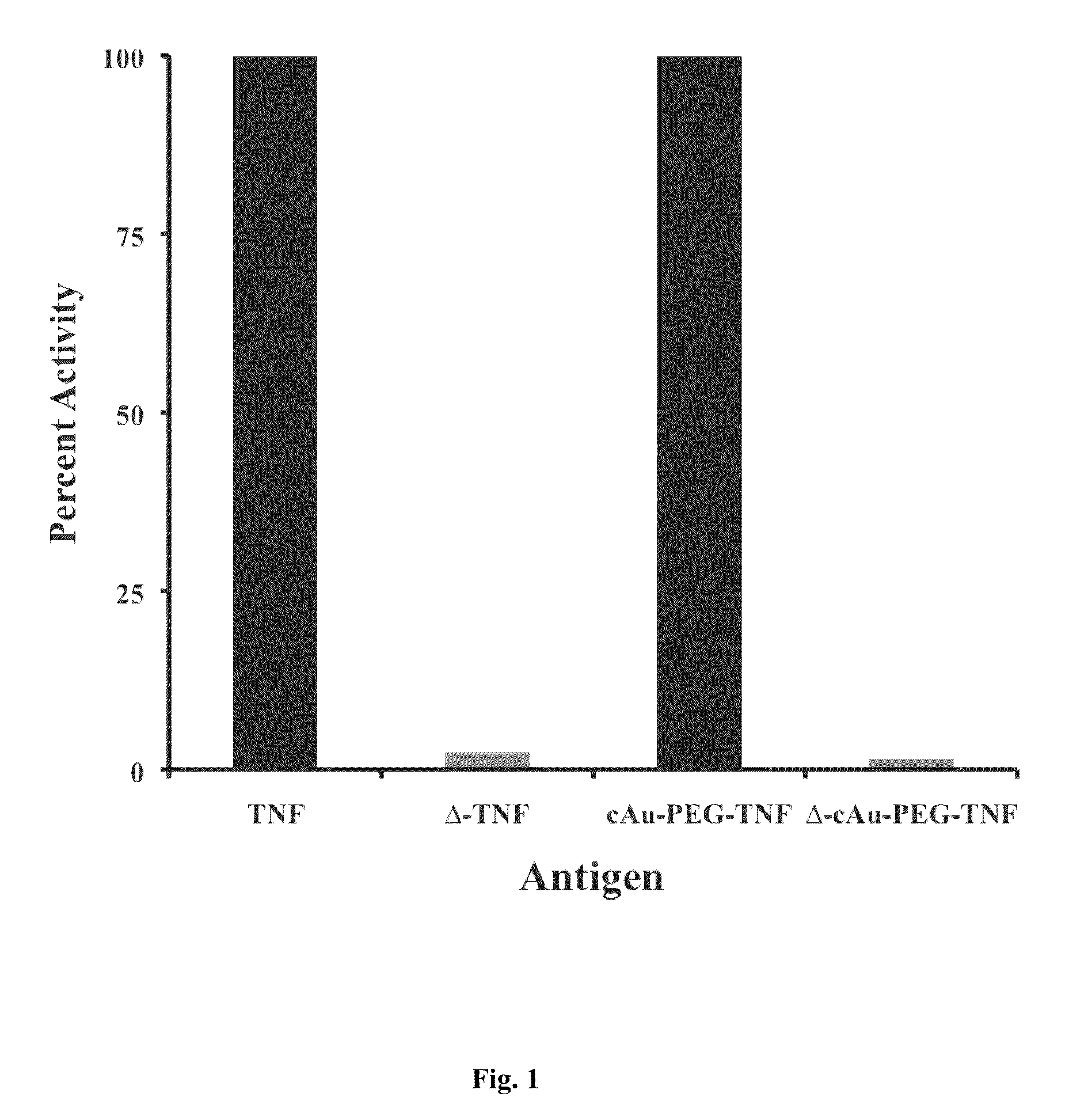
ActiveUS7960145B2Loss of activityLoss of detectionSugar derivativesImmunoglobulins against growth factorsAntiendomysial antibodiesPolyethylene glycol
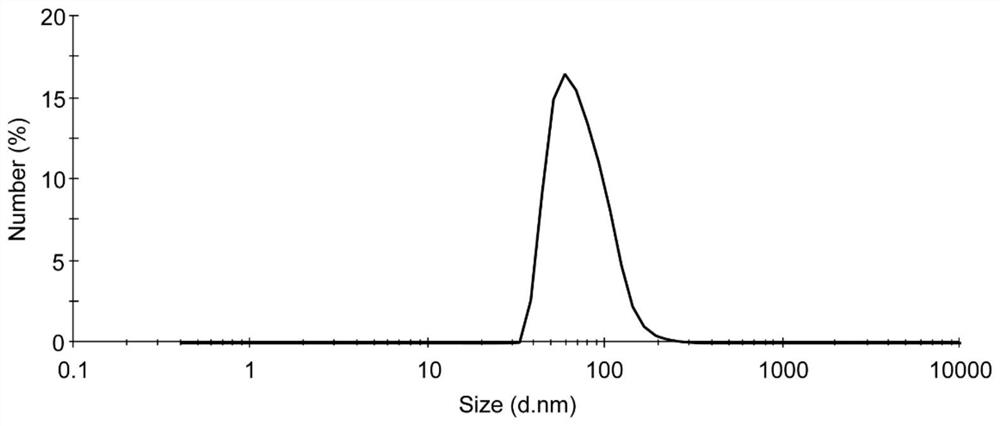
The compositions and methods of the present invention comprise the efficient and effective presentation of antigens to the appropriate components of the immune system resulting in the production of species-specific antibodies in vitro. In general, these compositions comprise one or more antigenic components together with a colloidal metal, optionally combined with derivatized PEG (polyethylene glycol) or other agents. The invention also comprises novel cytokine cocktails for generating desired antibodies.
Owner:CYTIMMUNE SCI
Preparation method of adipose tissue extracellular matrix
ActiveCN111603610ARetain activityStrong adipogenic abilityTissue regenerationProsthesisCell-Extracellular MatrixAdipogenesis
The invention discloses a preparation method of an adipose tissue extracellular matrix, and belongs to the technical field of tissue engineering, regenerative medicine and clinical medicine. The preparation method comprises the following steps: taking fresh adipose tissues, carrying out pretreating, and carrying out freeze thawing treatment; crushing the adipose tissue subjected to freeze thawingtreatment to obtain crushed adipose tissues; and then sequentially carrying out high-permeability sodium chloride solution cleaning, first-time sterile deionized water washing, TritonX-100 solution cleaning, second-time sterile deionized water washing, isopropanol grease precipitation, third-time sterile deionized water washing and alcohol RNA elution to obtain the adipose tissue extracellular matrix. Enzyme participation is not needed in the decellularization process, so that the preparation cost is reduced, the risk of rejection reaction caused by heterologous antigen components is reduced,the natural structure and active factors of extracellular matrixes can be reserved, more effective decellularization results are achieved, and the in-vivo adipogenesis capacity is higher.
Owner:PLASTIC SURGERY HOSPITAL CHINESE ACAD OF MEDICAL SCI
Process for producing antigenic substance
InactiveUS20060233789A1Wide rangeHinders its propagationBiocideSnake antigen ingredientsEmbryoMalaria
An object of the present invention is to provide a means for producing an antigenic component with the retained native antigenicity using a cell-free protein synthesis. In particular, it is an object to provide a means for producing an antigenic component without depending on codon usage, like expressing an antigenic component from a gene containing a large amount of AT. The present inventors have made a strenuous study to solve the matters described above and successfully completed the present invention by preparing an antigenic component with the retained antigenicity, in particular a malaria antigen useful for manufacturing a malaria vaccine, through a system with the use of a wheat embryo among cell-free protein synthesis means.
Owner:CELLFREE SCI
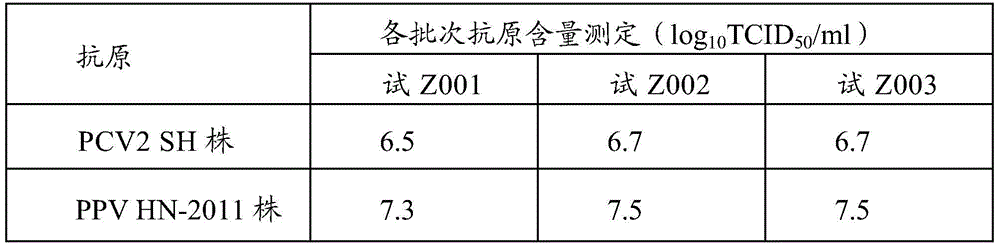
Bivalent inactivated vaccine for porcine circovirus type 2 and porcine parvovirus and preparation method thereof
InactiveCN105749273AHigh titer contentImmunization is convenient and fastViral antigen ingredientsAntiviralsImmune effectsAdjuvant
The invention relates to a multivalent vaccine for preventing and treating the infectious diseases of a pig, in particular to a combined vaccine for treating and preventing porcine circovirus type 2 and porcine parvovirus and a preparation method thereof. The multivalent vaccine selects and uses the porcine circovirus type 2 and the porcine parvovirus. The preparation method comprises the following steps of: culturing the porcine circovirus type 2, inactivating and concentrating; culturing the porcine parvovirus, inactivating and concentrating; mixing the two antigen components by proportion, and adding adjuvants to prepare into the vaccine. The bivalent vaccine prepared by the invention is convenient in use and is safer, and the immune effect is superior to that ofcombination of two single vaccines.
Owner:PU LIKE BIO ENG
Salmonella choleraesuis C500 strain having deletion of asd and realizing expression of lacI
The invention discloses a salmonella choleraesuis C500 strain having deletion of asd and realizing expression of lacI. According to the salmonella choleraesuis C500 strain, the salmonella choleraesuisC500 strain is adopted as the carrier, gene modification is carried out on the chromosomes of the salmonella choleraesuis C500 strain, so that the asd gene of the salmonella choleraesuis C500 has deletion, the salmonella choleraesuis C500 delta asd deletion strain is constructed, meanwhile, the araC PBAD-lacI sequence is placed in the deleted asd gene reading frame, so that while the C500 strainhas the deletion of the asd gene, the araC PBAD-lacI expression cassette is introduced, and the LacI protein can be expressed while arabinose exists; in addition, an EGFP expression plasmid pYA4514-EGFP containing the Ptrc promoter is constructed, and the negative regulation effect of LacI for the Ptrc promoter is detected through the western blot experiment. The invention provides the brand-new C500 strain realizing the regulatable expression of the exogenous antigenic gene based on a balanced-lethal system and the expression plasmid, and a foundation is laid for the expression of the exogenous antigenic component by utilizing the C500 strain in the future.
Owner:JILIN AGRICULTURAL UNIV
Method of separating and purifying various antigen ingredients of pertussis
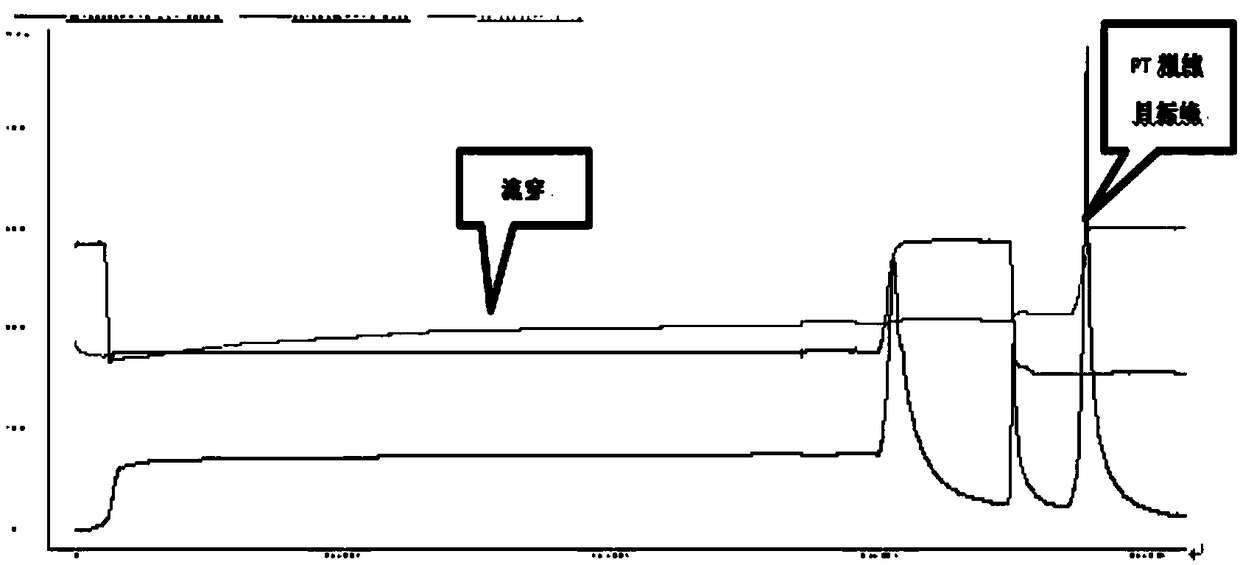
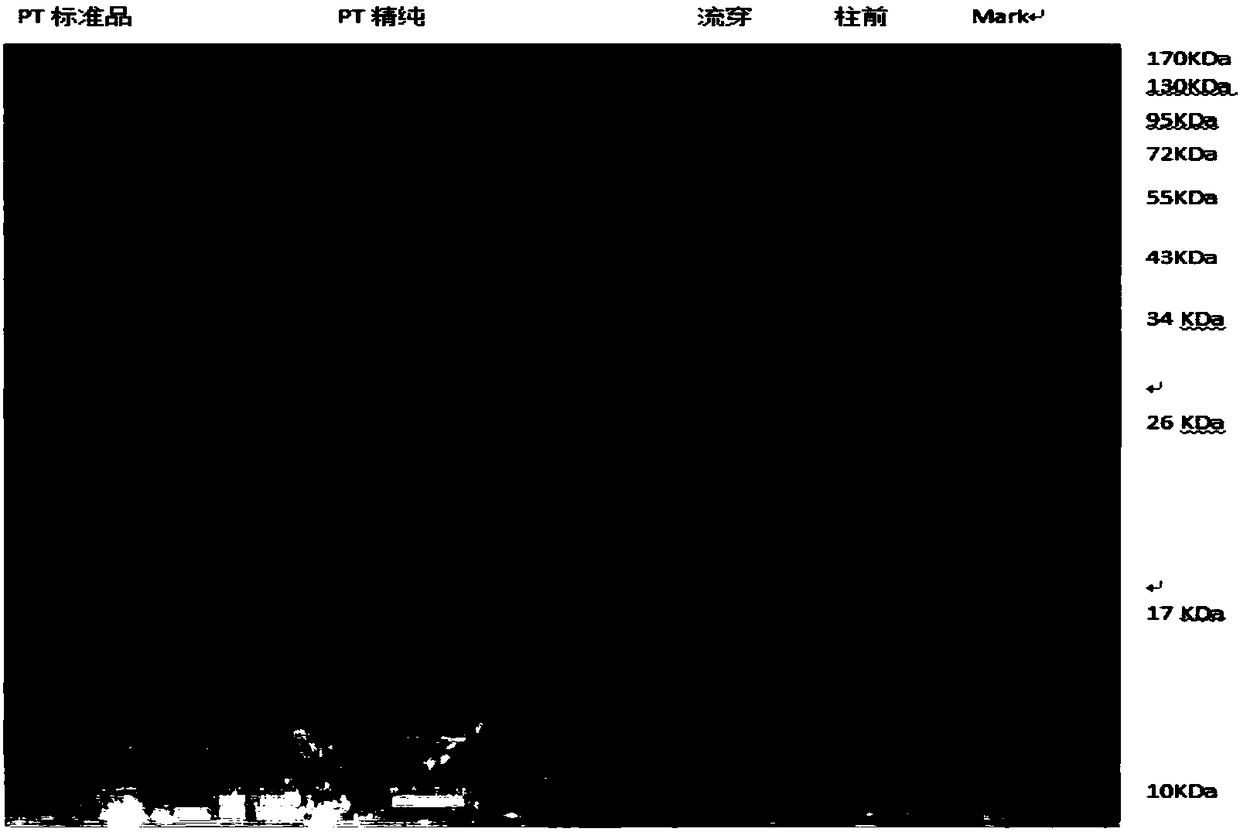
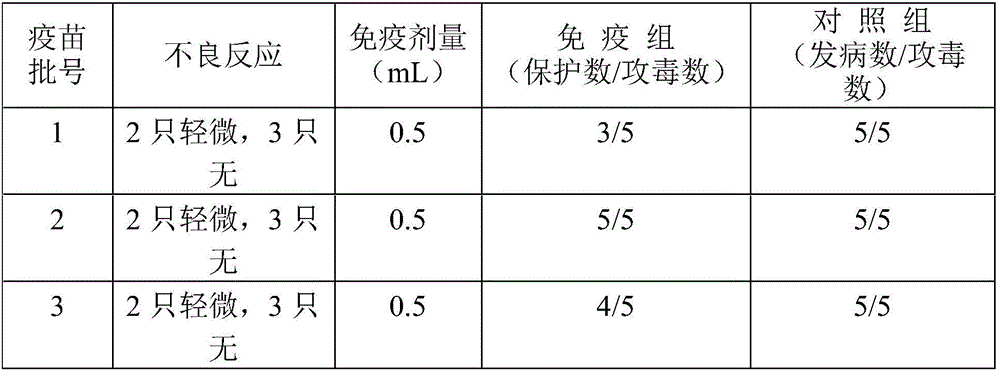
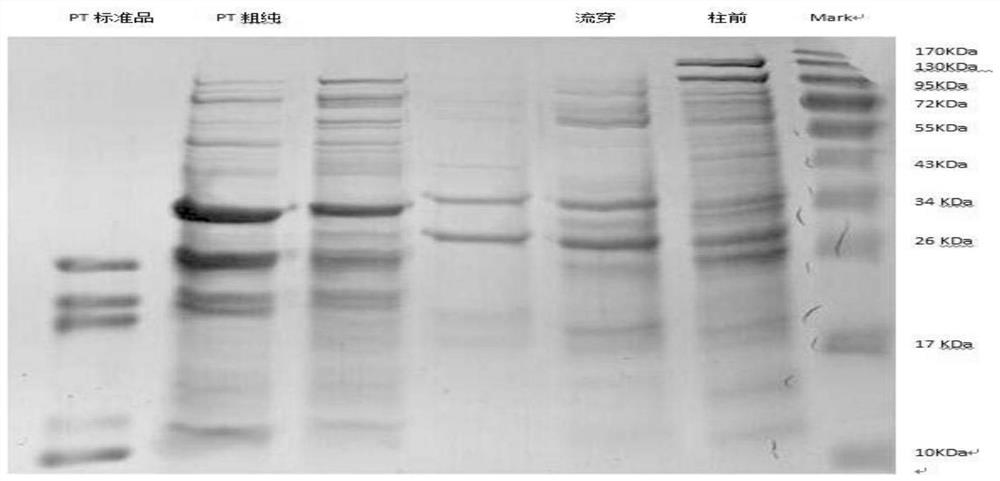
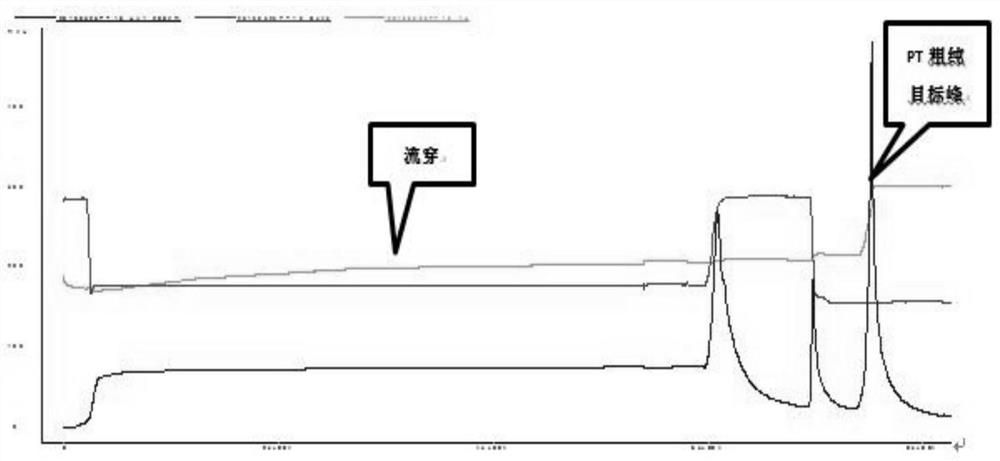
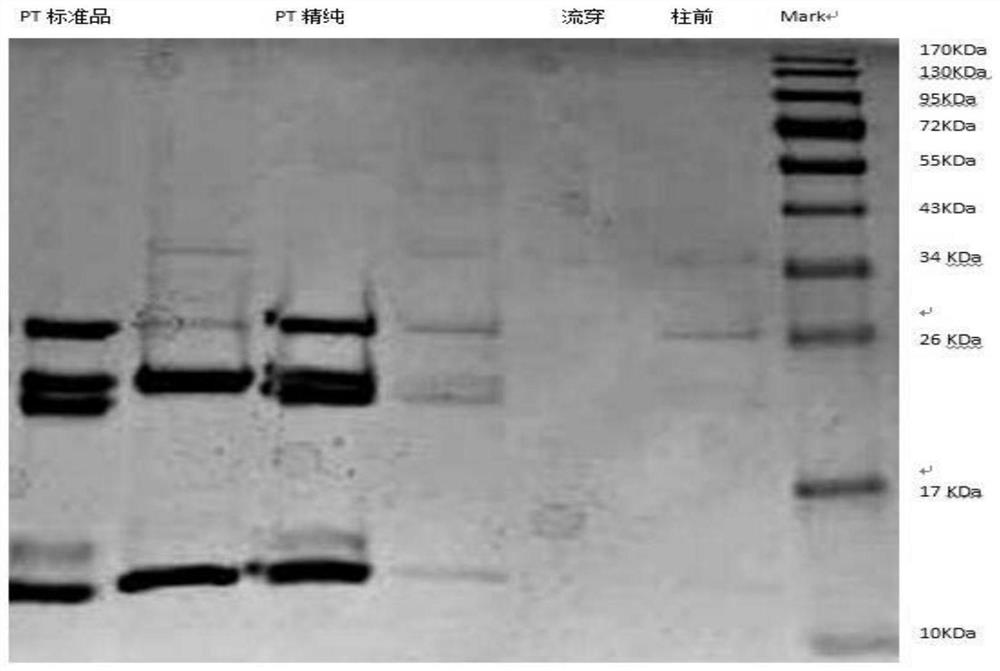
ActiveCN108570098AGuaranteed stabilityHigh recovery ratePeptide preparation methodsDepsipeptidesHemagglutininPertactin
The invention relates to a method of separating and purifying various antigen ingredients of pertussis. The various antigens include PT (pertussis toxin) antigen, FHA (filamentous hemagglutinin) antigen and PRN (pertactin) antigen. The method comprises the steps of (1) subjecting a pertussis strain to fermenting culture to acquire culture supernate and bacterial precipitate separately; (2) isolating and purifying PT antigen from the culture supernate, and separating and purifying PRN antigen and FHA antigen from the bacterial precipitation.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
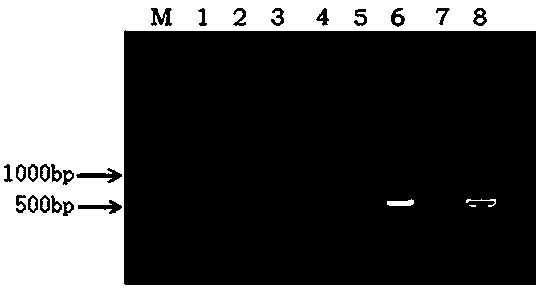
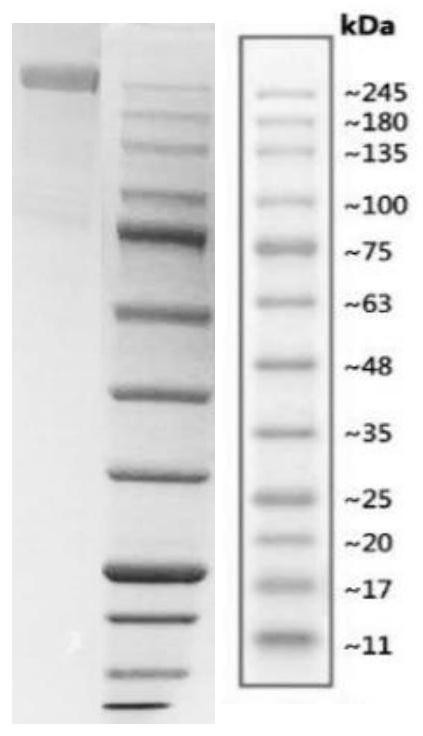
New coronavirus antigen and preparation method and application thereof
The invention provides a new coronavirus antigen and a preparation method and application thereof. The new coronavirus antigen comprises a fusion protein, wherein the fusion protein comprises a Spike protein S1 structural domain part and an HSA part, and the 685 arginine corresponding to Spike protein in the Spike protein S1 structural domain part is replaced with glycine. The prepared fusion protein serves as a key antigen component in a new coronavirus serum antibody detection kit and is used for detecting a new coronavirus antibody; and the fusion protein serves as a vaccine to be given to animals or human to generate antibodies and is used for preventing new coronavirus. The fusion protein serves as a therapeutic drug to be given to new coronavirus patients and is used for treating new coronavirus pneumonia.
Owner:思格(苏州)生物科技有限公司
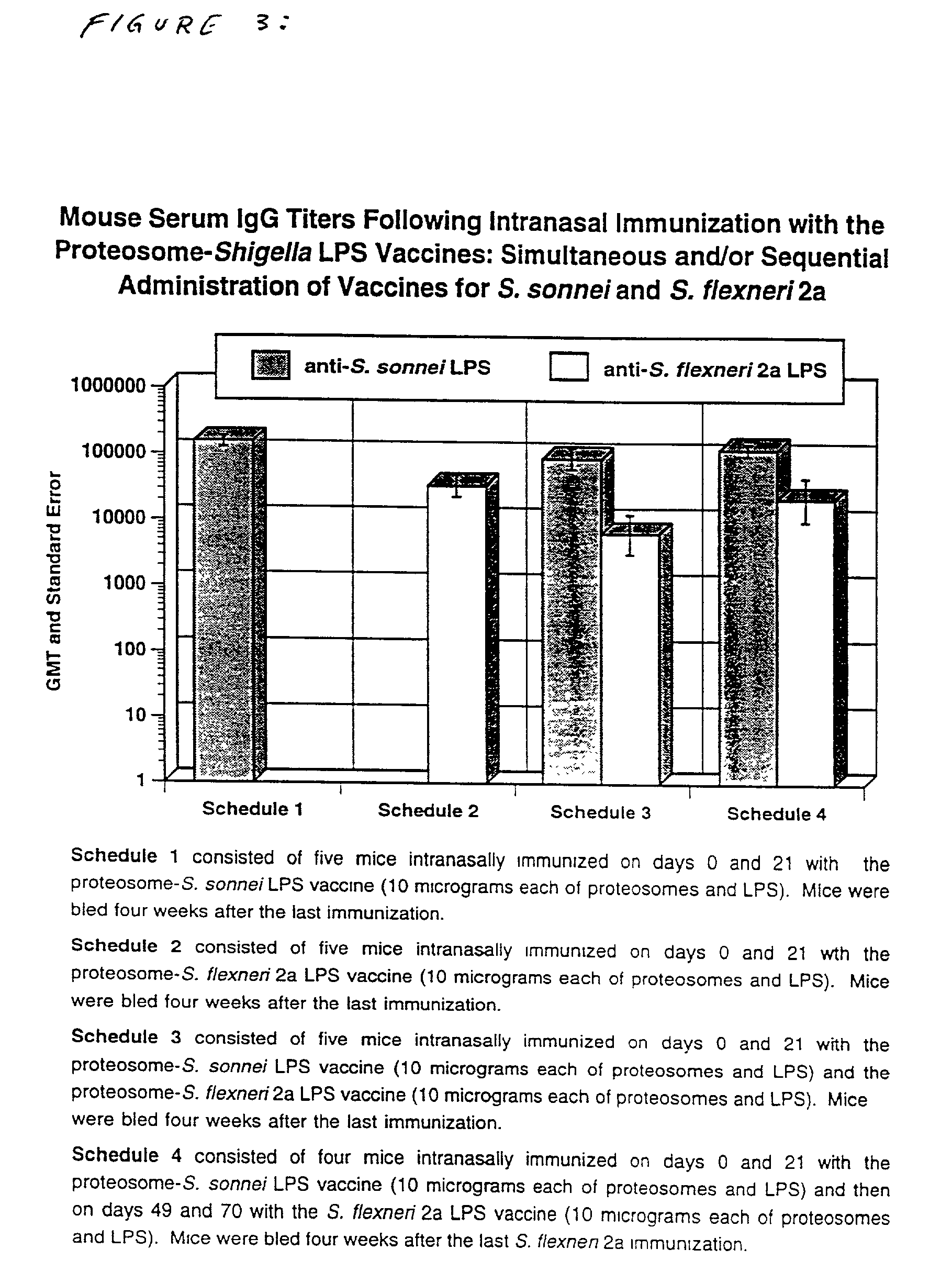
Methods for the production of non-covalently complexed and multivalent proteosome sub-unit vaccines
InactiveUS20020164357A1Shorten the timeIncrease temperatureAntibacterial agentsOrganic active ingredientsContinuous monitoringContamination
A method for preparing multivalent proteosomeamphiphilic determinant vaccines suitable for parenteral or mucosal administration using diafiltration or ultrafiltration technology. The amphiplilic determinants include lipopolysaccharides from gram negative bacteria, e.g. S. flexneri, P. shigelloides and S. sonnei. Proteosomes are obtained from group B type 2b meningococci. The active proteosomeamphiphilic determinant complexes (noncovalently complexes) of the vaccine are formed using defiltration or ultrafiltration to remove the detergent. The use of diafiltration or ultrafiltration decreases processing time and the opportunity for contamination and further permits the use of ambient temperature and efficient scaleup. In addition, the process permits the reliable and continuous monitoring of the dialysate which enhances the efficiency of the entire process. The time of dialysis for production of a lot of vaccine is reduced from 710 days to less than 72 hours and usually less than 48 or 4 hours. The use of the process optimizes the presence of each antigenic component in the preparation of multivalent vaccines.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
Composition and manufacturing of powders containing nanoadjuvants for mucosal vaccination
InactiveUS20200268880A1Reduce antigen activityEnhanced mixing processPowder deliveryInorganic non-active ingredientsMucosal vaccineTGE VACCINE
New preparative approach of dry powder vaccines for mucosal (e.g. nasal) administration for the purpose of human or animal immunization; it requires spraying a vaccine liquid dispersion, previously mixed with a sub-micron particulate adjuvant, onto a solid carrier while blending the mixture, followed by drying in mild conditions; the sub-micron particulate adjuvant is an O / W nanoemulsion stabilized with a polysaccharide. Improved dry powder vaccines are obtained in form of aggregated antigen-carrier particles, whereby the antigen is finely and firmly dispersed within the carrier; once in contact with the mucosal surface, the product quickly dissociates and releases the antigen component.
Owner:UNIV DEGLI STUDI DI PARMA
Helicobacter pylori mutant strain capable of stimulating immune response and construction method and application thereof
PendingCN111849850AHas adjuvant activityGood service for antigen presentationAntibacterial agentsBacterial antigen ingredientsVaccine PotencyVaccine efficacy
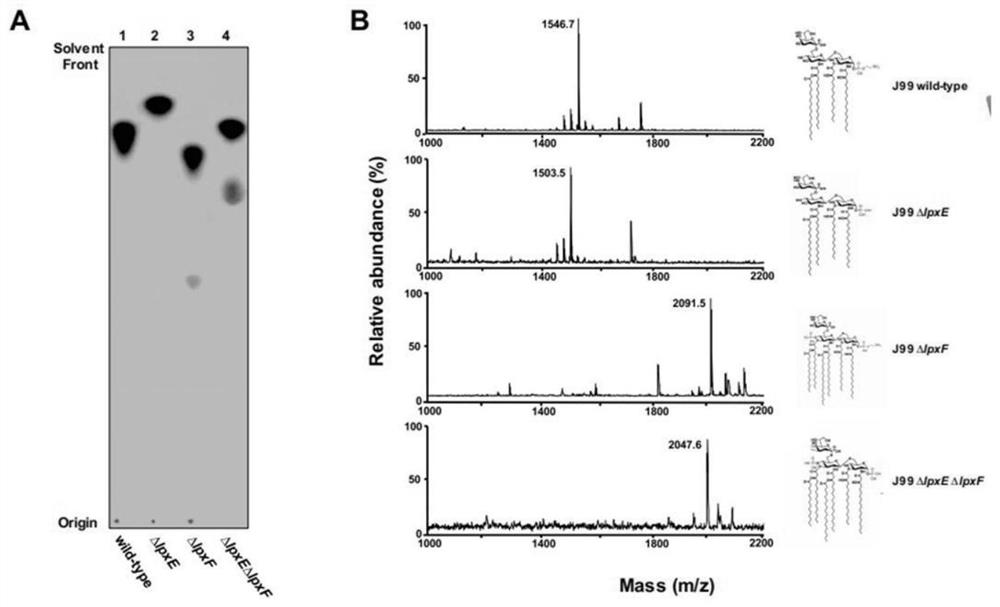
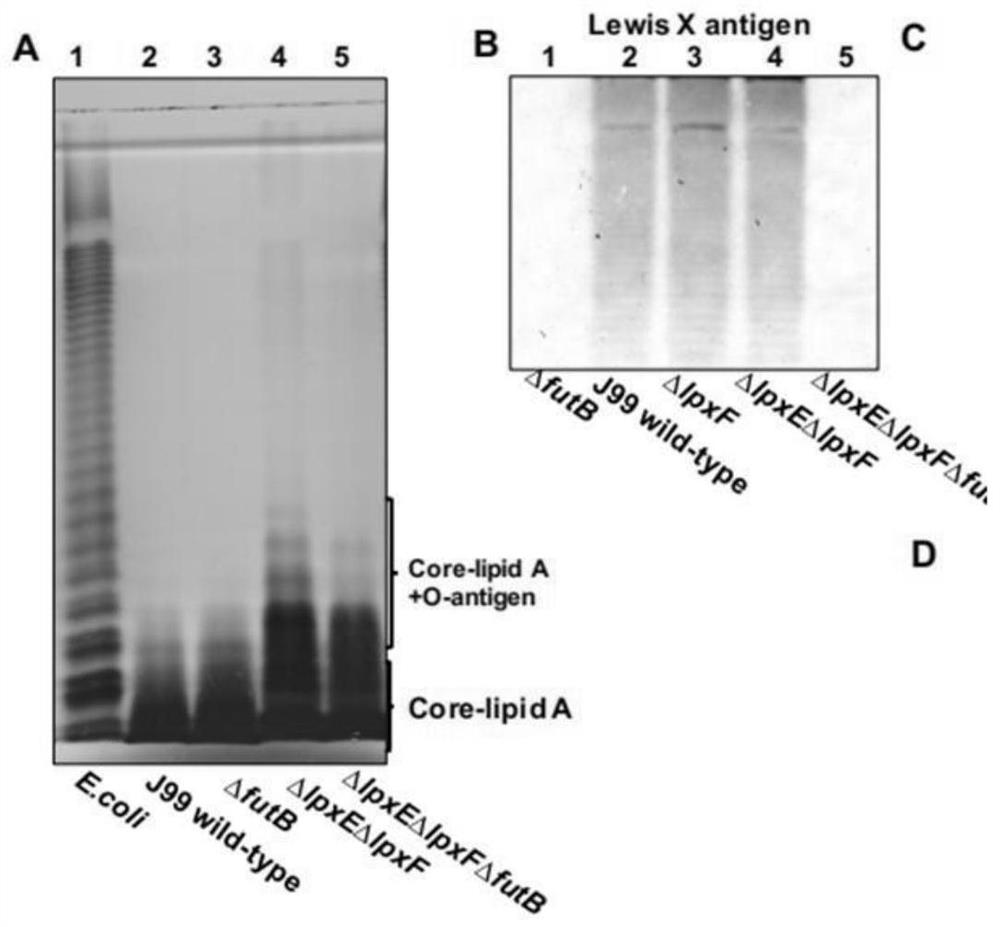
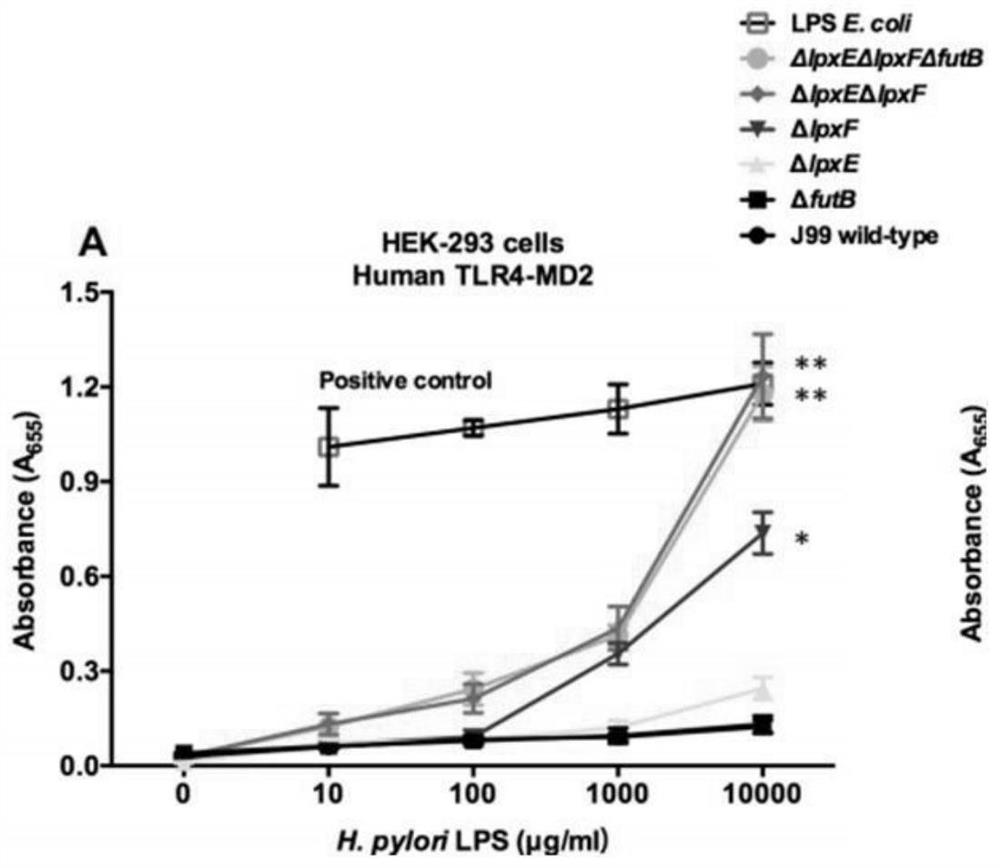
The invention provides a helicobacter pylori mutant strain capable of stimulating immune response and a construction method and application of the helicobacter pylori mutant strain, and belongs to thetechnical field of bioengineering. The mutant strain provided by the invention does not contain a gene futB for encoding glycosyltransferase in an O antigen synthesis process, meanwhile, a lipid A structure is modified, related synthesis genes lpxE and lpxF are knocked out, and the preservation number of the strain is CCTCC NO: M 2020028. The helicobacter pylori outer membrane vesicle vaccine canefficiently stimulate a host to generate immune response and secrete the outer membrane vesicle, so that the helicobacter pylori outer membrane vesicle has the characteristic of high vaccine efficacy. Meanwhile, the method can effectively enable helicobacter pylori lipid A to be recognized by TLR4 again, enables lipid A to have adjuvant activity, and can better serve antigen presentation. The method can be used for constructing novel helicobacter pylori antigen presenting plasmids, antigen protein is presented to periplasmids of bacteria or exposed to the surface of an outer membrane of the bacteria through different strategies, and therefore the efficiency of immunoreaction generated by host recognition of a target antigen is improved.
Owner:NANCHANG UNIV
Foot-and-mouth disease vaccine
ActiveUS20200078456A1Reduce frequencySsRNA viruses positive-senseViral antigen ingredientsDiseaseAdjuvant
Compositions for prevention of Foot and Mouth Disease (FMD) are provided, comprising an antigen component in the amount equivalent to 0.5-20 μg FMD virus and an adjuvant component comprising oil, an immunostimulatory oligonucleotide, and a polycationic carrier. Methods of using the composition, as well as the methods of reducing FMD persistence are also provided.
Owner:UNITED STATES OF AMERICA +1
Vaccination with immuno-isolated cells producing an immunomodulator
InactiveUS20060024318A1SsRNA viruses positive-sensePeptide/protein ingredientsAdjuvantVaccine antigen
The present invention relates to immuno-protected encapsulated cells producing an immunomodulator, for example GM-CSF (granulocyte-macrophage colony stimulating factor). The cells of the invention are particularly well adapted for providing an active adjuvant or immunomodulator, for example in the context of immunisation in humans and animals. These cells can be used for vaccination where they provide the immunomodulator in an active form, in a continuous, non-immunogenic manner in the immediate vicinity of the vaccine antigen(s). The invention also relates to a vaccine composition comprising immuno-protected encapsulated cells producing an immunomodulator and an antigenic component. The invention also relates to a kit comprising a cell as described and an antigenic component. The strategy of the invention is perfectly suited for both cancer immunotherapy and vaccination against infectious agents.
Owner:NOVIMMUNE
Nucleotide specific for bacillus coli O167 type and Sh.boydii 3 type O-antigen
InactiveCN1570114ABacterial antigen ingredientsMicrobiological testing/measurementNucleotideGene cluster
The invention provides an O-antigen specific nucleotide to Escherichia coli O167 and Shigella boydii3, which is the whole sequence of gene cluster controlling O-antigen synthesis in Escherichia coli O167 and Shigella boydii3, such as separating nucleotide sequence of SEQ ID NO:1 , Escherichia coli O167 being 12864 base, Shigella boydii3 being 12199 base, or one or several base substituted, defected or inserted SEQ ID NO:1 and at same time have same function with SEQ ID NO:1, also comprise oligonucleotide of glycosyltransferase gene and oligosaccharide disposing gene derived from O-antigen gene cluster of Escherichia coli O167 and Shigella boydii3. It is validated that oligonucleotide is highly specific to Escherichia coli O167 and Shigella boydii3 O-antigen. The invention also discloses Escherichia coli O167 and Shigella boydii3 detection and identification method using inventive oligonucleotide.
Owner:TIANJIN BIOCHIP TECH CO LTD
Connective tissue derived polypeptides
InactiveUS7919456B2Avoid problemsAvoid symptomsPeptide/protein ingredientsAntipyreticAnti arthriticAntigen
The present invention relates to compositions comprising one or more connective tissue derived polypeptides having a molecular weight of less than 30,000 Da that are capable of tolerising individuals to antigenic components of cartilage and prevent the appearance of and / or treat symptoms of arthritis and other musculoskeletal degenerative conditions. The present invention provides methods for recovering polypeptides having a molecular weight of less than 30,000 Da from connective tissue and having anti-arthritic or anti-inflammatory activity. The present invention further relates to compositions comprising a polypeptide containing an NC4 domain of collagen type IX alpha 1 chain or fragment thereof, having a molecular weight of less than 30,000 Da, where the polypeptide is capable of tolerising individuals to antigenic components of cartilage, preventing the appearance of arthritic symptoms, and / or treating the symptoms of arthritis.
Owner:PROTEOBIOACTIVES PTY LTD
Polyether sulfone membrane with separation/adsorption dual functions and preparation method thereof
The invention discloses a polyethersulfone membrane with separation / adsorption dual functions and a preparation method thereof, and solves the problems of single function, low purification precision and the like in the field of vaccine purification in the existing membrane technology. According to the technical scheme, adsorption functional particles are uniformly dispersed in a polyethersulfone membrane matrix, cells and a virus culture solution are in contact with the adsorption functional particles in the membrane after being filtered through membrane holes, and target antigen components are adsorbed and enriched; a hydrophilic macromolecule is adopted as a pore-forming additive, so that the hydrophilic and separation properties of the membrane are synchronously improved; the polyethersulfone membrane for vaccine purification is obtained.
Owner:TIANJIN POLYTECHNIC UNIV
Fish broad-spectrum vibrio subunit vaccine and preparation method
InactiveCN102512674BHigh homologyImprove the immunityAntibacterial agentsMicroorganism based processesVibrio anguillarumFlagellin
The invention discloses a broad-spectrum vibrio subunit vaccine for preventing fish pathogenic vibrio infection and a preparation method, belonging to the technical field of biology. The fusion protein OmpK-FlaA of an outer membrane protein OmpK with a conserved structure and a flagellin protein FlaA on the vibrio parahaemolyticus surface serves as an antigen component of the vaccine. The method is characterized by comprising the following steps of: superposing and extending Ppolymerase chain reaction (PCR) to the ompK and flaA genes of the vibrio parahaemolyticus to obtain a fusion protein gene flaA-ompK; constructing flaA-ompK-pET-28a recombinant plasmids; and obtaining the fusion FlaA-OmpK with high purity through exogenous induction expression and purification. The vaccine prepared bythe invention is safe and nontoxic and has no side effects; injection immune can be adopted; and the immune can also be realized by taking enteric microsphere vaccine orally; cross immunity protection can be acted to fish pathogenic vibrio (vibrio parahaemolyticus, vibrio alginolyticus, vibro harveyi, vibrio anguillarum and vibrio vulnificus).
Owner:FUZHOU UNIV
Live salmonella vaccine and methods to prevent fowl typhoid
InactiveUS20170049872A1PeptidesAntibody medical ingredientsVirulent characteristicsRecombinant vaccines
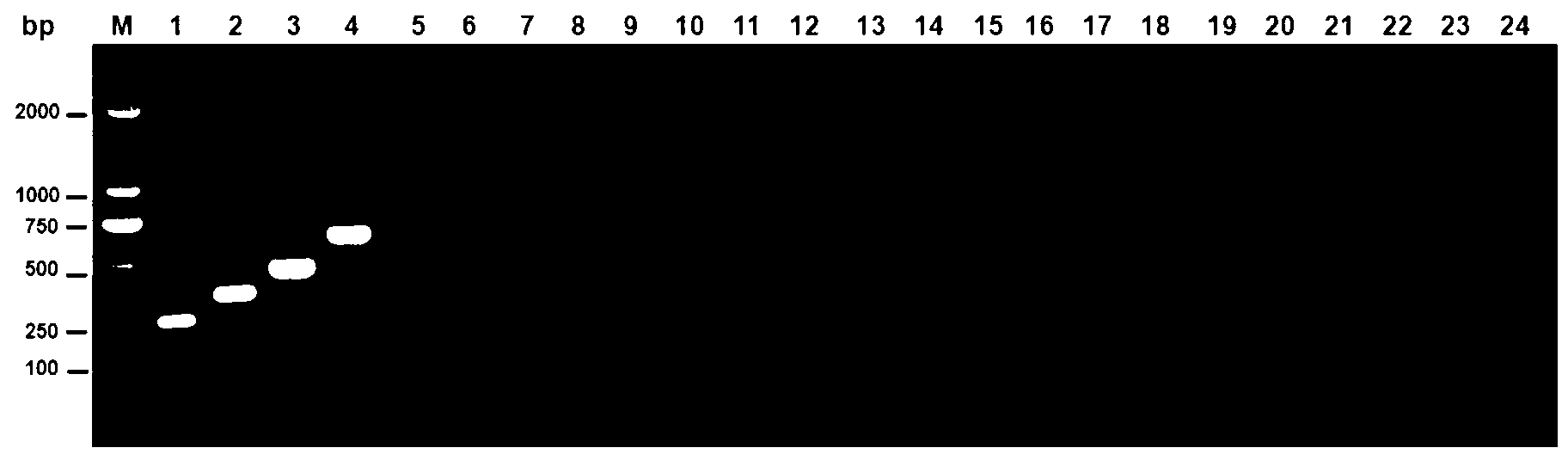
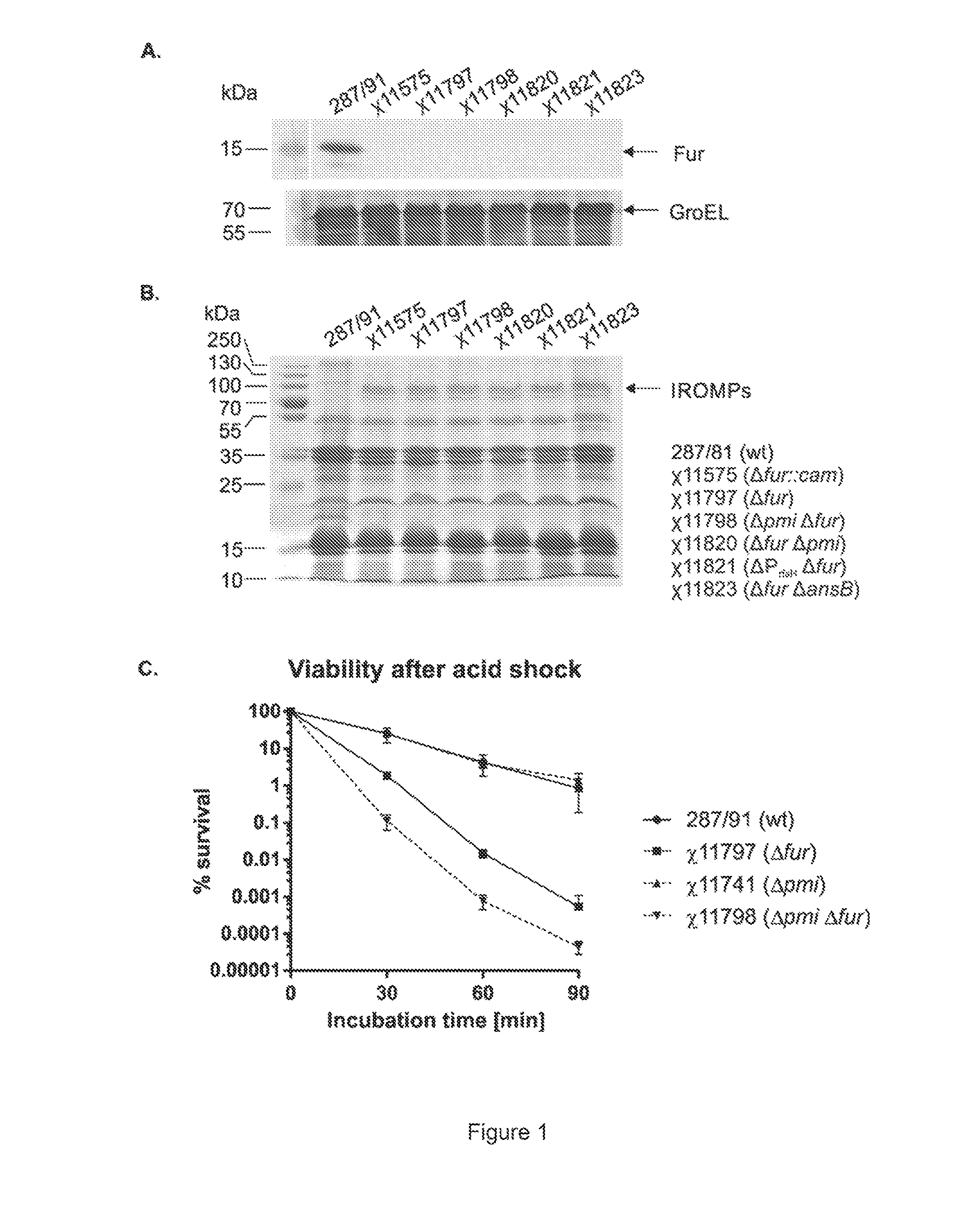
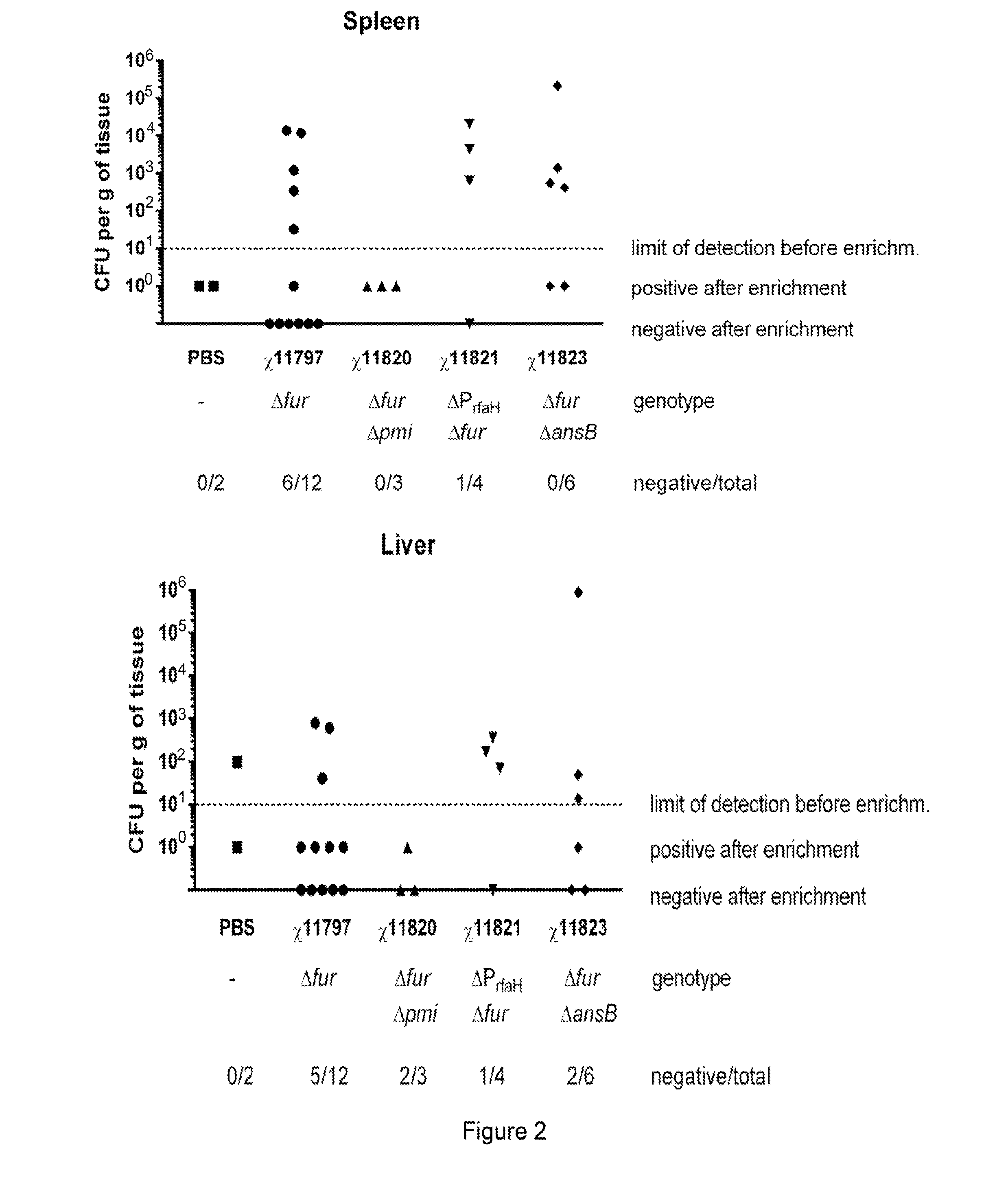
We constructed S. Gallinarum strains deleted for the global regulatory gene fur (FIG. 1) and evaluated their virulence and protective efficacy in Rhode Island Red chicks and Brown Leghorn layers. The fur deletion mutant was a virulent and, when delivered orally to chicks, elicited excellent protection against lethal S. Gallinarum challenge. We also examined the effect of a pmi mutant and a combination of fur deletions with mutations in the pmi and rfaH genes, which affect O-antigen synthesis, and ansB, whose product inhibits host T cell responses. The ΔAfur Δpmi and Δfur ΔansB double mutants were attenuated, but not protective when delivered orally to chicks. However, a Δpmi Δfur strain was substantially immunogenic when administrated intramuscularly. Altogether our results show that the fur gene is essential for virulence of S. Gallinarum and the fur mutant is effective as a live recombinant vaccine against fowl typhoid.
Owner:ARIZONA STATE UNIVERSITY
Escherichia coli O1, O2, O18 and O78 serotype detection kit and detection method thereof
ActiveCN103305627BCross reactionReduce sensitivityMicrobiological testing/measurementMicroorganism based processesESCHERICHIA COLI ANTIGENEscherichia coli serotype
The invention belongs to the technical field of biology detection and relates to an escherichia coli O1, O2, O18 and O78 serotype detection kit and a detection method thereof. Forward primers of a primer group of the kit are highly conservative escherichia coli O antigen combined relative gene sequences, and backward primers are four serotype specific primers designed on the basis of four O1, O2, O18 and O78 serotype escherichia coli O antigen synthesis relative genes. The detection kit containing the primer group and the detection method of the detection kit have the advantages of being fast, sensitive, specific, low in cost, easy to operate and capable of well overcoming the shortcomings in the current escherichia coli O1, O2, O18 and O78 serotype detection method, can meet the requirements for the current escherichia coli O1, O2, O18 and O78 serotype detection, can be popularized and used easily in a wide range and have wide market prospect and large economic benefit.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Cra4s1 gene, encoded cra4s1 protein, and application
PendingUS20220204570A1Manufactured successfullyAntibacterial agentsOrganic active ingredientsPharmaceutical drugMicrobiology
Provided are a cra4S1 gene, an encoded cra4S1 protein, and a vaccine or drug containing the cra4S1 protein or a fragment thereof. A nucleotide sequence of the cra4S1 gene is represented by SEQ ID NO. 1. The vaccine combines the specific target of an outer membrane protein of Porphyromonas gingivalis and the antigen component of the bacterial conserved region, which has an immune prevention and protection effect on the body.
Owner:ZHENJIANG YANGTZE GREEN BIOTECHNOLOGY CO LTD
Pseudomonas antigens and antigen combinations
An effective Pseudomonas aeruginosa vaccine may require one or several antigenic components, and so various antigens of P. aeruginosa are identified for use in immunization. These polypeptides may optionally be used in combination with other nosocomial antigens.
Owner:OSPEDALE SAN RAFFAELE SRL +1
Fusobacterium necrophorum antigen, preparation method thereof, and vaccine prepared by adopting fusobacterium necrophorum antigen
PendingCN106237321AInhibit side effectsRetain effective antigenic componentsAntibacterial agentsBacterial antigen ingredientsAntigenEndotoxin removal
The invention relates to the field of fusobacterium necrophorum vaccines, and in particular relates to a fusobacterium necrophorum antigen, a preparation method of the fusobacterium necrophorum antigen, and a vaccine prepared by adopting the fusobacterium necrophorum antigen. The preparation method of the fusobacterium necrophorum antigen comprises the following steps: carrying out enzymolysis on fusobacterium necrophorum by adopting pancreatin, carrying out centrifugation to obtain a supernate, and carrying out inactivation treatment on the supernate to obtain the fusobacterium necrophorum antigen. Through multiple times of experiments, the method of lysing the thallus by adopting pancreatin is adopted, the obtained lysed product is separated, so that endotoxin and other invalid ingredients are removed, and the effective antigenic components are reserved; the vaccine prepared by adopting the fusobacterium necrophorum antigen obtained through the inactivation treatment overcomes the defects that after the inoculation of the existing vaccine, hardening easily forms at the injected part, and the side reactions including local swelling and pain, heating and the like occur; and the prepared vaccine is stable and reliable in immune performance.
Owner:JL TEYAN BIOLOGICAL TECH LIMITED LIABILITY +1
A method for separation and purification of multiple antigenic components of pertussis
ActiveCN108570098BGuaranteed stabilityHigh recovery rateDepsipeptidesPeptide preparation methodsPertussis toxinCulture fluid
The present invention relates to a method for separating and purifying multiple antigenic components of pertussis, wherein the various antigens are pertussis toxin (PT) antigen, filamentous hemagglutinin (FHA) antigen and pertussis adhesin (PRN) antigen, and the The method comprises the following steps: (1) fermenting and culturing the pertussis strain, and harvesting the culture supernatant and bacterial precipitate respectively; (2) separating and purifying PT antigen from the culture supernatant, and separating and purifying PRN antigen and FHA antigen from the bacterial precipitate.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com