Fusobacterium necrophorum antigen, preparation method thereof, and vaccine prepared by adopting fusobacterium necrophorum antigen
A fusobacterium necroptosis antigen technology, applied in the field of fusobacterium necroptosis antigen and its preparation method and prepared vaccine, can solve the problem of vaccine containing endotoxin, achieve stable immune performance, stable and reliable immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
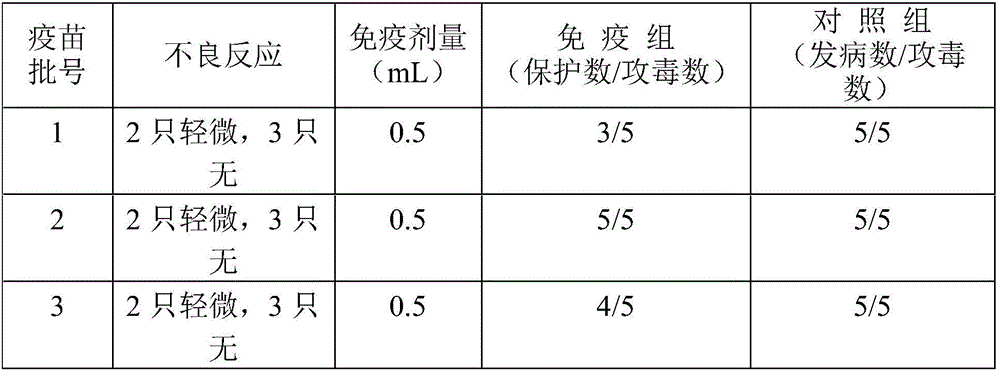
Embodiment 1
[0041] A Fusobacterium necroticum antigen prepared by:
[0042] Bovine-derived Fusobacterium necroptosis was inoculated in anaerobic medium thioglycollate medium, the inoculum amount of Fusobacterium necroptosis was 5%, the culture temperature was 39°C, and the culture time was 24 hours, and the necrosis in the logarithmic growth phase was obtained by culture. Fusobacterium;
[0043] Centrifuge the cultured Fusobacterium necroticus bacterial liquid, and use a continuous flow centrifuge for centrifugation. When using a continuous flow centrifuge to centrifuge the culture of Fusobacterium necroticus, the centrifuge rotor and infusion tube need to be sterilized by boiling or autoclaving. Install the rotor and infusion tube;
[0044] The flow rate of the bacterial liquid into the continuous flow centrifuge is about 100mL / min, the centrifugation speed is 12000rpm, and the centrifugation is 5min to obtain bacterial sediment;
[0045] The bacterial cell precipitate is resuspended w...
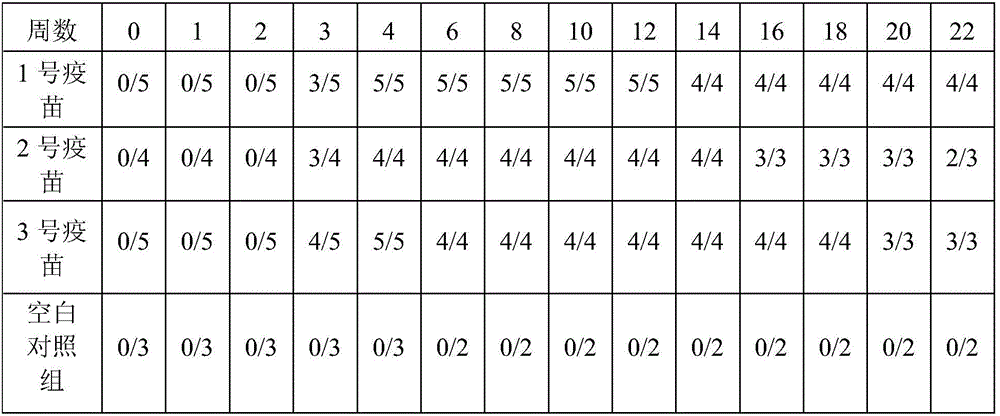
Embodiment 2
[0052] A Fusobacterium necroticum antigen prepared by:
[0053] Bovine-derived Fusobacterium necroptosis was inoculated in the anaerobic medium brain-heart extract broth medium, the inoculum amount of Fusobacterium necroptosis was 8%, the temperature of cultivation was 37°C, and the cultivation time was 12h, and the culture was obtained in the logarithmic growth phase. Fusobacterium necroptosis;
[0054] Centrifuge the cultured Fusobacterium necroticus bacterial liquid, and use a continuous flow centrifuge for centrifugation. When using a continuous flow centrifuge to centrifuge the culture of Fusobacterium necroticus, the centrifuge rotor and infusion tube need to be sterilized by boiling or autoclaving. Install the rotor and infusion tube;
[0055] The flow rate of the bacterial liquid into the continuous flow centrifuge is about 110mL / min, the centrifugation speed is 12000rpm, and the centrifugation is 5min to obtain bacterial sediment;
[0056] The bacterial cell precipi...
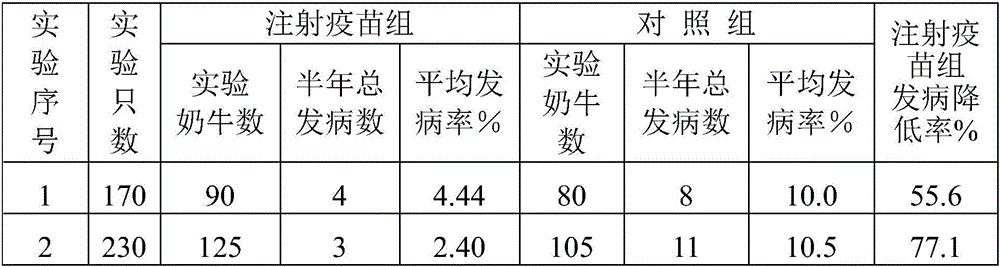
Embodiment 3
[0063] A Fusobacterium necroticum antigen prepared by:
[0064] Bovine-derived Fusobacterium necroptosis was inoculated in the anaerobic medium brain-heart extract broth medium, the inoculation amount of Fusobacterium necroptosis was 10%, the temperature of cultivation was 35°C, and the cultivation time was 8 hours, and the culture was obtained in the logarithmic growth phase. Fusobacterium necroptosis;
[0065] Centrifuge the cultured Fusobacterium necroticus bacterial liquid, and use a continuous flow centrifuge for centrifugation. When using a continuous flow centrifuge to centrifuge the culture of Fusobacterium necroticus, the centrifuge rotor and infusion tube need to be sterilized by boiling or autoclaving. Install the rotor and infusion tube;
[0066] The flow rate of the bacterial liquid into the continuous flow centrifuge is about 120mL / min, the centrifugation speed is 13000rpm, and the centrifugation is 3min to obtain bacterial sediment;
[0067] The bacterial cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com