Patents
Literature
68 results about "Post vaccination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
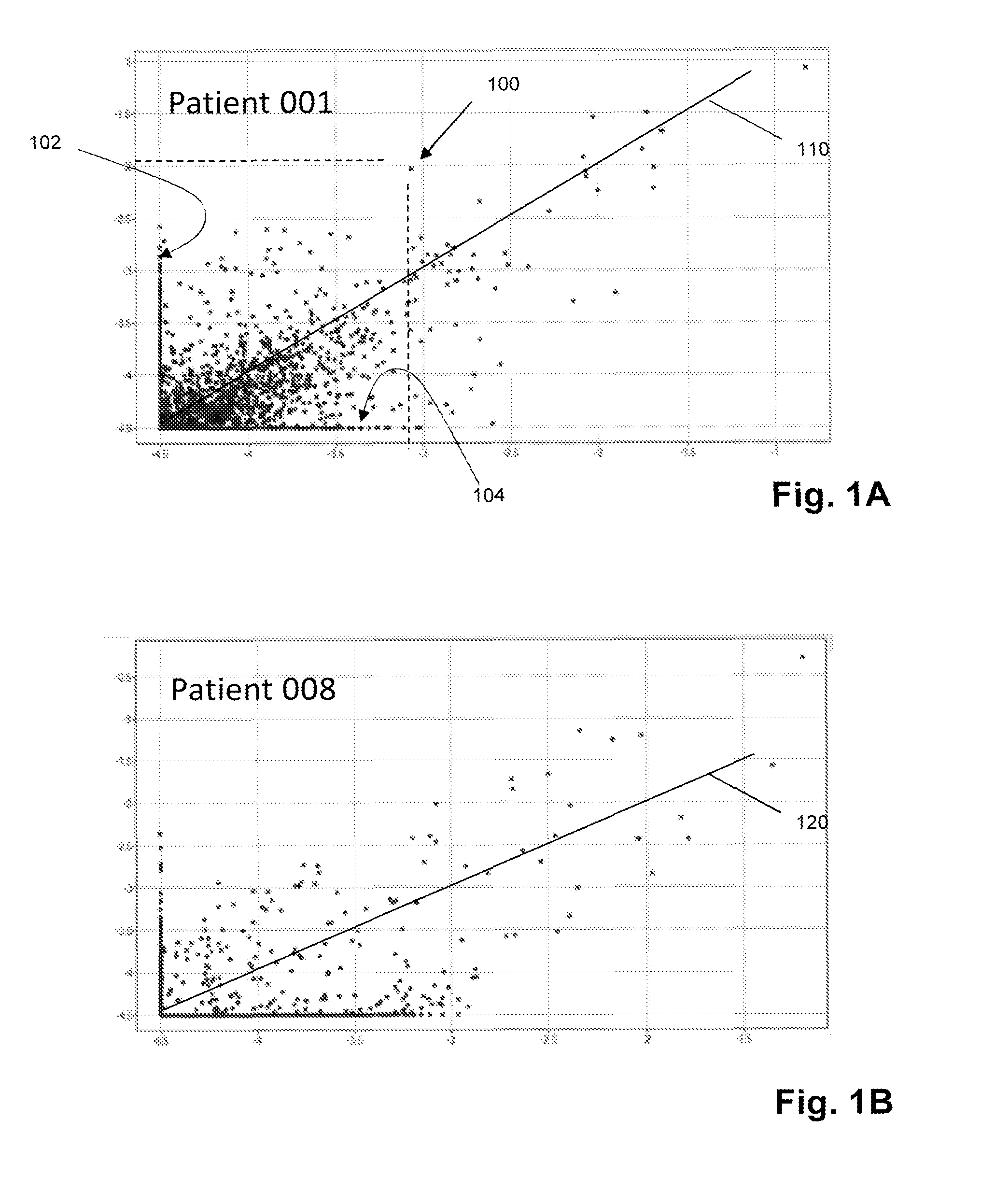
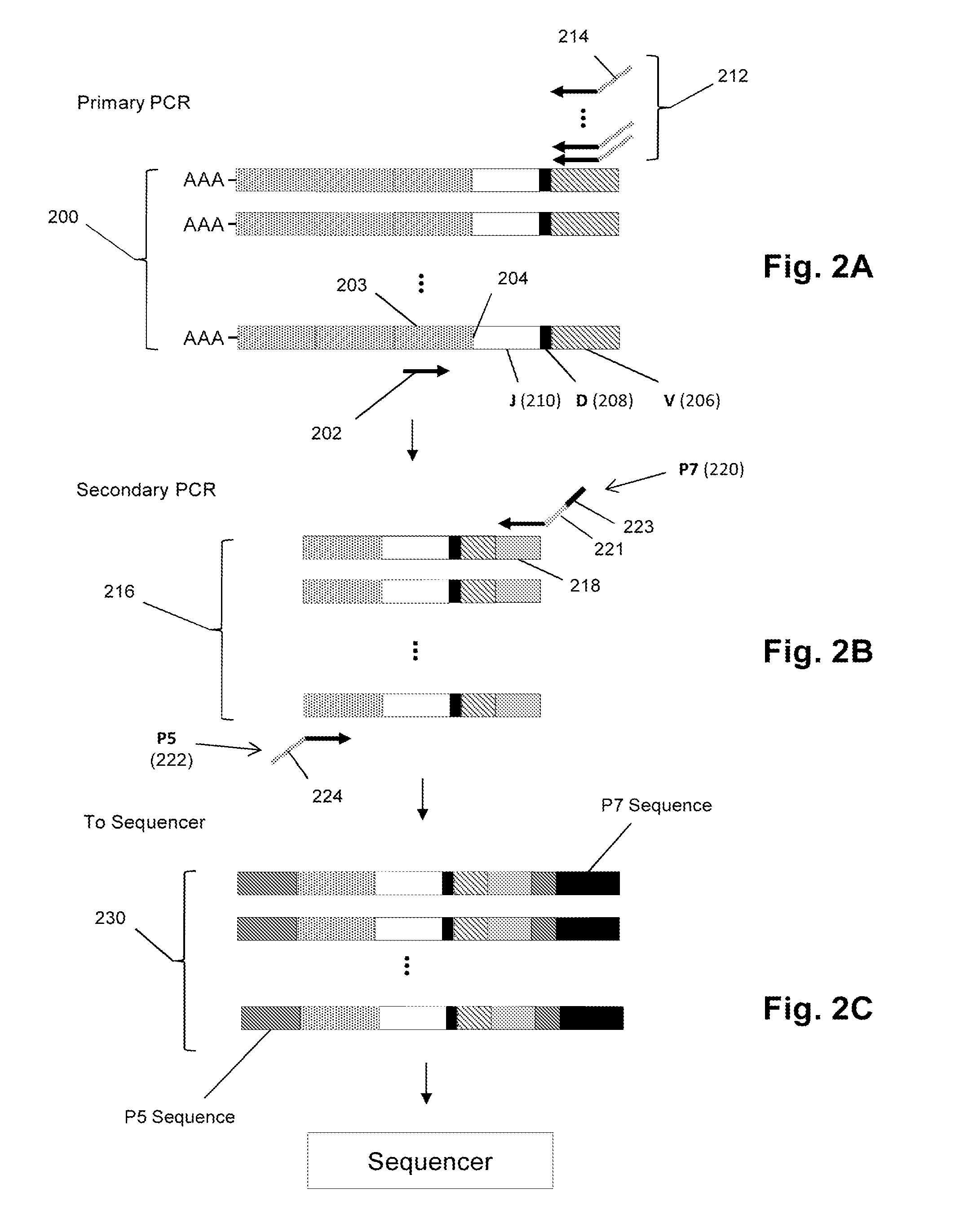
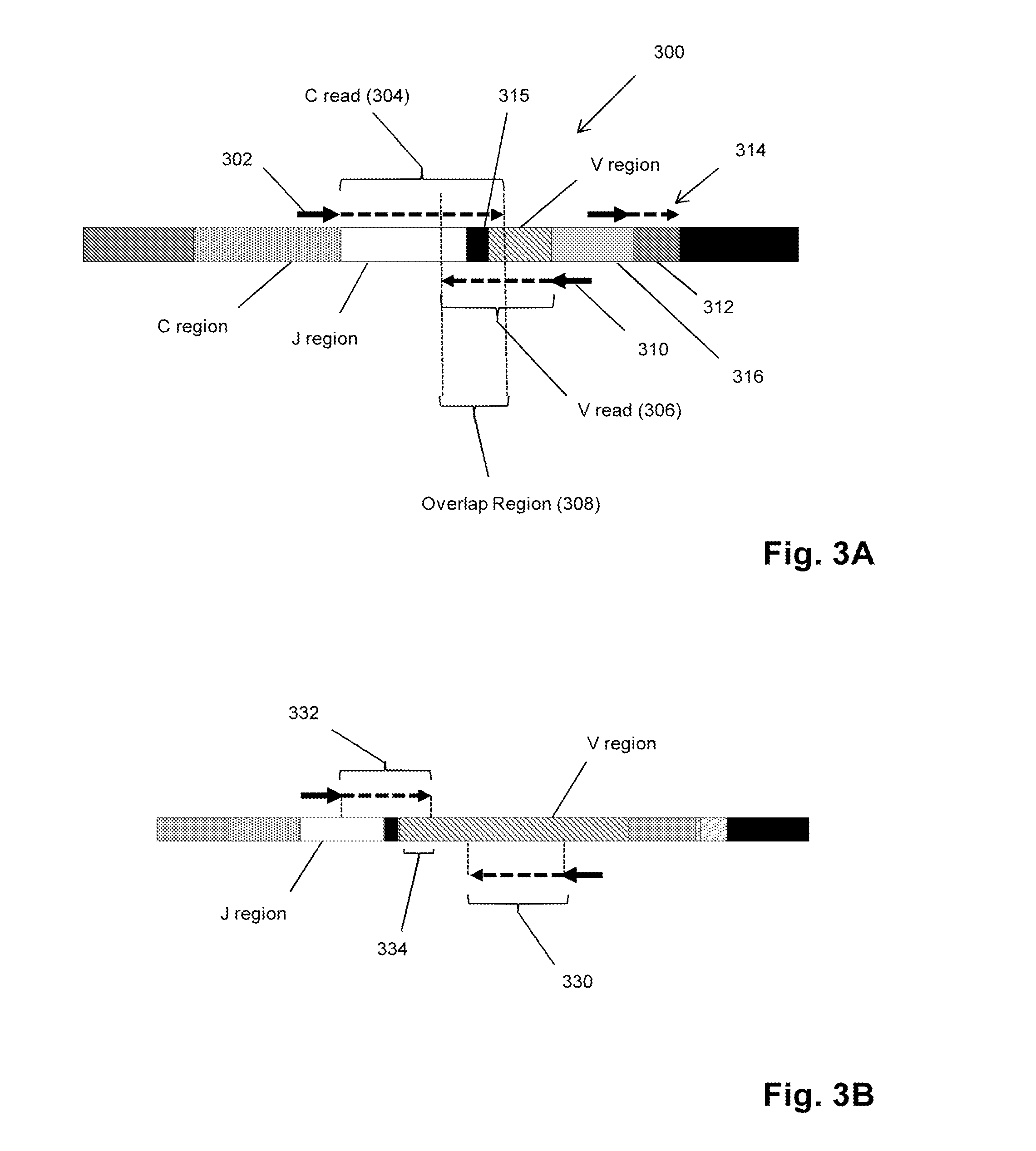
Sequence-based measures of immune response
InactiveUS20140356339A1Evaluate effectivenessBiocideMicrobiological testing/measurementTreatment successImmunotherapy
The invention is directed to methods of measuring an immune response by comparing sequence-based clonotype frequency data from successively measured clonotype profiles. In particular, the invention includes immunotherapies of cancers, such as lymphomas, that include sensitive pre- and post-vaccination sequence-based measurements of changes in a patient's immune repertoire, thereby providing a sensitive measure of the likelihood of treatment success.
Owner:ADAPTIVE BIOTECH
Monitoring immune responsiveness to cancer vaccination
InactiveUS20150038346A1Microbiological testing/measurementDisease diagnosisPost vaccinationMonitoring immune
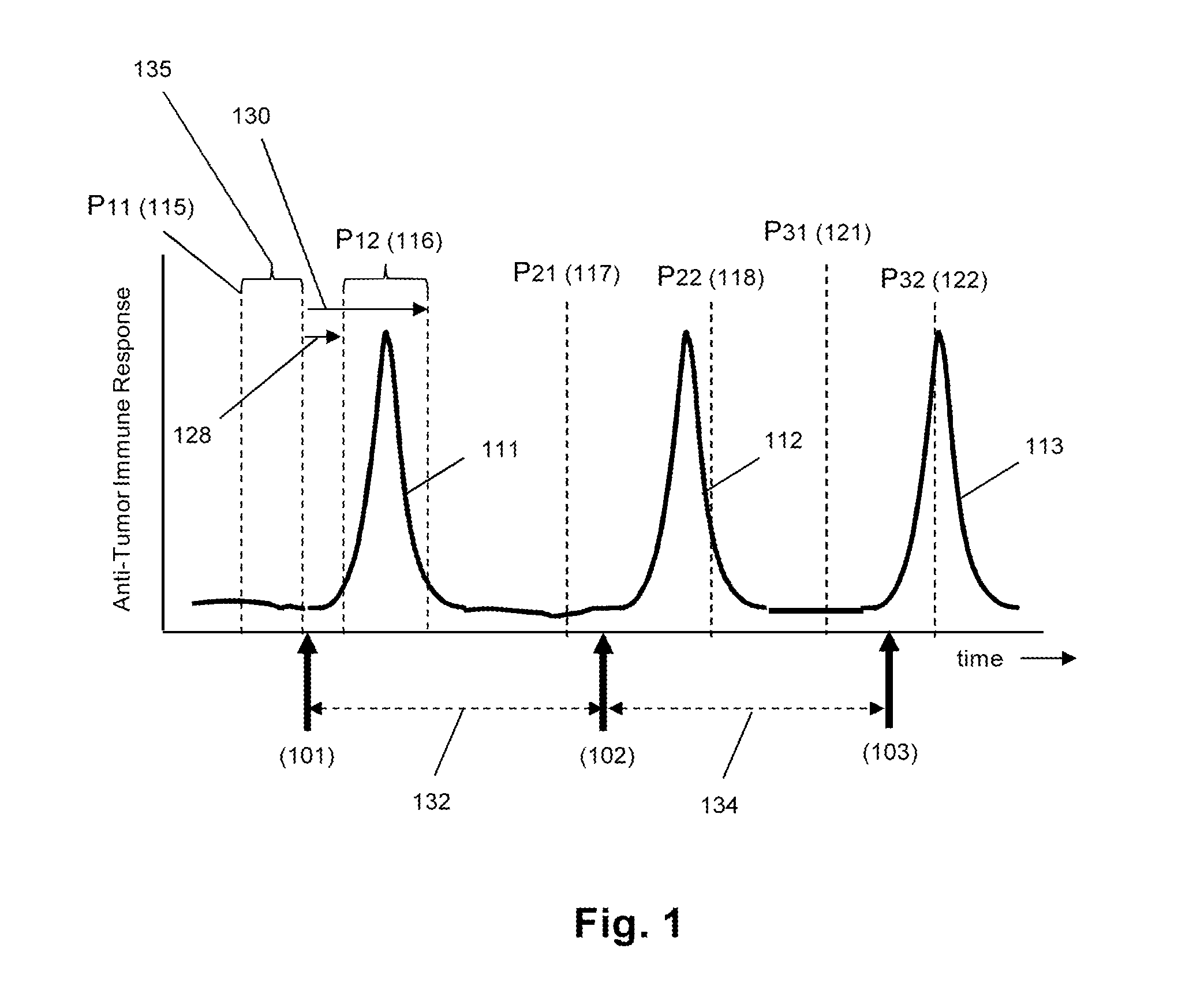
The invention is direct to a method for determining a cancer patient's immune responsiveness to anti-cancer vaccination. In one aspect, for each of a plurality of vaccinations, pairs of clonotype profiles are obtained, one immediately prior to vaccination and one during the period of peak immune response, usually within two to twenty days after the vaccination. Responsiveness is correlated to successive increases in identical clonotypes within each pair of clonotype profiles in at least two successive vaccinations.
Owner:ADAPTIVE BIOTECH
Annexin a2 as immunological target
InactiveUS20110293608A1Prevent intrusionCompound screeningApoptosis detectionAntigenCalcium-dependent phospholipid binding
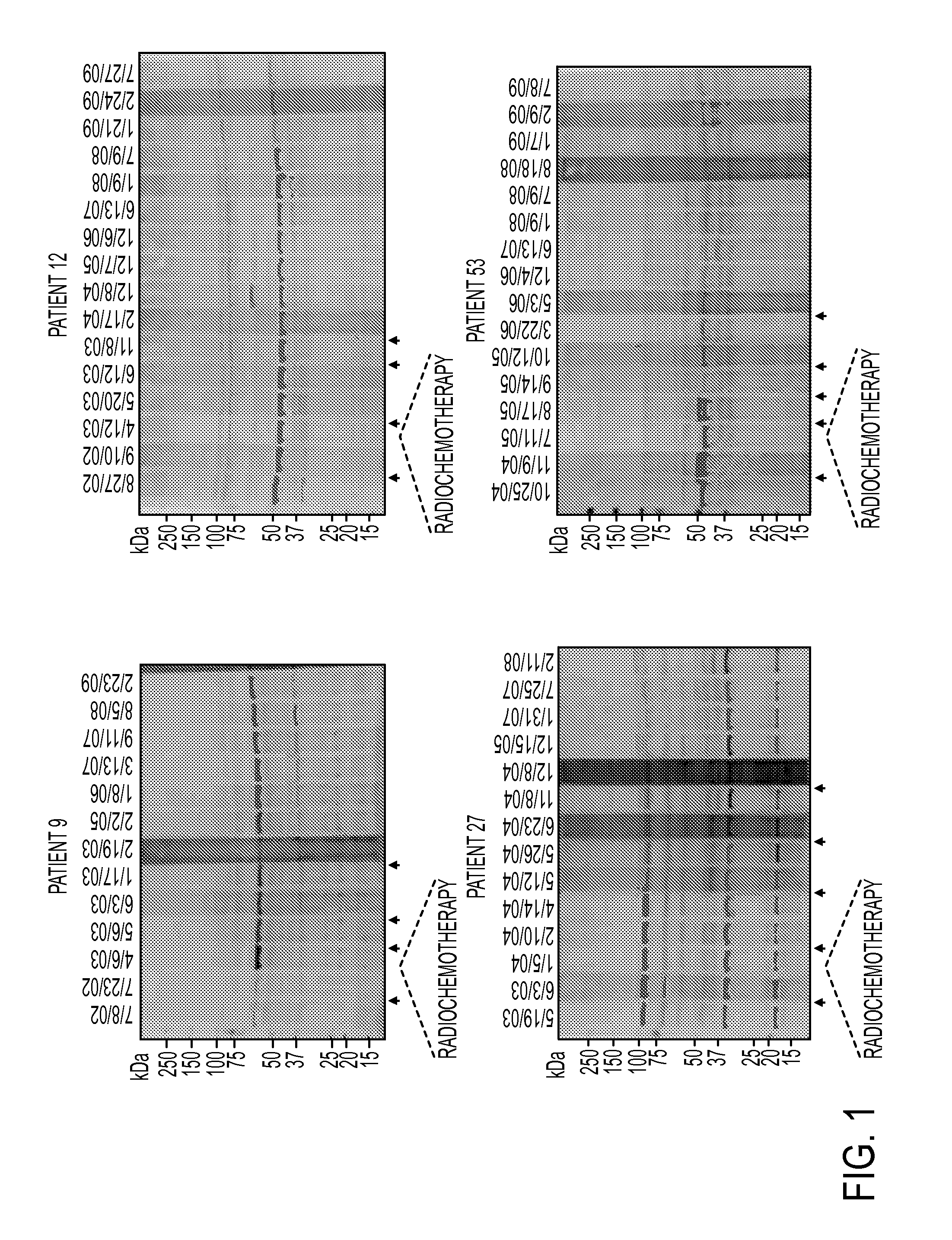
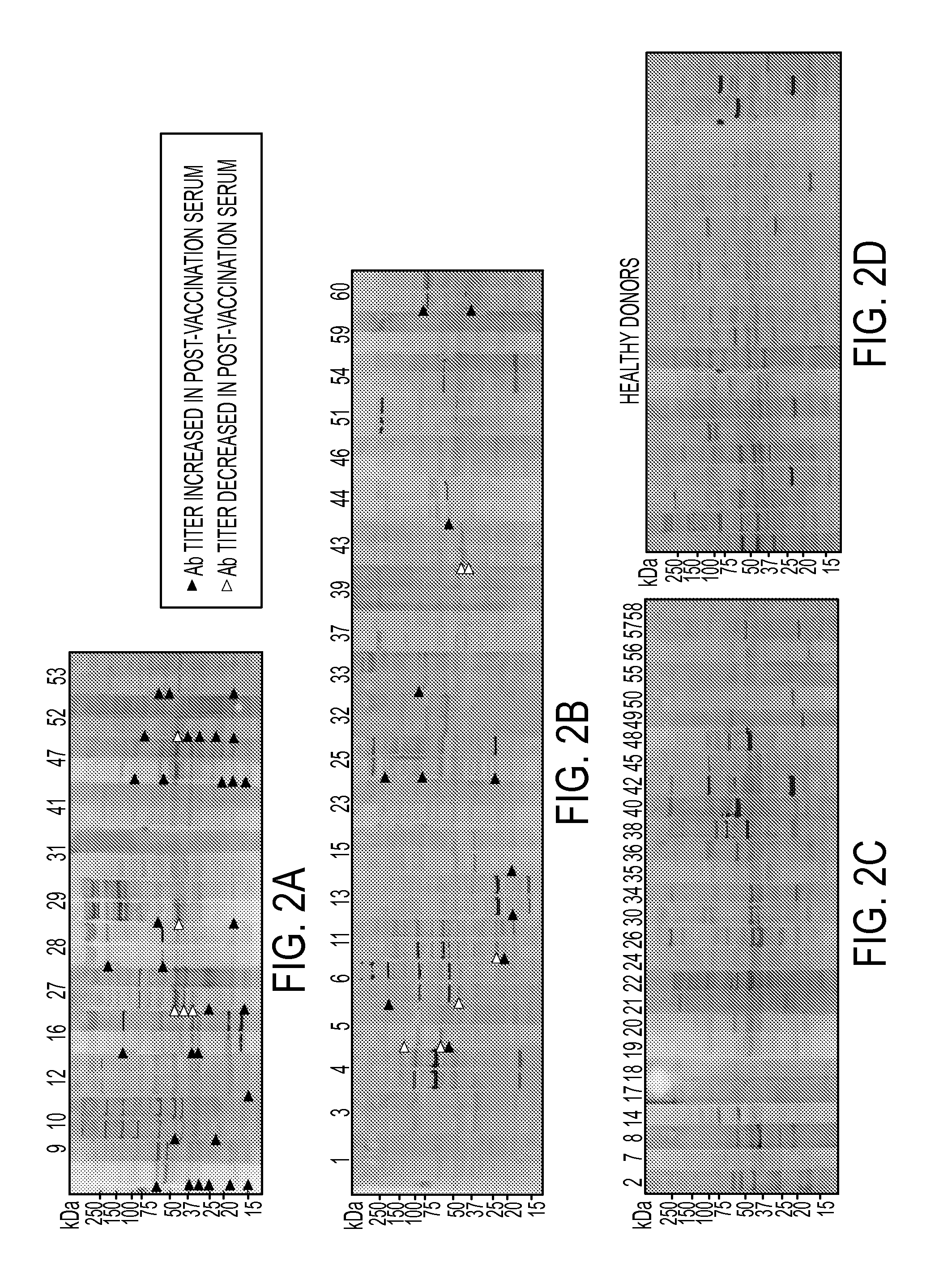
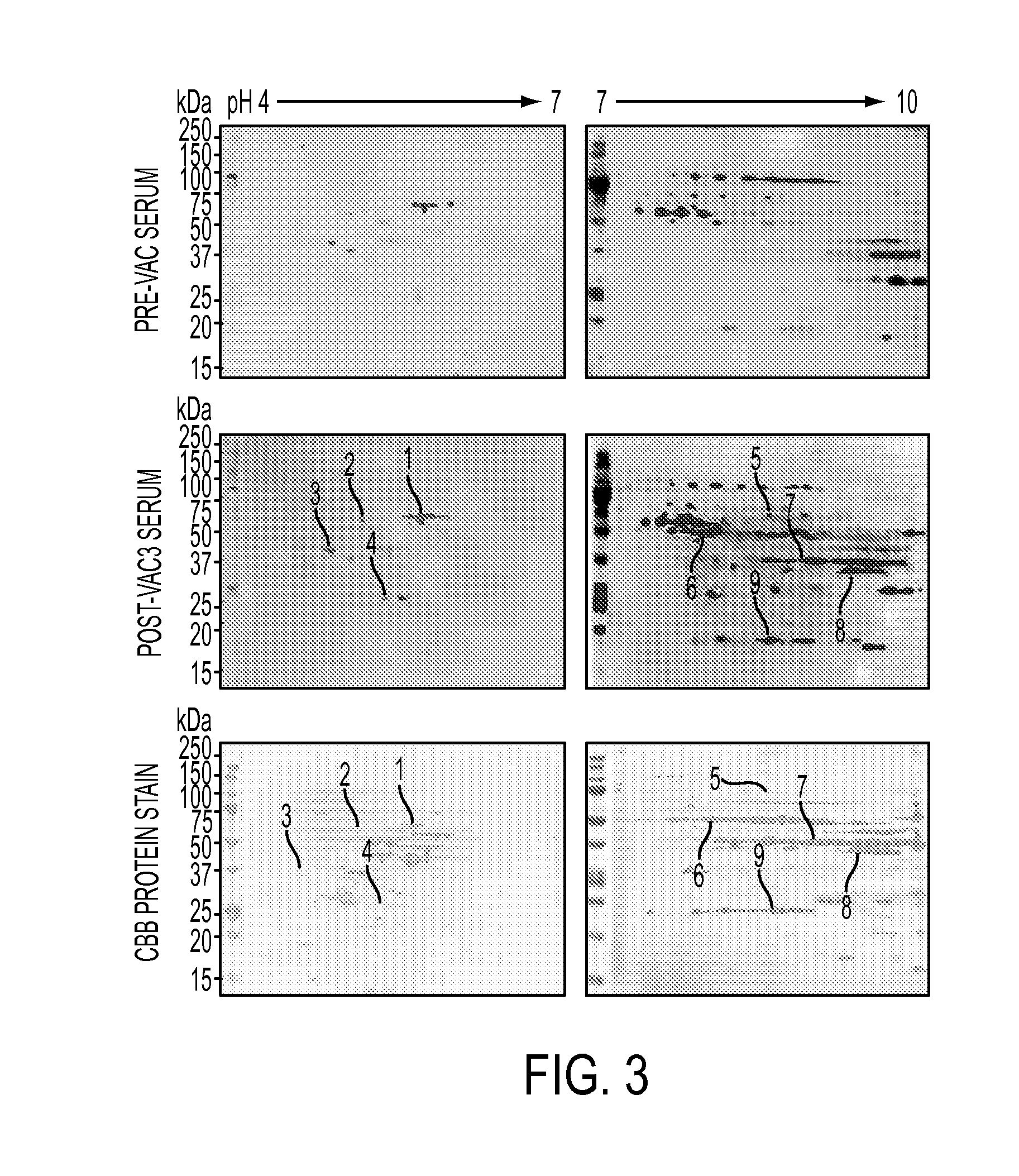
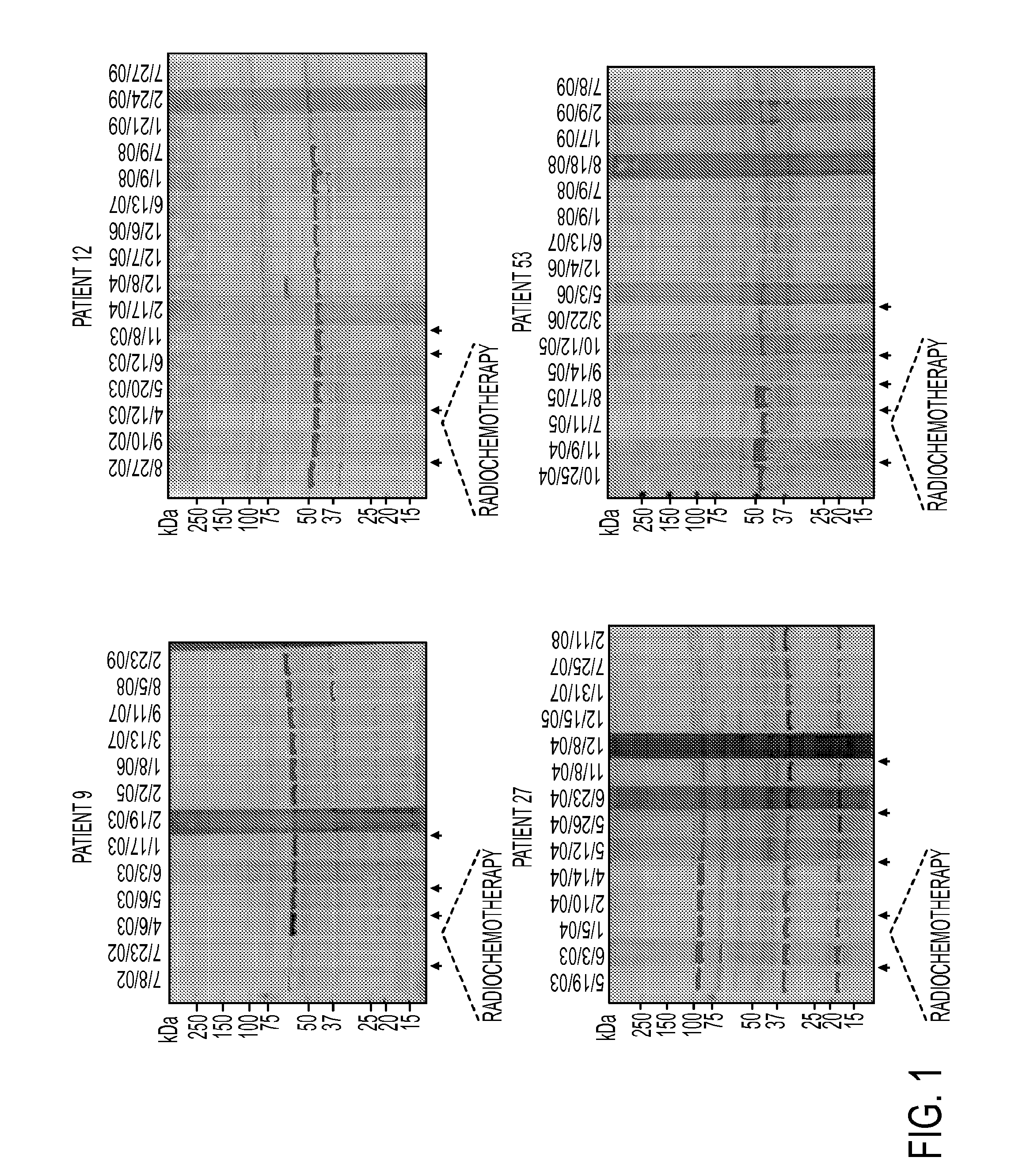
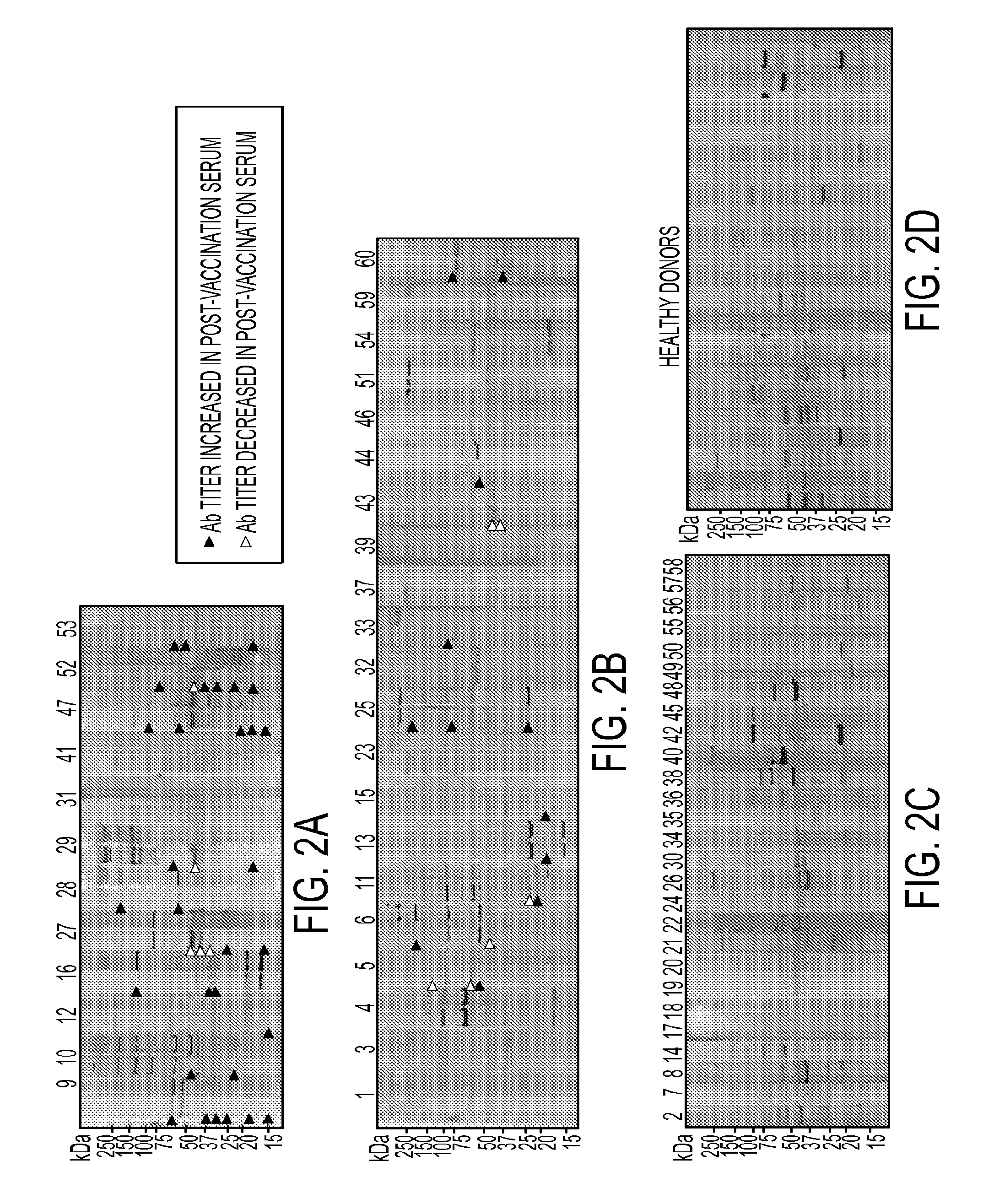
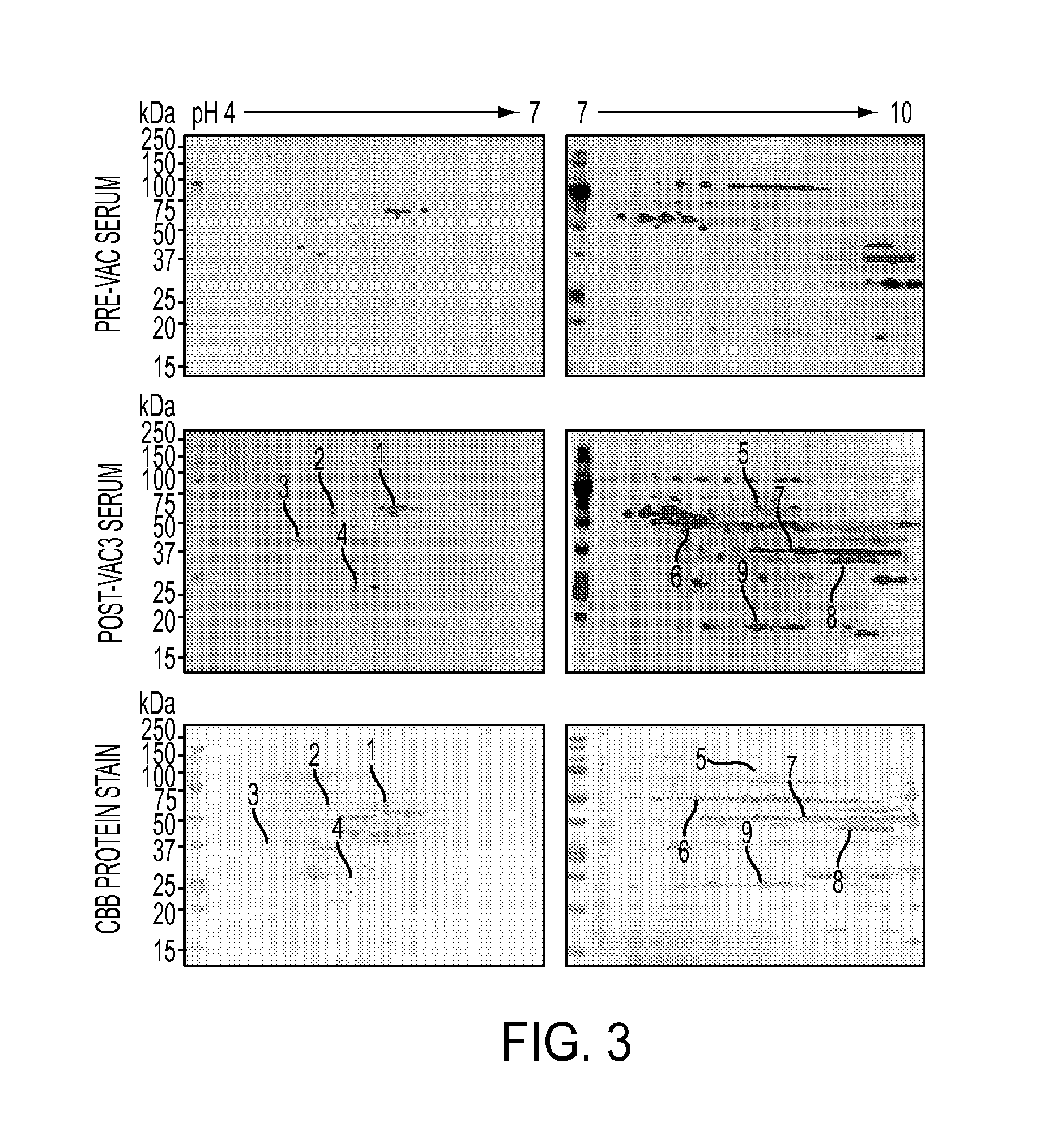
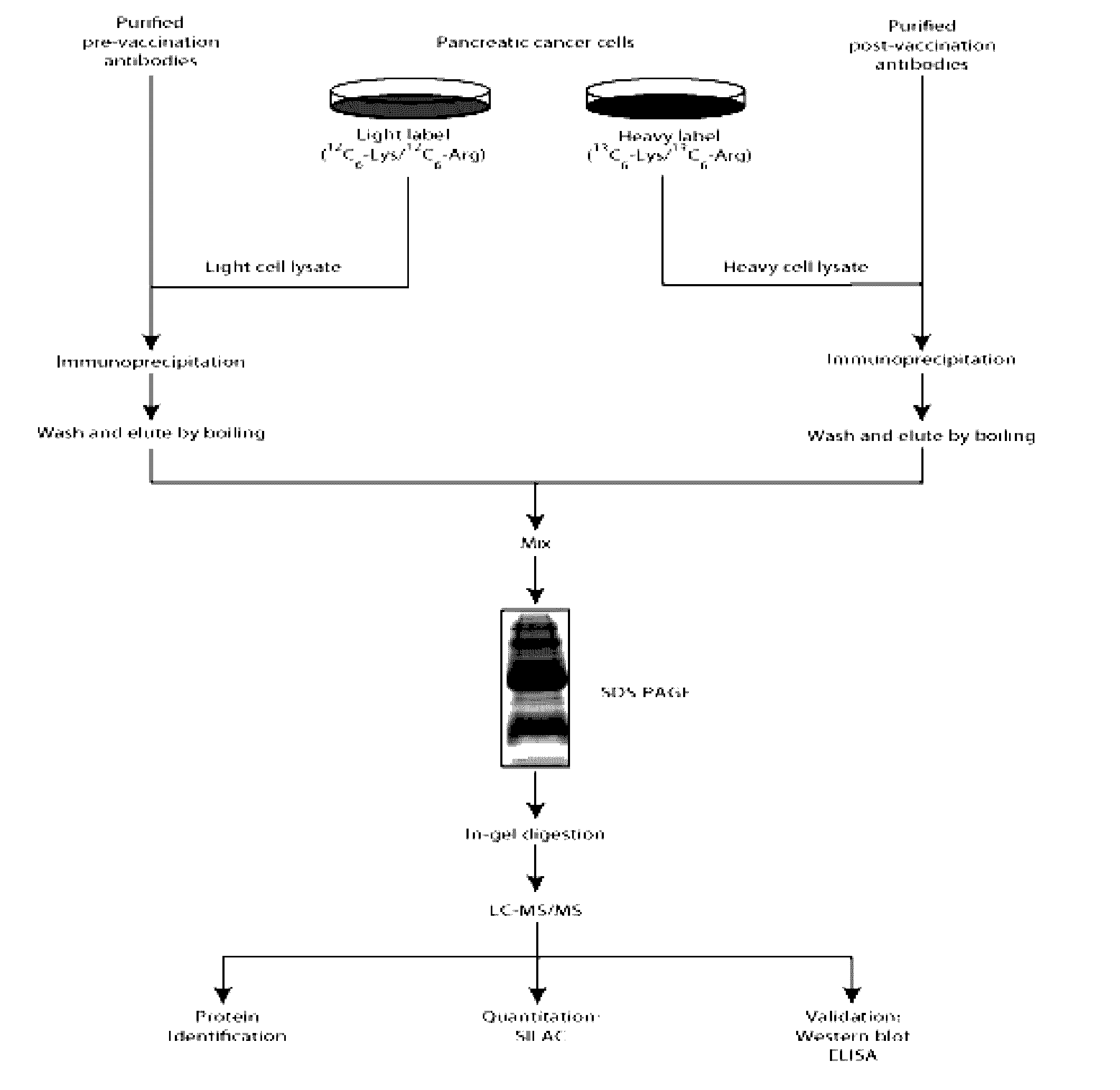
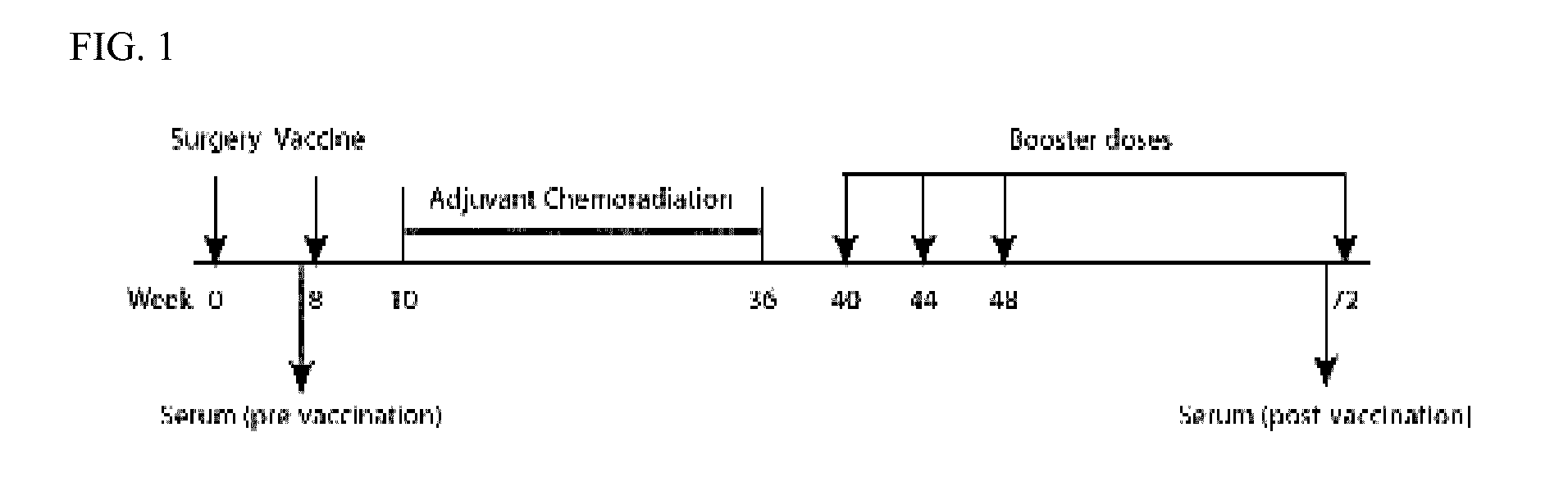
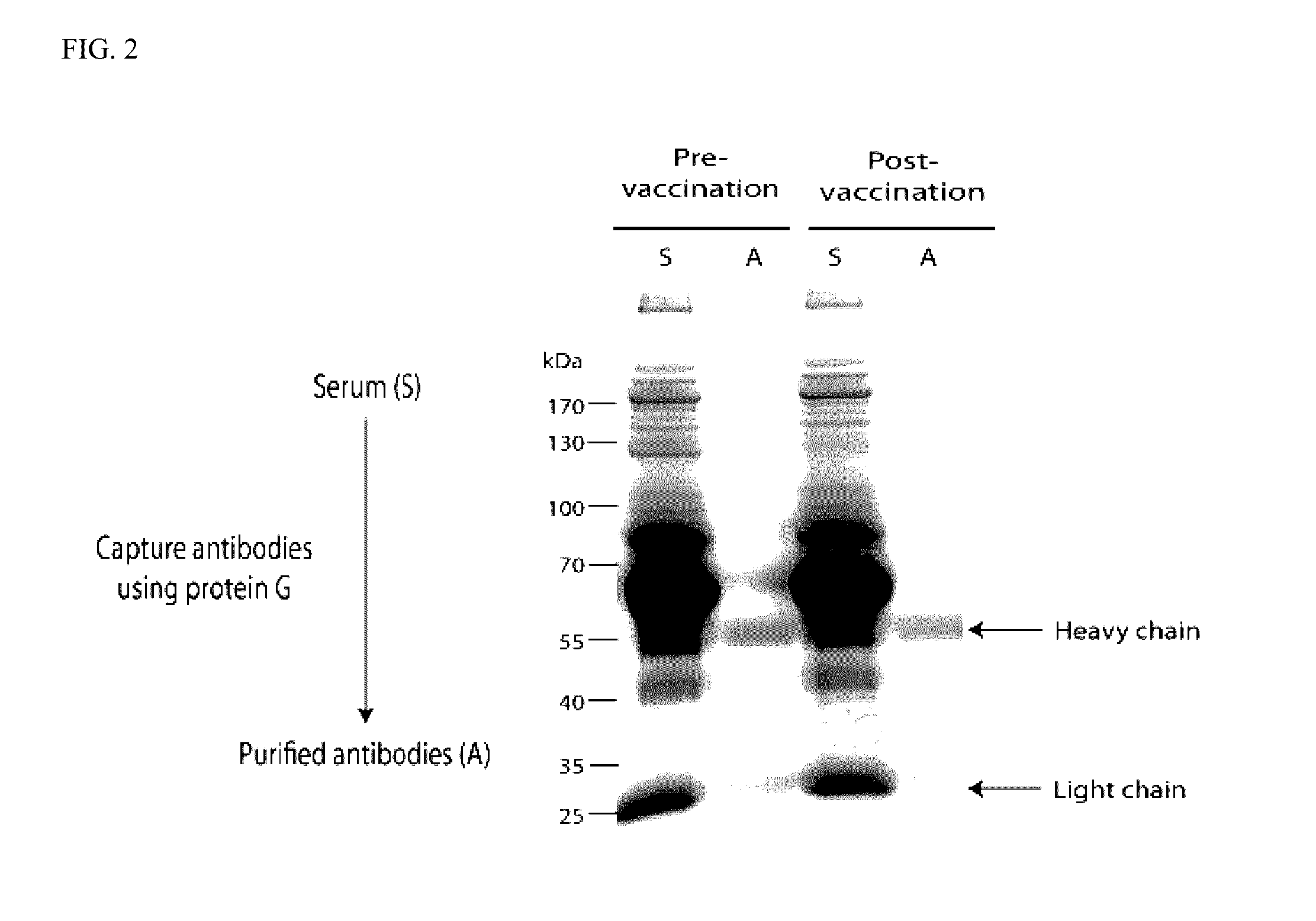
AnnexinA2 (ANXA2), a member of the Annexin family of calcium-dependent, phospholipid binding proteins, is one of a panel of identified antigens recognized by the post-vaccination sera of patients who demonstrated prolonged disease-free survival following multiple vaccinations. AnnexinA2 is abundantly expressed in pancreatic adenocarcinomas and cell surface / membrane AnnexinA2 increases with the progression from premalignant lesions to invasive pancreatic adenocarcinomas. The cytoplasmic to cell surface translocation of AnnexinA2 expression is critical for pancreatic cancer cell invasion. In addition, phosphorylation of AnnexinA2 at Tyrosine 23 is important for its localization to the cell surface and for the invasion of pancreatic cancer cells. Finally, loss of AnnexinA2 leads to the loss of the Epithelial-Mesenchymal Transition.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Rapid Detection of Post-Vaccination Antibody Response
InactiveUS20100322823A1Component separationBiological material analysisAbsorbent materialMechanical engineering
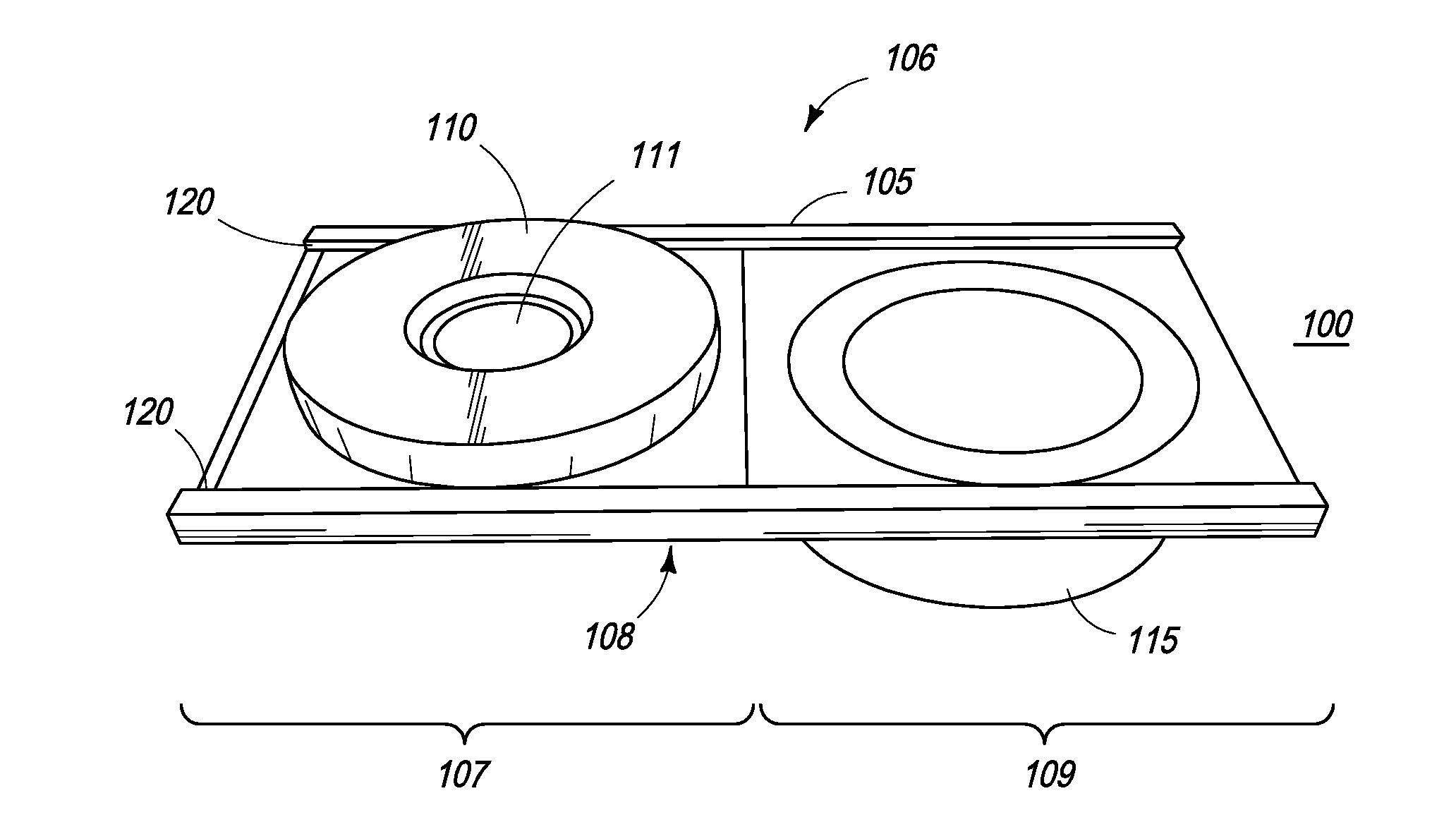
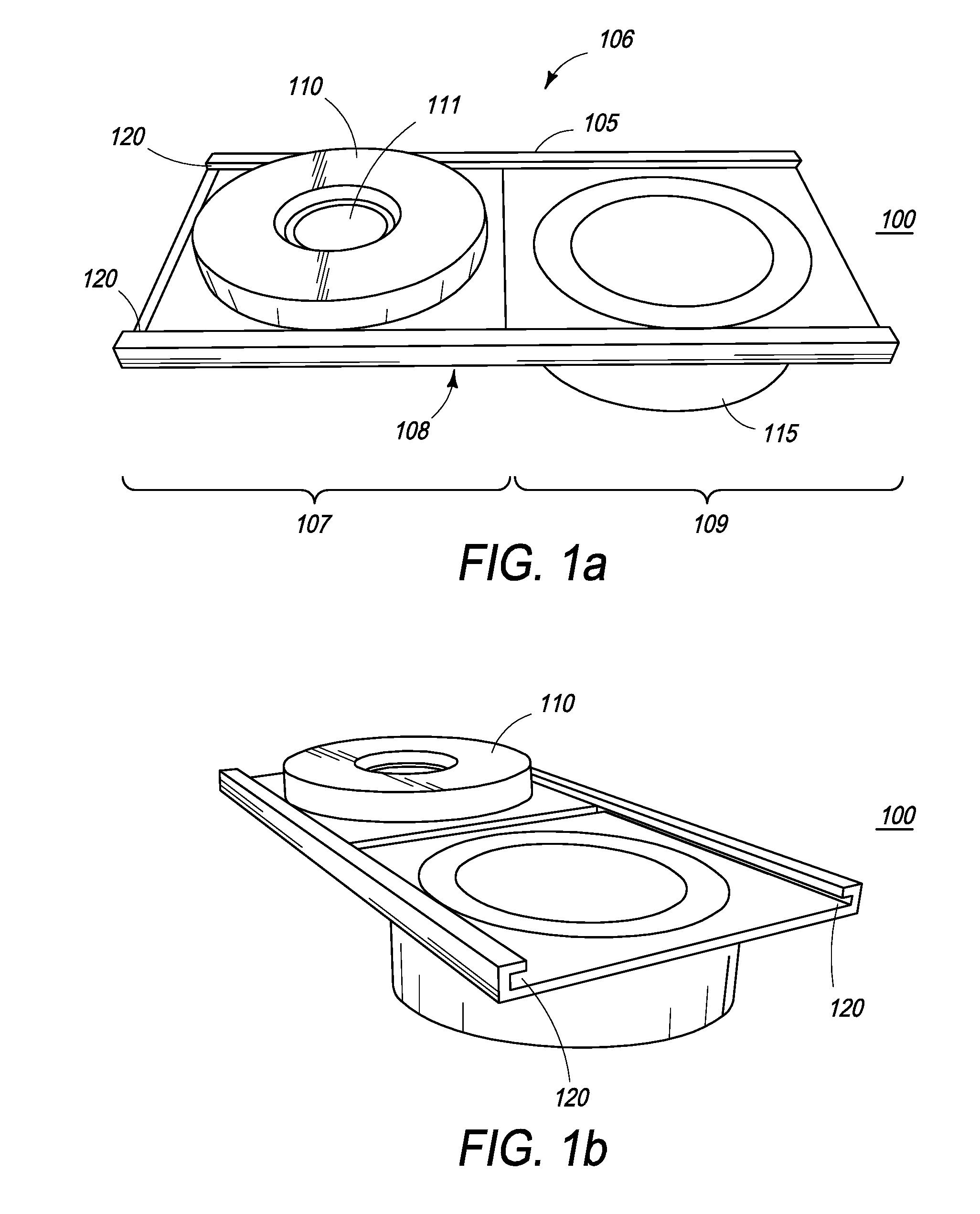
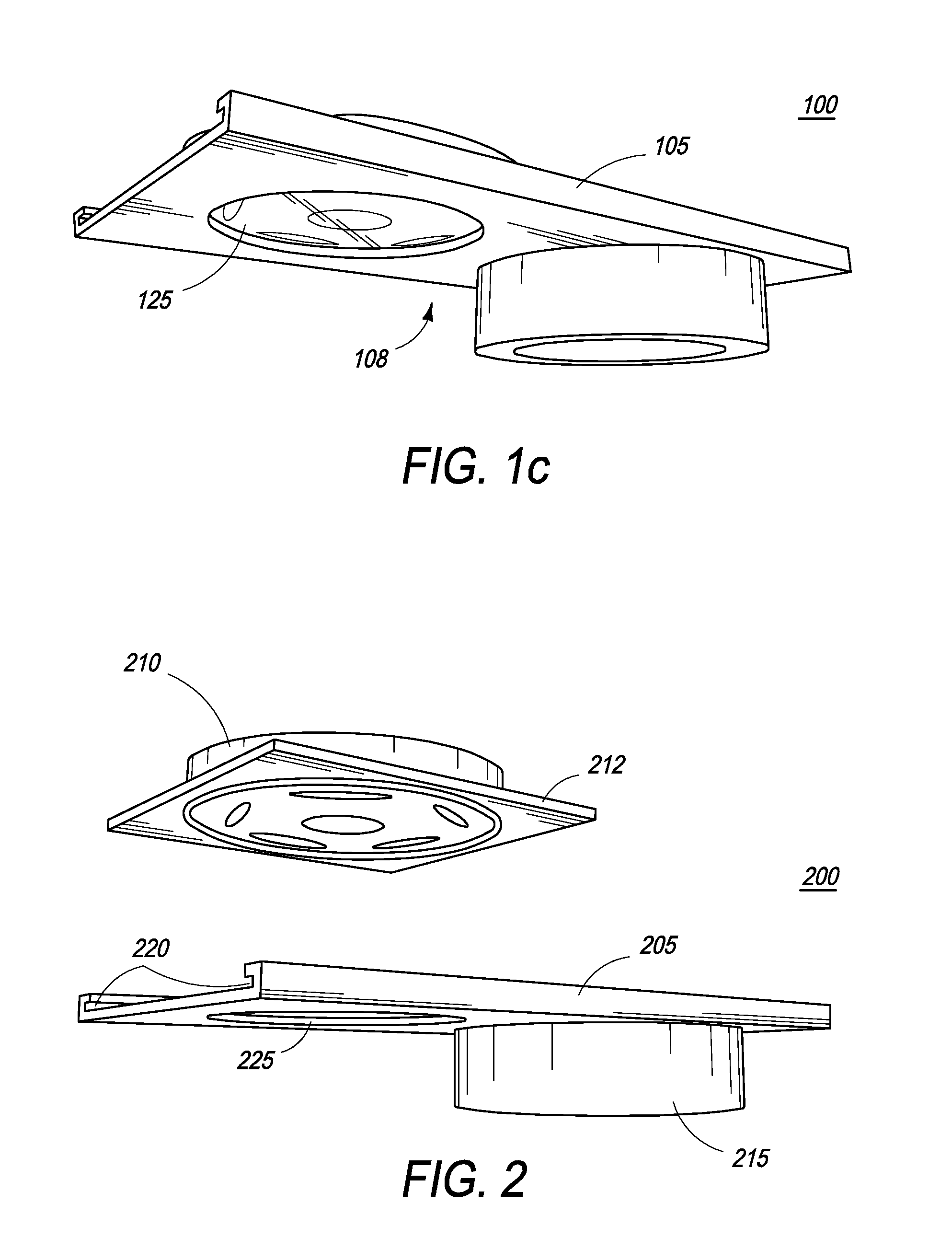
The present inventions are directed to apparatuses for rapidly measuring post-vaccination immune status. In one version, the apparatus has a support platform, with a top side, a bottom side, a first portion, and a second portion. A first void is integrally formed in the first portion. A container is configured to be removably affixed to the top side of the support platform. The container has a housing, a base, and at least one reactant. The container base can be viewed through the first void when the container is removably affixed to the first portion of the top side. An absorbent material is affixed to the second portion, where the base of the container comes into contact with the absorbent material when the container is removably affixed to the second portion of the top side.
Owner:SURAPANENI KRISHNA P +3
Vaccine clinical trial management method and system
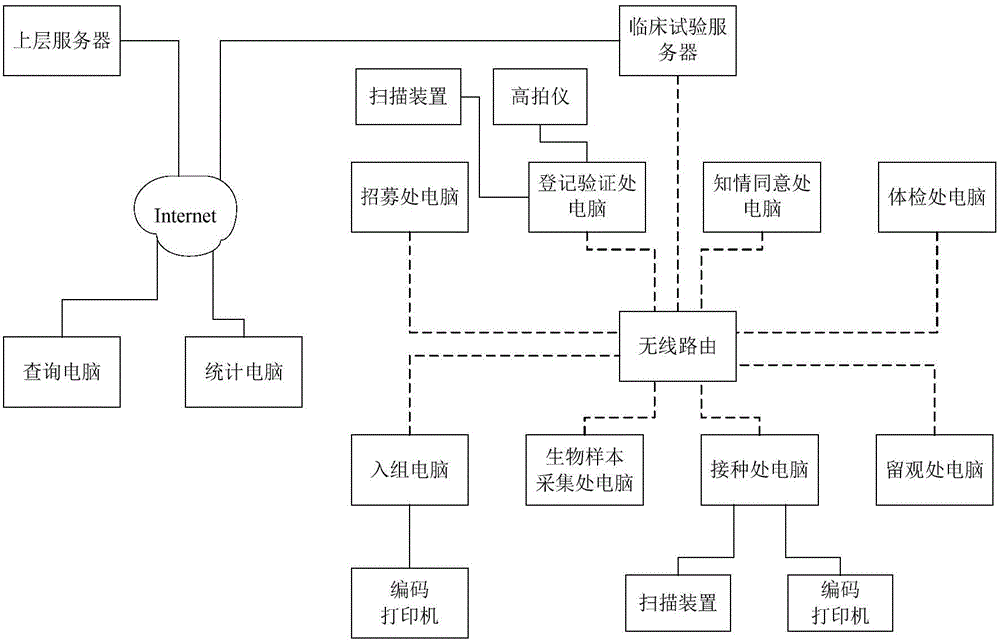
ActiveCN104318355ARealize network managementHigh degree of automationResourcesApproaches of managementMedicine
The invention discloses a vaccine clinical trial management method and system. The method comprises the following steps: scanning intelligent tags on immunization certificates held by subjects, acquiring pictures of the subjects and correspondingly storing in a clinical trial database; performing physical examination on the subjects according to preset physical examination items and recording physical examination data, then screening the subjects, further grouping and numbering the subject, acquiring the types and doses of inoculated vaccines of the subjects and then automatically calling the subjects to perform biological sample collection; scanning intelligent tags on flow cards of the subjects, acquiring vaccine inoculation information corresponding to the subjects according to corresponding data stored in the clinical trial database, performing vaccine inoculation of the subjects, recording the inoculation information and storing in the clinical trial database, and finally performing observing registration and measuring the body temperature of the subjects. The method and the system have the advantages of high degree of automation, high informationization degree and high efficiency, and can be widely applied to the field of clinical trial of the vaccines.
Owner:GUANGDONG PROVINCIAL INST OF BIOLOGICAL PROD & MATERIA MEDICA
Polynucleotide herpes virus vaccine
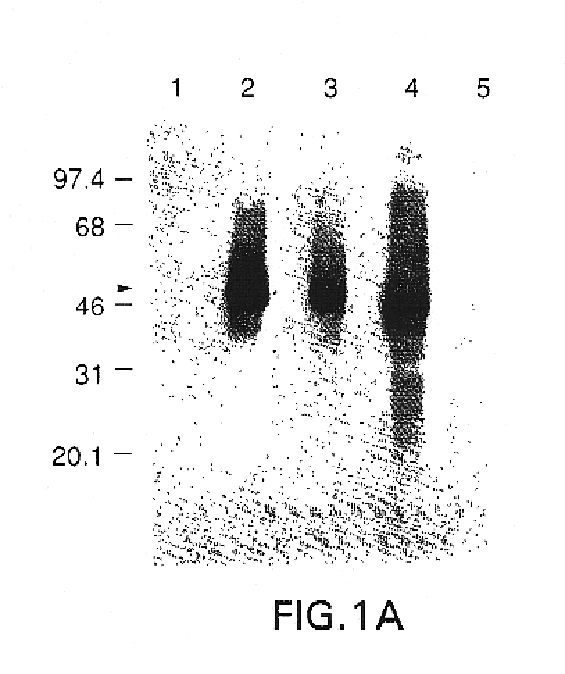
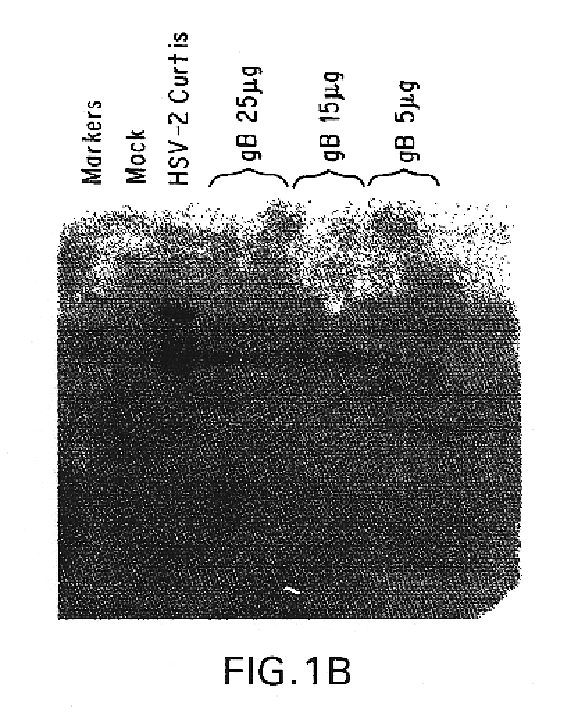
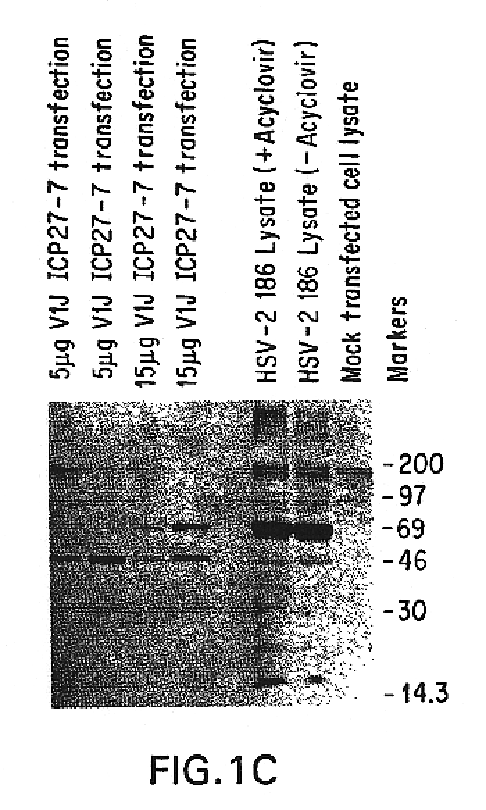
InactiveUS7094767B2Avoid infectionAmeliorate HSV-related diseaseSugar derivativesGenetic material ingredientsMammalSeroconversion
Genes encoding herpes simplex virus type 2 (HSV-2) proteins were cloned into eukaryotic expression vectors to express the encoded proteins in mammalian muscle cells in vivo. Animals were immunized by injection of these DNA constructs, termed polynucleotide vaccines or PNV, into their muscles. In a DNA titration, it was found that a single immunization of ≧0.5 μg of (one) PNV, gave >90% seroconversion by ten weeks post immunization. Immune antisera neutralized both HSV-2 and HSV-1 in cell culture. When animals were challenged with HSV-2, significant (p<0.001) protection from lethal infection was achieved following PNV vaccination. DNA constructs may be full-length, truncated and / or mutated forms and may be delivered along or in combination in order to optimize immunization and protection from HSV infection.
Owner:MERCK SHARP & DOHME CORP
Annexin A2 as Immunological Target
ActiveUS20140227286A1Compound screeningApoptosis detectionAntigenCalcium-dependent phospholipid binding
AnnexinA2 (ANXA2), a member of the Annexin family of calcium-dependent, phospholipid binding proteins, is one of a panel of identified antigens recognized by the post-vaccination sera of patients who demonstrated prolonged disease-free survival following multiple vaccinations. AnnexinA2 is abundantly expressed in pancreatic adenocarcinomas and cell surface / membrane AnnexinA2 increases with the progression from premalignant lesions to invasive pancreatic adenocarcinomas. The cytoplasmic to cell surface translocation of AnnexinA2 expression is critical for pancreatic cancer cell invasion. In addition, phosphorylation of AnnexinA2 at Tyrosine 23 is important for its localization to the cell surface and for the invasion of pancreatic cancer cells. Finally, loss of AnnexinA2 leads to the loss of the Epithelial-Mesenchymal Transition.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
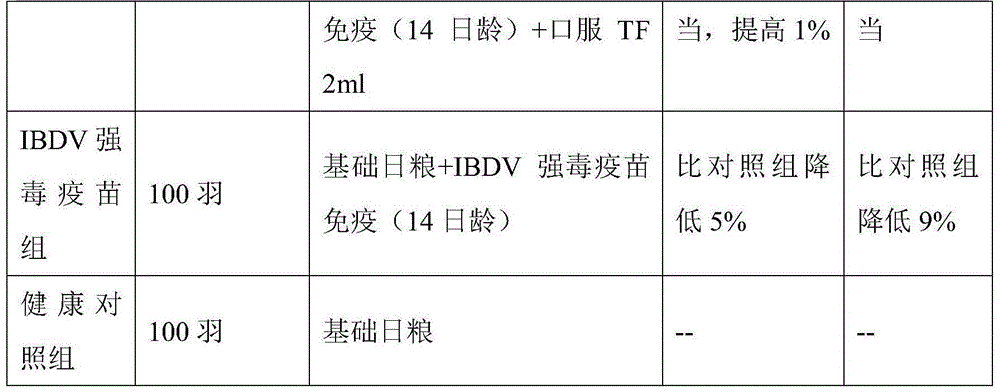
Transfer factor solution for livestock and poultry, and preparation method thereof
InactiveCN101537169ARealize storage at room temperatureImprove disease resistanceAntibacterial agentsPeptide/protein ingredientsDiseaseGlycerol
The invention provides a transfer factor solution for livestock and poultry. Frozen pig spleens are washed clean by use of distilled water, cut into small pieces, added with distilled water free from heat source, subjected to cell disruption by use of a tissue bruiser and prepared into homogenate; the homogenate passes through a high-pressure homogenizer under the pressure of 60 kPa and then is added with a flocculating agent for flocculation; the flocculated homogenate is centrifuged at a centrifugation ambient temperature of 4 DEG C and at a speed of 7,000 r / min; precipitate is removed; supernatant is left on standby; the supernatant is filtered by use of a microporous filtering membrane 0.45 mu m in pore diameter, and then is ultrafiltered by use of a ultrafiltration membrane 5,000 Dalton in molecular weight cutoff so as to obtain a stock solution; after viruses are removed, nicotinamide is added as a stabilizer; the stock solution is subjected to aseptic filtration by use of the microporous filtering membrane; and the obtained product is added with glycerol as a heat-resisting protective agent and then is directly and separately packed. The transfer factor solution can be applied to livestock and poultry. The preparation method is characterized by directly using the pig spleen to extract the transfer factor solution so as to save manpower and materials and improve extraction efficiency. The preparation method combines a heat-resisting protection technique with transfer factors, thereby realizing the normal-temperature preservation of the transfer factors. The transfer factor solution can improve the disease-resisting capability of organisms, relieve immunologic suppression and immunologic tolerance, can play a role in emergency adjuvant treatment, and makes up for immunization blank after vaccination before antibody production.
Owner:TIANJIN RINGPU BIO TECH
MERS-CoV specific polypeptides and application thereof
ActiveCN106397549AImprove featuresIncreased sensitivitySsRNA viruses positive-senseAntibody mimetics/scaffoldsT cellPost vaccination
The invention discloses MERS-CoV specific polypeptides and application thereof, belongs to the field of immunodetection and provides three MERS-CoV specific polypeptides aiming at BABL / c mice. An MHC-tetramer is prepared by using corresponding polypeptides, affinity between polypeptide-MHC and specific T cell surface TCR is improved effectively, and the MHC-tetramer can serve as an effective tool for T cell evaluation. The technology can be used for separating and cloning of specific T cells and performing in-situ dyeing on mouse corresponding tissue slices after MERS-CoV infection or vaccination and has high application value in the studying aspect of T cells of a mouse model after MERS-CoV infection or vaccination.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT +1
Test paper strip for rapidly detecting morbilli and rubella virus IgG antibody colloidal gold
ActiveCN101363856AHigh sensitivityImprove featuresMaterial analysisRubulavirus InfectionsSpecific igg
The invention provides a test strip for simultaneous detection of measles and rubella virus specific IgG antibodies, which comprises a reaction film and a conjugate release pad. The reaction film has a detection band simultaneously coated with measles virus H antigen and rubella virus E1 specific antigen, and a quality control band coated with double-antibody IgG. The conjugate release pad is coated with colloidal gold labeled anti-human IgG. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base and site detection and epidemiological investigation, has auxiliary and differential diagnosis effects on measles and rubella virus infection, and can be used for the immune effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
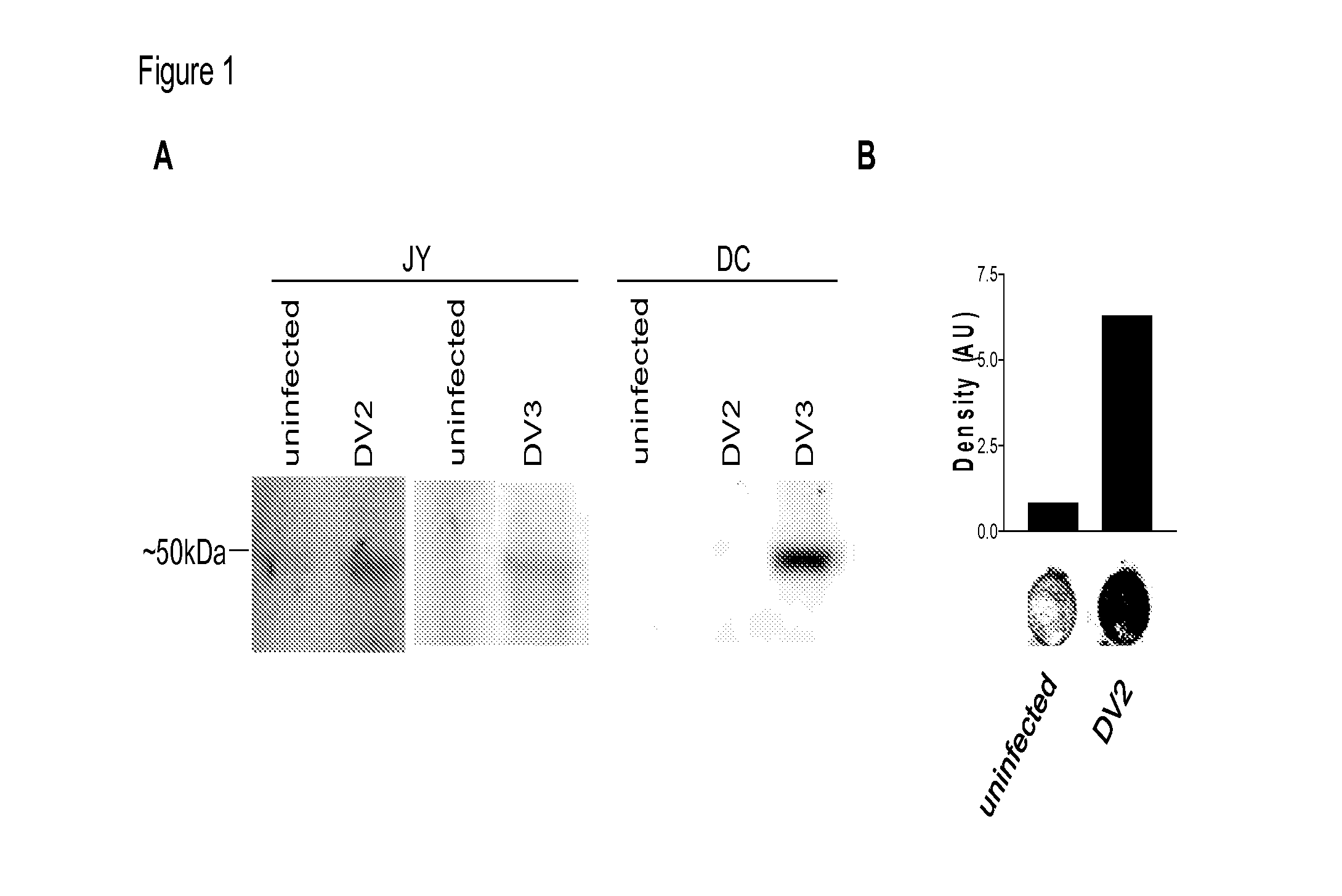
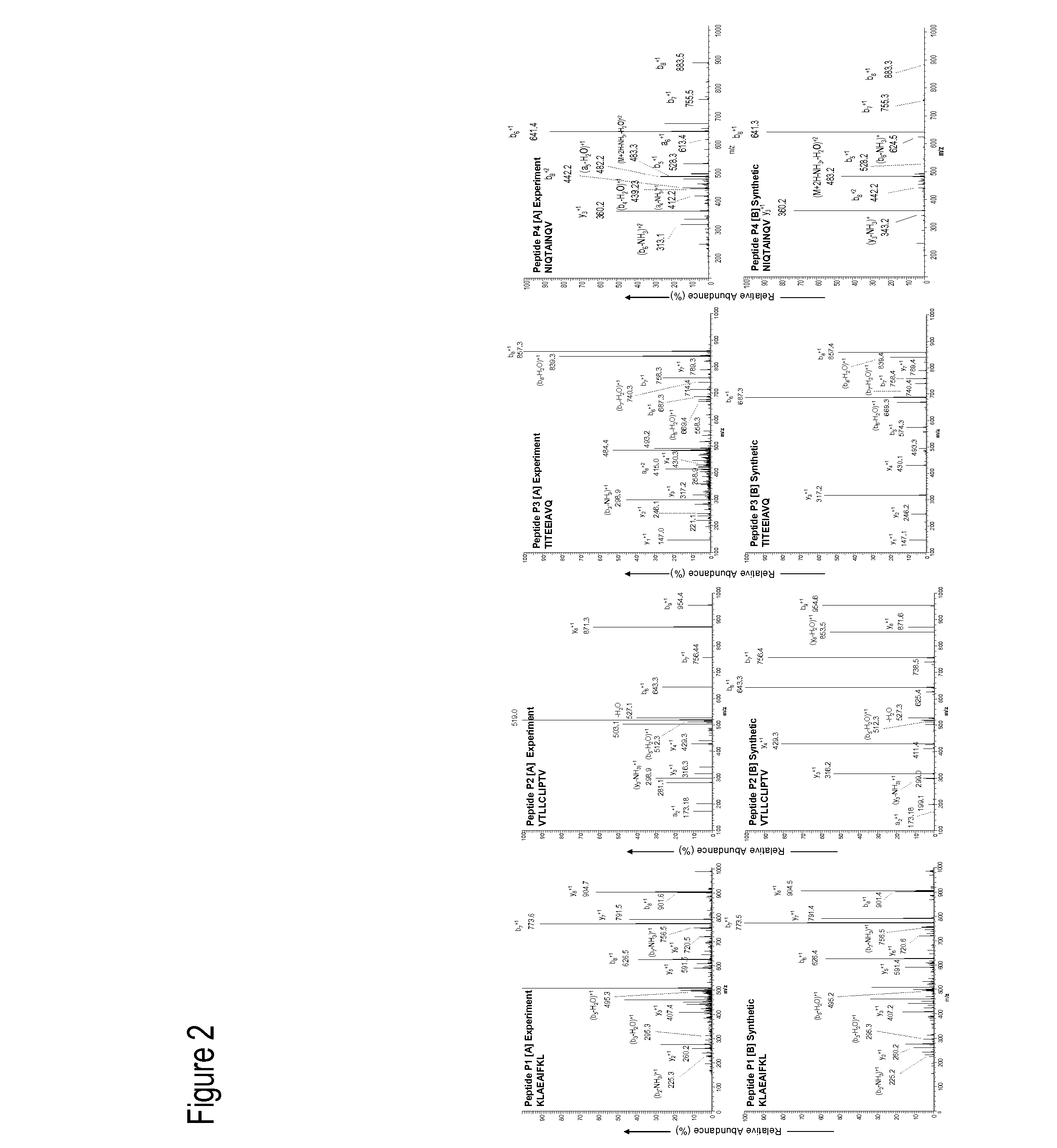
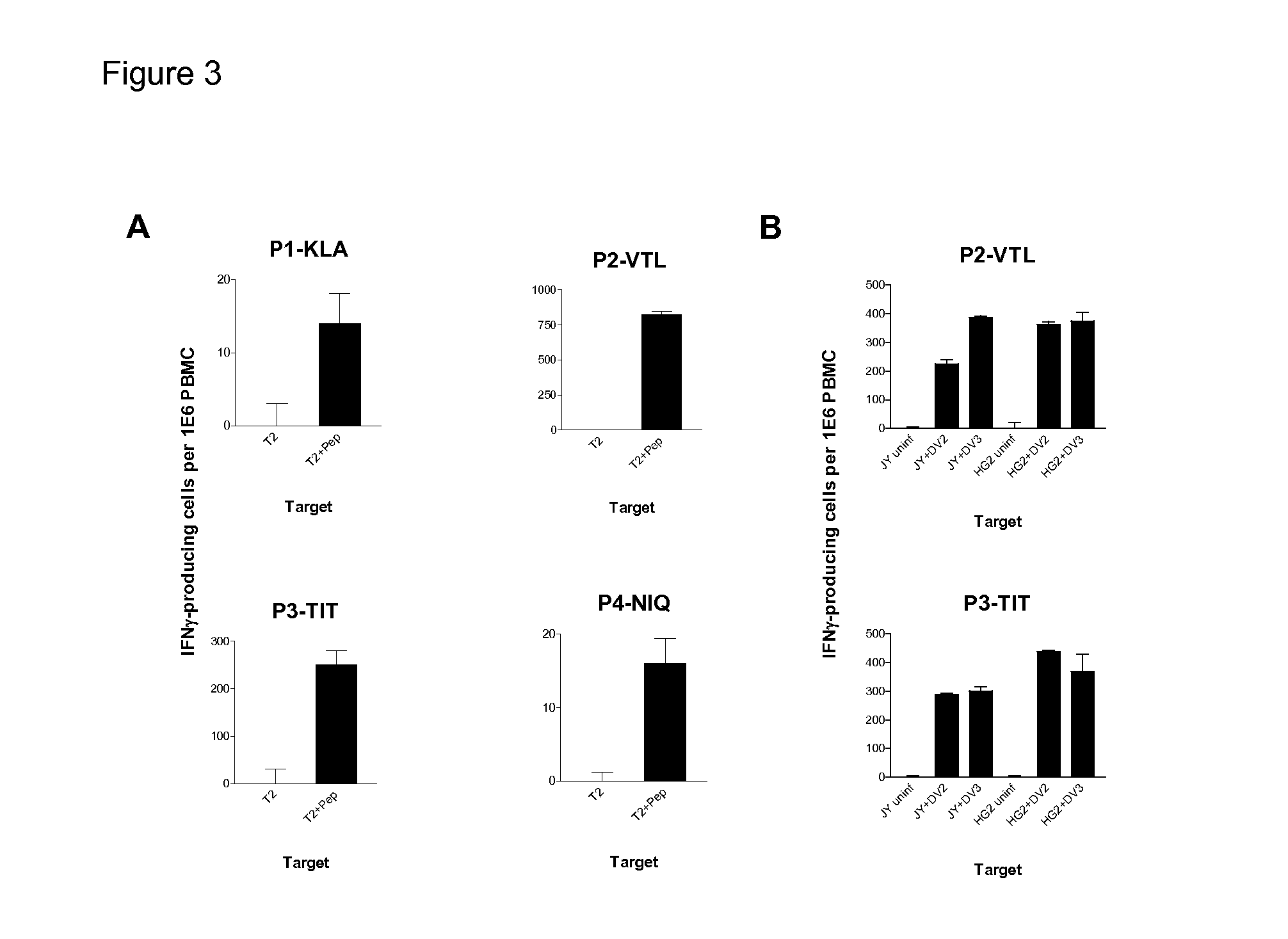
Cytotoxic T Lymphocyte Inducing Immunogens For Prevention Treatment and Diagnosis of Dengue Virus Infection
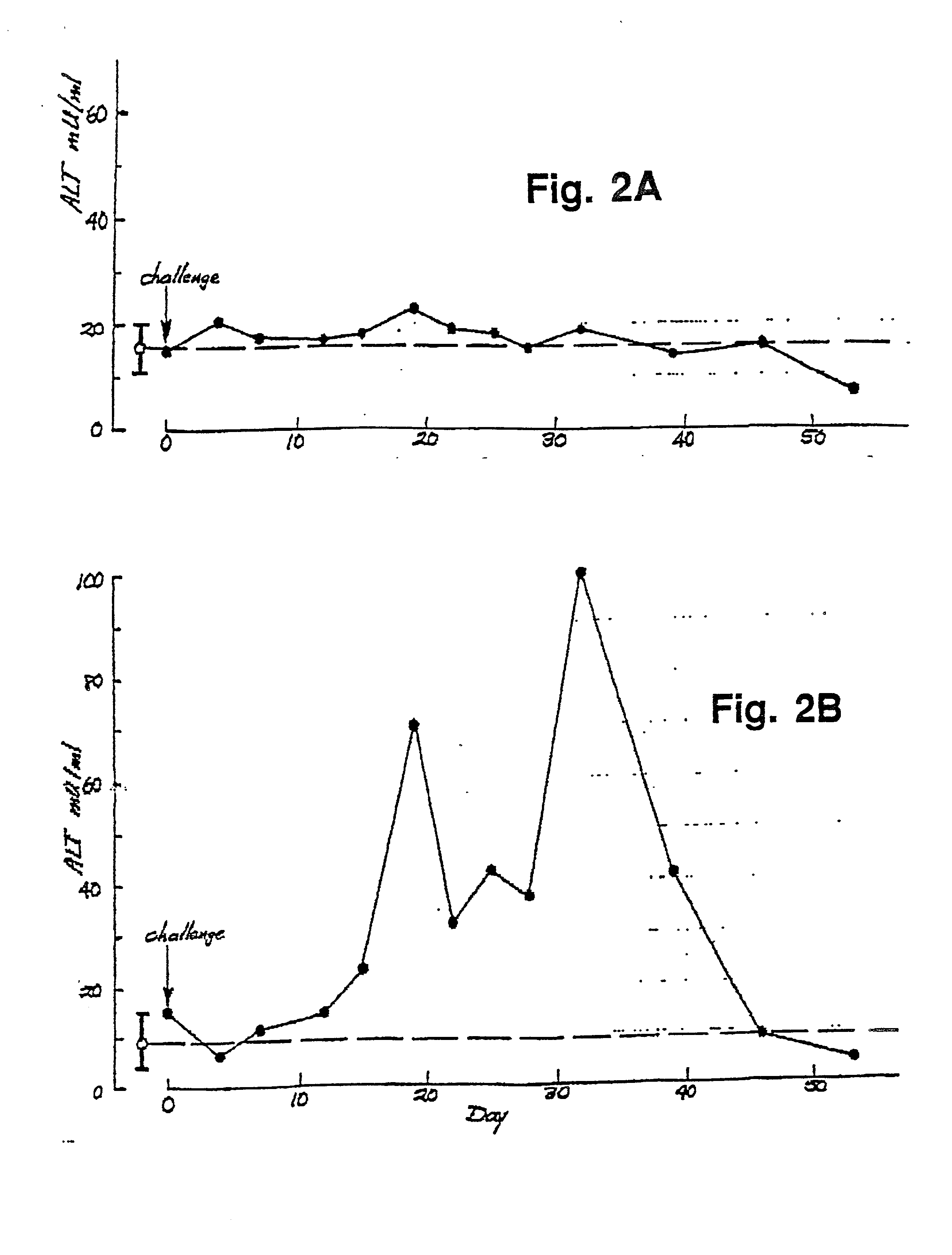
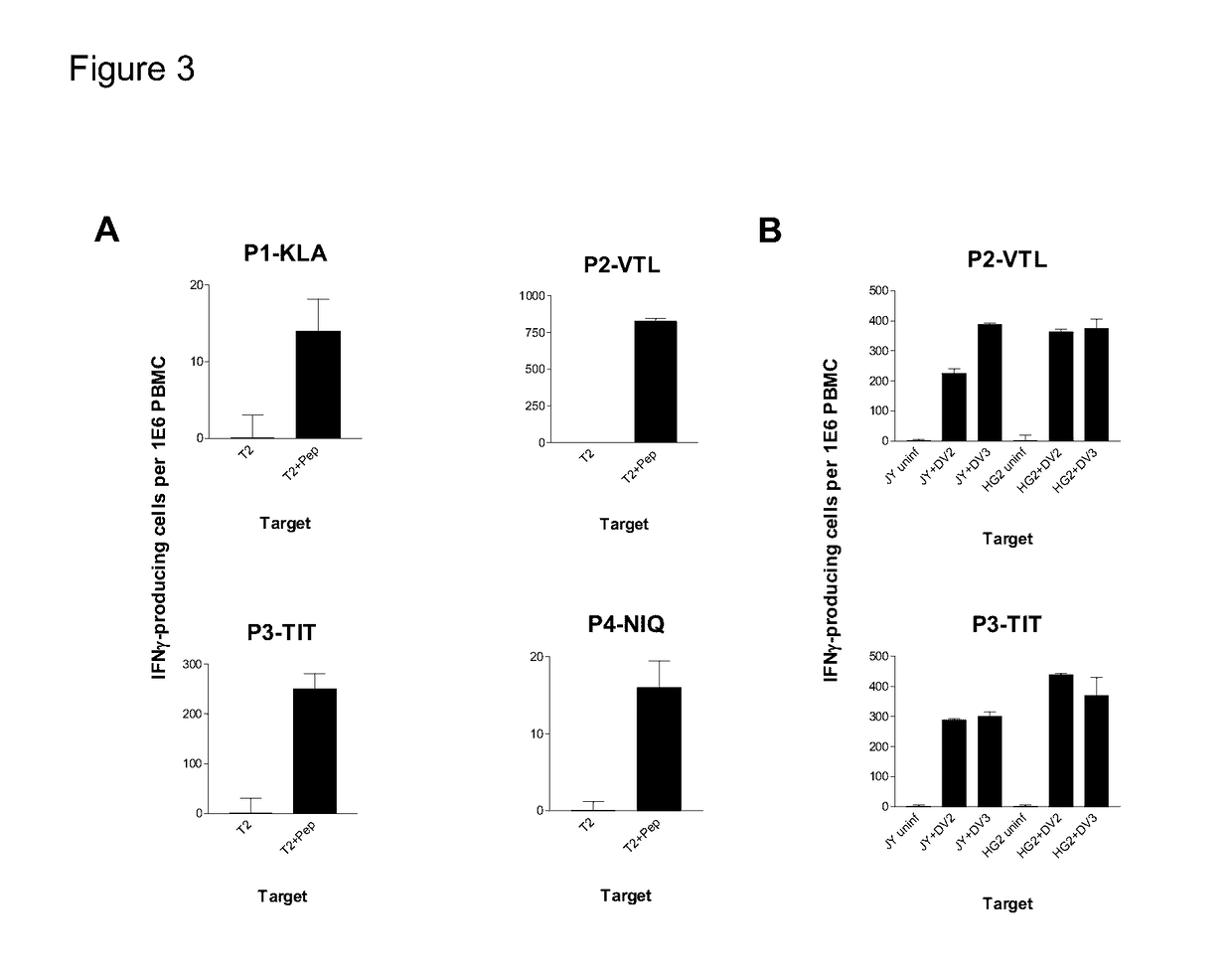
Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHR) are significant global public health problems and understanding the overall immune response to infection will contribute to appropriate management of the disease and its potentially severe complications. Live attenuated and subunit vaccine candidates, which are under clinical evaluation, induce primarily an antibody response to the virus and minimal cross-reactive T cell responses. Currently, there are no available tools to assess protective T cell responses during infection or post vaccination. The present invention incorporates immunoproteomics to uncover novel HLA-A2 specific epitopes derived from Dengue Virus (DV)-infected cells. These epitopes are conserved with epitope-specific CTLs cross-reacting against all four DV serotypes. These epitopes have potential as new informational and diagnostic tools to characterize T cell immunity in Dengue virus (DV) infection, and serves as a universal vaccine candidate complementary to current vaccines.
Owner:EMERGEX VACCINES HLDG LTD
Transfer factor solution applied in livestock and poultry, its preparation method and application thereof
InactiveCN1864742ARaise storage temperatureImprove disease resistancePeptide/protein ingredientsImmunological disordersUltrafiltrationDistilled water
Disclosed is a transfer factor solution for animals, wherein its preparing process consists of washing freeze chicken spleens and chopping, charging distilled water and comminuting cells to homogenate, freezing eight times at minus 30 deg C, centrifuging the homogenated liquid, separating deposition and filtering supernatant fluid with microporous filtering film, carrying out hyperfiltration with ultrafiltration membrane to obtain stock solution, removing viruses, charging niacinamide as stabilizer, filtering and degerming through microporous filtering film, charging glycerine and split charging directly.
Owner:TIANJIN RINGPU BIO TECH
Recombinant live attenuated foot-and-mouth disease (FMD) vaccine containing mutations in the L protein coding region
ActiveUS8846057B2Reduce severityReduce probabilitySsRNA viruses positive-senseViral antigen ingredientsNeutralizing antibodyAttenuated Live Vaccine
Previously we have identified a conserved domain (SAP, for SAF-A / B, Acinus, and PIAS) in the foot-and-mouth disease virus (FMDV) leader (L) protein coding region that is required for proper sub-cellular localization and function. Mutation of isoleucine 55 and leucine 58 to alanine (I55A, L58A) within the SAP domain resulted in a viable virus that displayed a mild attenuated phenotype in cell culture, along with altered sub-cellular distribution of L and failure to induce degradation of the transcription factor nuclear factor kappa-B. Here we report that inoculation of swine and cattle with this mutant virus results in the absence of clinical disease, the induction of a significant FMDV-specific neutralizing antibody response, and protection against subsequent homologous virus challenge. Remarkably, swine vaccinated with SAP mutant virus are protected against wild type virus challenge as early as two days post-vaccination suggesting that a strong innate as well as adaptive immunity is elicited. This variant could serve as the basis for construction of a live-attenuated FMD vaccine candidate.
Owner:UNITED STATES OF AMERICA
Traditional Chinese medicine composition and preparation method and applications thereof
InactiveCN104666399AReduce appetiteSlow weight gainOrganic active ingredientsDigestive systemImmune effectsFowl
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Adjuvanted vaccine which is substantially free of non-host albumin
InactiveUS20050232948A1Improve scalabilitySufficient protectionBiocideSsRNA viruses positive-senseAdjuvantSystemic reaction
Disclosed herein is a serum-based adjuvanted vaccine which is substantially free of non-host albumin and the use thereof in reducing or preventing post-vaccination systemic reactions.
Owner:HENNESSY KRISTINA J +3
Artificial immune algorithm based on RBF neural network and adaptive search
ActiveCN109870909AExpedited screeningSpeed up the efficiency of calculating antigensAdaptive controlArtificial immune algorithmSelf adaptive
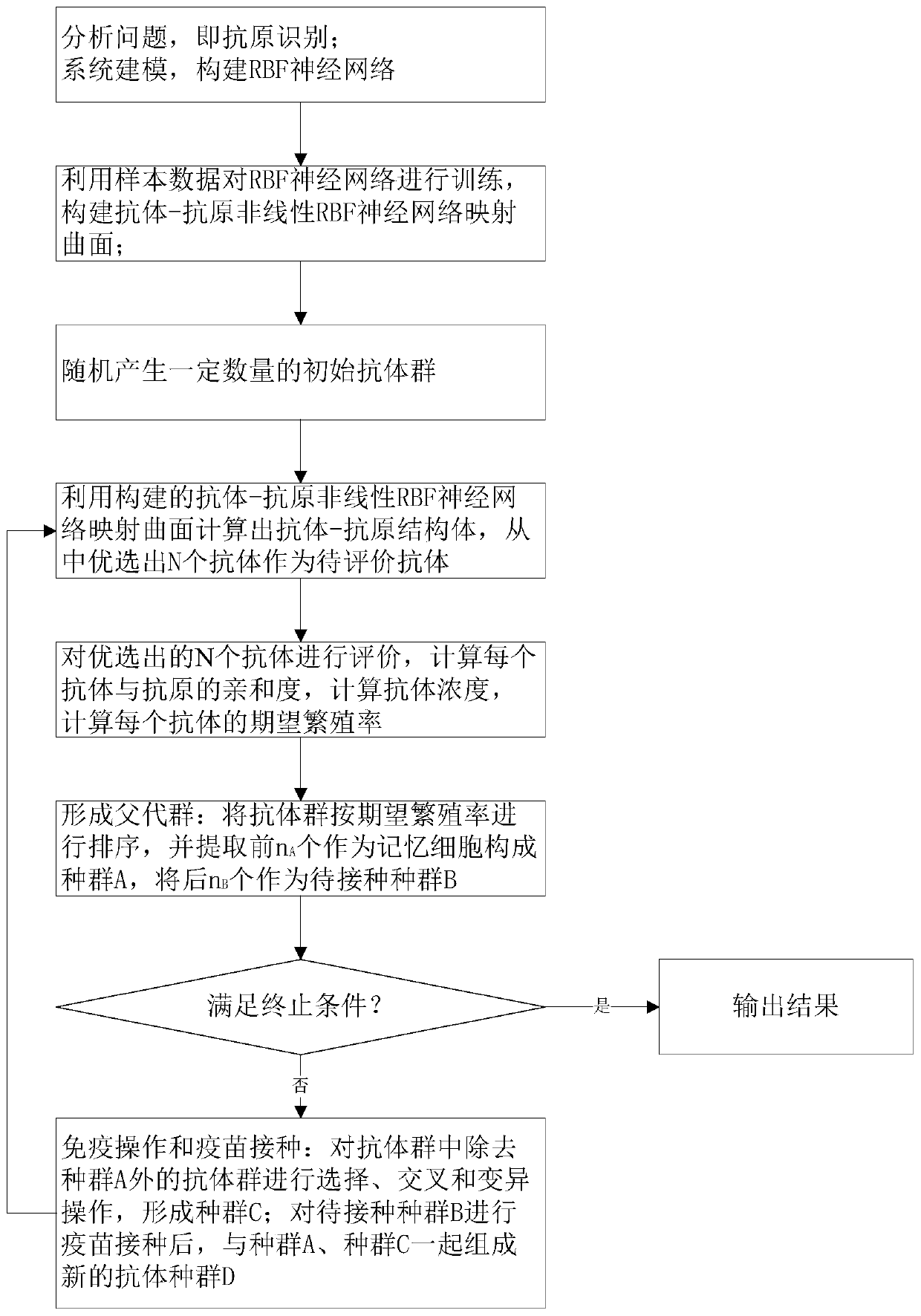
The invention provides an artificial immune algorithm based on an RBF neural network and adaptive search. The artificial immune algorithm comprises the following steps: S1, performing antigen recognition, and constructing the RBF neural network; S2, constructing an antibody-antigen nonlinear mapping curved surface; S3, randomly generating a certain number of initial antibody groups; S4, calculating an antibody-antigen structural body, and preferably selecting N antibodies to serve as antibodies to be evaluated; S5, evaluating the antibodies; S6, sorting the antibody groups, extracting the previous nA antibody groups to serve as memory cells to form a population A, and extracting subsequent nB antibody groups to serve as populations B to be inoculated; S7, judging a termination condition, outputting a result if the termination condition is satisfied, or otherwise, executing S8; and S8, performing selection, crossover and mutation operations on the antibody groups excluding the population A in the S6 to form a population C, after vaccination is performed on the populations B to be inoculated, forming an antibody population D via the populations B together with the populations A and C, and skipping to S4. The invention aims at providing the artificial immune algorithm based on the RBF neural network and adaptive search, which is high in local search capability, high in convergencespeed, high in algorithm efficiency and high in precision.
Owner:ARMOR ACADEMY OF CHINESE PEOPLES LIBERATION ARMY +1
Identification of gene sequences and proteins involved in vaccinia virus dominant T cell epitopes
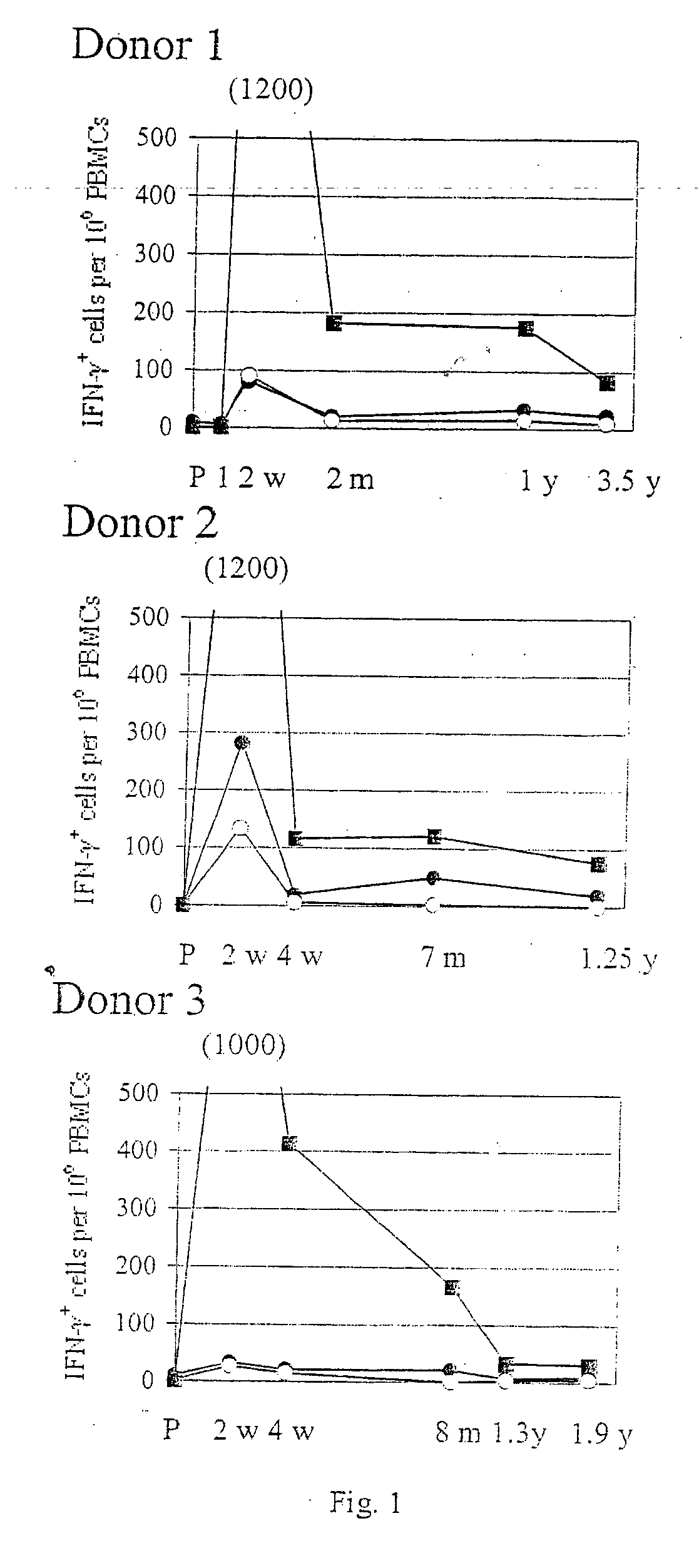
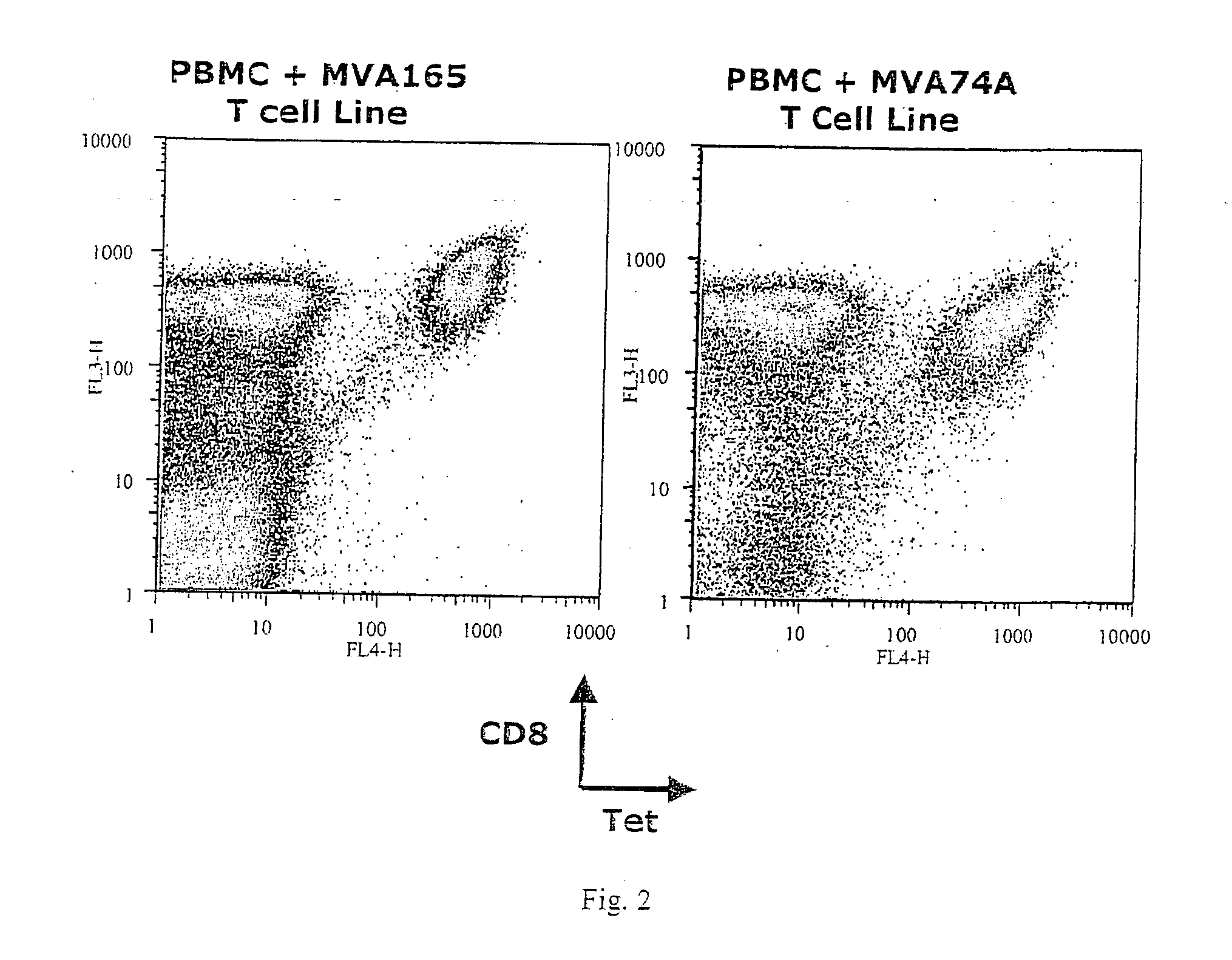
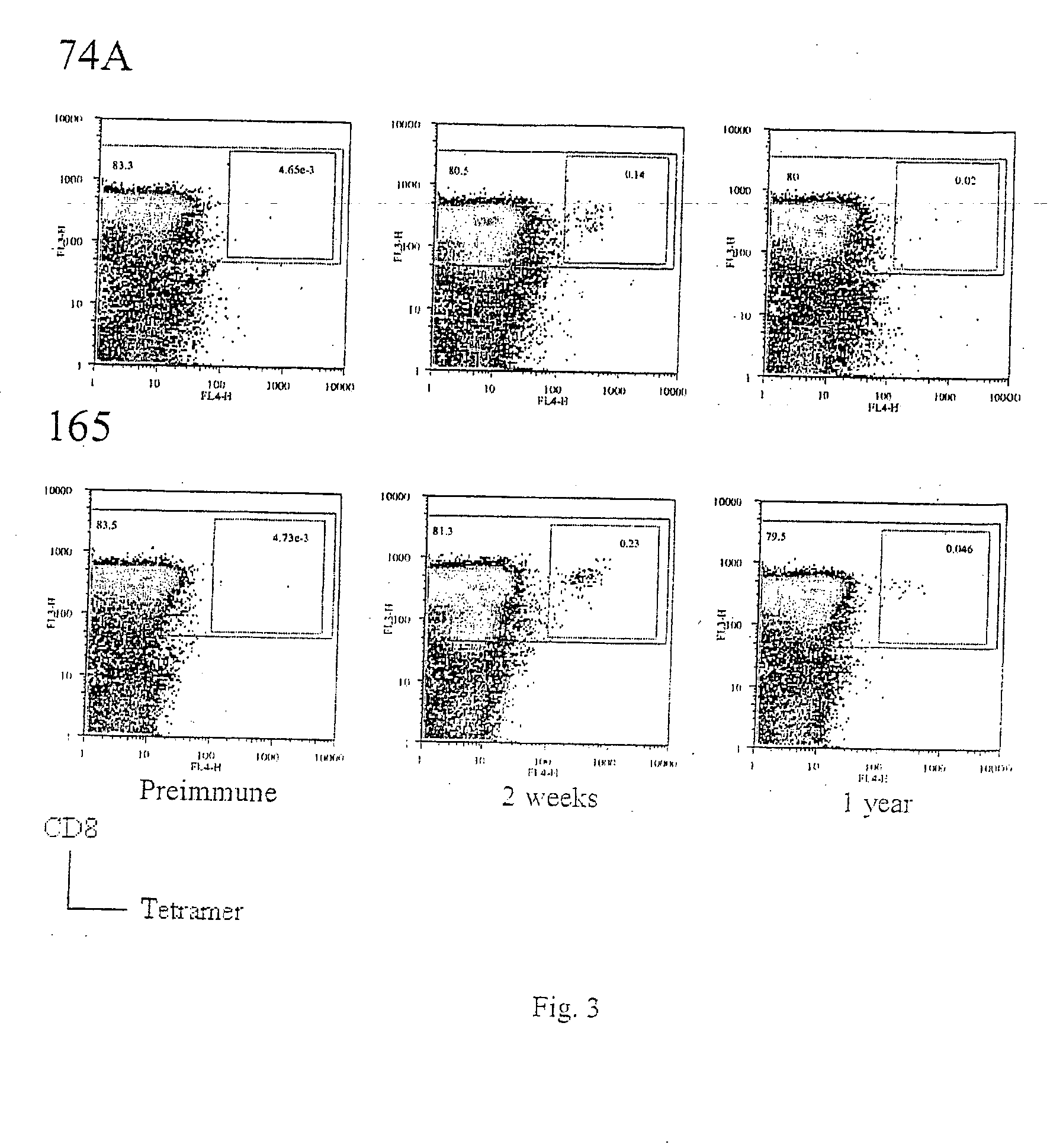
The present invention relates to the identification of gene sequences and proteins involved in vaccinia virus dominant T cell epitopes. Two vaccinia virus CD8+ T cell epitopes restricted by the most common human MHC class I allele, HLA-A0201 have been identified. Both epitopes are highly conserved in vaccinia and variola viruses. The induction of the T cell responses following primary vaccination is demonstrated by the kinetics of epitope specific CD8+ T cells in 3 HLA-A0201 individuals. This information will be useful for the design and analyses of the immunogenicity of experimental vaccinia vaccines, and for basic studies of human T cell memory.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Test paper strip for detecting encephalitis virus specificity IgG antibody, method for making same and applications
ActiveCN101363864ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseSpecific igg
The invention provides a colloidal gold test strip for the detection of Japanese encephalitis virus specific IgG antibody. A Japanese encephalitis virus E gene antigen domain III and an anti III polyclonal antibody are coated on a nitrate cellulose film (NC film), and a membrane chromatography double antigen sandwich method is adopted to detect the Japanese encephalitis virus specific IgG antibody in an animal or human body serum specimen in combination with a colloidal gold labeled Japanese encephalitis virus E gene antigen domain III. Or the Japanese encephalitis virus E gene antigen domain III and an anti-mouse IgG are coated on the nitrate cellulose film (NC film), and a capture method is adopted to detect the Japanese encephalitis virus specific IgG antibody in the human body serum specimen in combination with a colloidal gold labeled antihuman monoclonal antibody. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, has auxiliary effect on the diagnosis of Japanese encephalitis virus infection, and can be used for the effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
Virulent aeromonas vaccines and methods
ActiveUS20200030428A1Bacterial antigen ingredientsMultivalent vaccineHighly pathogenicVaccine antigen
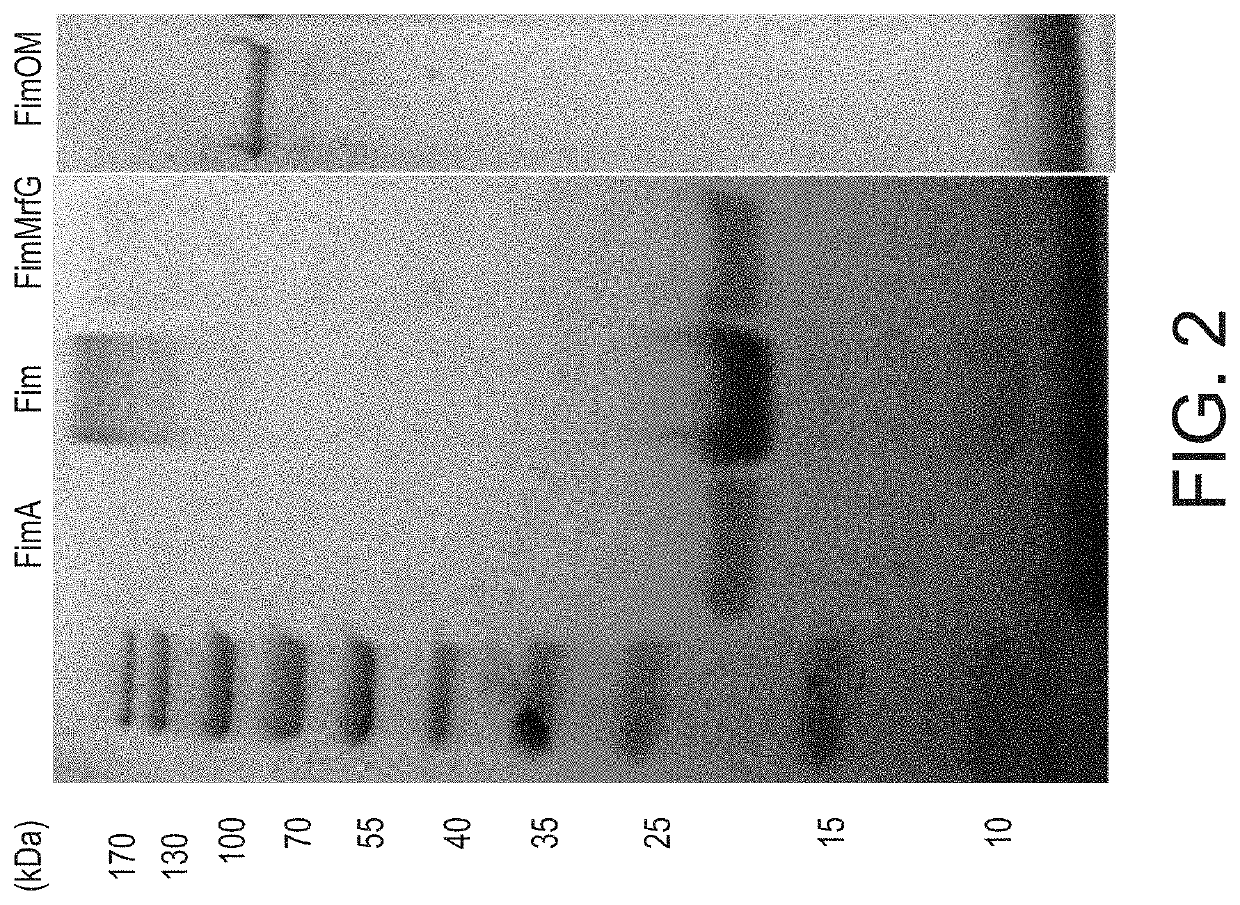
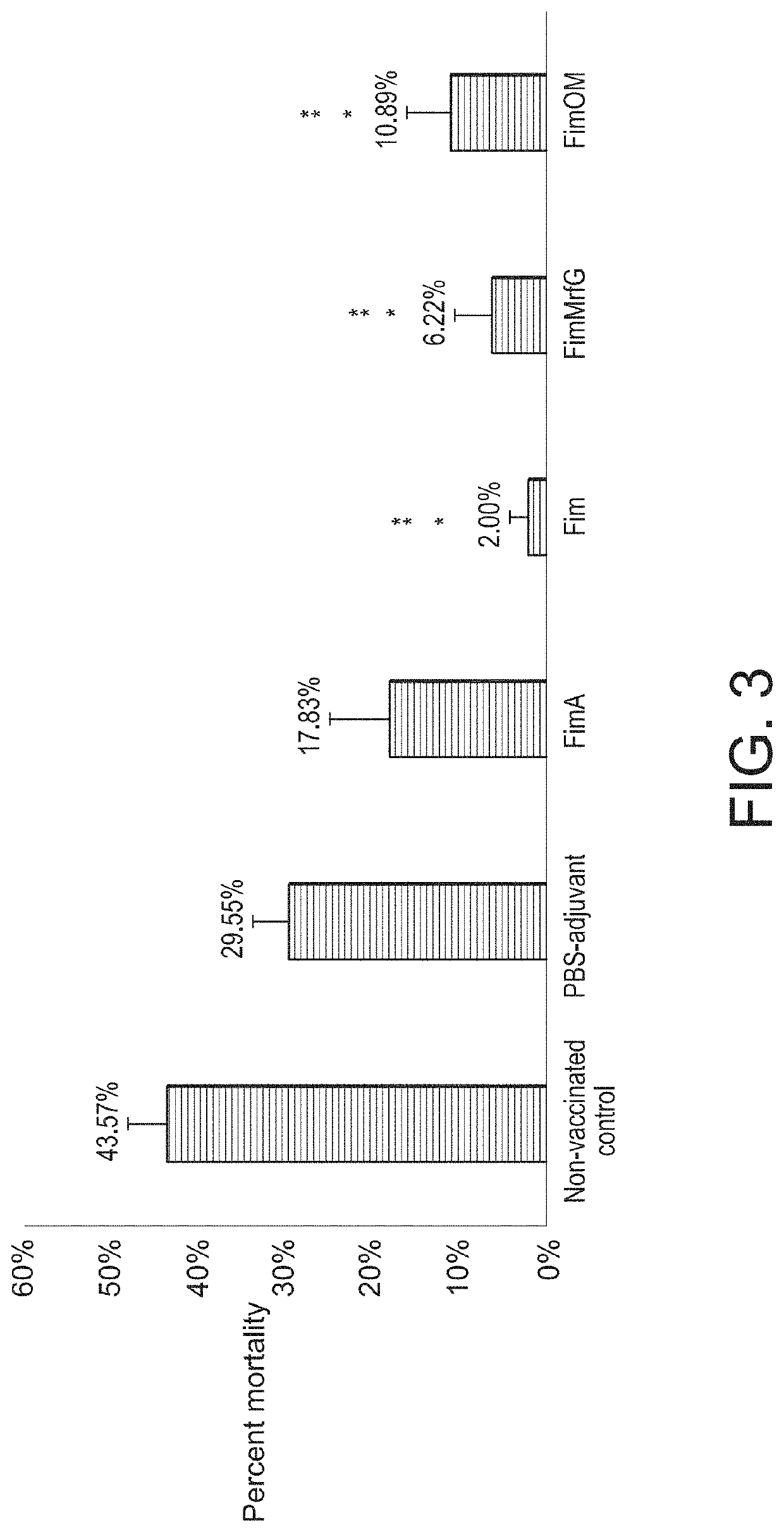
Aeromonas hydrophila is a reemerging pathogen of channel catfish (Ictalurus punctatus); recent outbreaks from 2009 to 2014 have caused the loss of more than 12 million pounds of market size catfish in Alabama and Mississippi. Genome sequencing revealed a clonal group of A. hydrophila isolates with unique genetic and phenotypic features that is highly pathogenic in channel catfish. Comparison of the genome sequence of a representative catfish isolate (ML09-119) from this virulent clonal group with lower virulence A. hydrophila isolates revealed four fimbrial proteins unique to strain ML09-119. In this work, we expressed and purified four A. hydrophila fimbrial proteins (FimA, Fim, MrfG, and FimOM) and assessed their ability to protect and stimulate protective immunity in channel catfish fingerlings against A. hydrophila ML09-119 infection for vaccine development. Our results showed catfish immunized with FimA, Fim, FimMrfG, and FimOM exhibited 59.83%, 95.41%, 85.72%, and 75.01% relative percent survival, respectively, after challenge with A. hydrophila strain ML09-119. Bacterial concentrations in liver, spleen, and anterior kidney were significantly (p<0.05) lower in vaccinated fish compared to the non-vaccinated sham groups at 48 h post-infection. However, only the Fim immunized group showed a significantly higher antibody titer in comparison to the non-vaccinated treatment group (p<0.05) at 21 days post-vaccination. Altogether, Fim and FimMrfG recombinant proteins have potential for vaccine development against virulent A. hydrophila infection. Genomic subtraction revealed three outer membrane proteins present in strain ML09-119 but not in the low virulence reference A. hydrophila strain; the major outer membrane protein OmpAI (OmpA1), TonB-dependent receptor (TonB-DR), and transferrin-binding protein A (TbpA). Here, the genes encoding OmpAI, tonB-DR, and tbpA were cloned from A. hydrophila ML09-119 and were expressed into Escherichia coli. The purified recombinant OmpA, TonB-DR, and TbpA proteins had estimated molecular weights of 37.26, 78.55, and 41.67 kDa, respectively. Catfish fingerlings vaccinated with OmpA1, TonB-DR, and TbpA emulsified with non-mineral oil adjuvant were protected against the subsequent A. hydrophila ML09-119 infection with 98.59%, 95.59%, and 47.89% relative percent survival (RPS), respectively. Furthermore, the mean liver, spleen, and anterior kidney bacterial loads were significantly lower in catfish vaccinated with the OmpA1 and TonB-DR than the non-vaccinated control group. ELISA demonstrated that catfish immunized with OmpA1, TonB-DR, and TbpA produce significant antibody response by 21 days post-immunization. Therefore, data generated during the study suggest that OmpAI and TonB-DR proteins could be used as potential candidates for vaccine development against A. hydrophila epidemic strain infection. However, TbpA protein failed to provide such strong protection. Recombinant ATPase from A. hydrophila also showed promise as a vaccine antigen. A live attenuated vaccine was prepared that combined the advantages of a live attenuated vaccine (ESC-NDKL1 (ΔgcvPΔsdhCΔfrdA) mutant of Edwardsiella ictaluri) against enteric septicemia of catfish (ESC) and three immunogenic recombinant proteins (Fim, FimMrfg, and ATPase) against A. hydrophila infection. Our results showed channel catfish fingerlings immersion-vaccinated with ESC-NDKL1::pETfim, ESC-NDKL1::pETmrfG, and ESC-NDKL1::pETATPase exhibited 100%, 91.67%, and 100% percent survival after challenge with the A. hydrophila ML09-119, which was significantly less than non-vaccinated group (88.89% mortality). In a second study, Catfish immunized with NDKL1::pETfim, ESC-NDKL1::pETmrfG, ESC-NDKL1::pETATPase had significantly (p<0.05) lower mortalities than sham-vaccinated group.
Owner:MISSISSIPPI STATE UNIVERSITY
Recombinant Live Attenuated Foot-and-Mouth Disease (FMD) Vaccine Containing Mutations in the L Protein Coding Region
ActiveUS20110177123A1More attenuationReduce probabilitySsRNA viruses positive-senseViral antigen ingredientsSubcellular distributionWild type virus
Previously we have identified a conserved domain (SAP, for SAF-A / B, Acinus, and PIAS) in the foot-and-mouth disease virus (FMDV) leader (L) protein coding region that is required for proper sub-cellular localization and function. Mutation of isoleucine 55 and leucine 58 to alanine (I55A, L58A) within the SAP domain resulted in a viable virus that displayed a mild attenuated phenotype in cell culture, along with altered sub-cellular distribution of L and failure to induce degradation of the transcription factor nuclear factor kappa-B. Here we report that inoculation of swine and cattle with this mutant virus results in the absence of clinical disease, the induction of a significant FMDV-specific neutralizing antibody response, and protection against subsequent homologous virus challenge. Remarkably, swine vaccinated with SAP mutant virus are protected against wild type virus challenge as early as two days post-vaccination suggesting that a strong innate as well as adaptive immunity is elicited. This variant could serve as the basis for construction of a live-attenuated FMD vaccine candidate.
Owner:US SEC AGRI
Preparation method of anti-human Nogo-66 receptor protein vaccine and application thereof
InactiveCN101288771APromote regenerationPromote functional recoveryNervous disorderAntibody ingredientsEscherichia coliEukaryotic plasmids
The invention provides a method for preparing antihuman Nogo-66 receptor protein vaccine. An hNgR gene in full size is obtained by the PCR amplification from human neurobalstoma (SK-N-SH) cDNA. The amplified segment is inserted in a cloning vector and is sub-cloned to an expression vector pET28a (+) containing six His labels to obtain a recombinant expression plasmid pET28a-NgR. The recombinant plasmid is transformed into a coli expression strain BL21 (DE3) for the induced expression to obtain hNgR protein. By closing the effect of NgR and blocking the effect of inhibitory factor, the NgR protein vaccine can promote the regeneration of neurons axon after the spinal cord injury, can reduce the tissue necrosis in a damage zone, can promote the functional recovery of the injured spinal cord and express that the NgR protein vaccine is provided with a potential value for treating the spinal cord injury. The defect that exogenous biological agent can be easily eliminated by an autoimmune system is avoided. In addition, the characters that the cellular immunity is mainly induced after the DNA vaccination of NgR and only few individuals can induce humoral immunity are overcome.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Test strip for double-line detection of antibody after novel coronavirus vaccine inoculation
PendingCN112904021AHigh sensitivityEasy to detectBiological testingImmunoassaysCoronavirus vaccinationNeutralising antibody
The invention discloses a test strip for detecting novel coronavirus vaccination antibodies, which comprises a bottom plate, a sample pad, a combination pad, a reaction pad and a water absorption pad, the sample pad, the combination pad, the reaction pad and the water absorption pad are sequentially overlapped on the bottom plate, the reaction pad is provided with a control band and two detection bands, one of the detection bands is used for detecting whether an organism generates an immune reaction after the novel coronavirus vaccine is inoculated and generating a corresponding antibody; and the other detection band is used for detecting whether the neutralizing antibody generated by the body can reach a certain titer or not to form a protective effect. The test strip is good in sensitivity, good in detection performance, low in cost and simple in use method, and can be used for rapidly, simply and conveniently detecting the effect of the novel coronavirus vaccine after inoculation without professional technicians and professional equipment in multiple scenes such as hospitals, clinics and even at home; the sample demand is low; the effective vaccination rate of the vaccine can be improved, infection caused by poor vaccination effect is prevented, and the vaccine has a good clinical application prospect.
Owner:HENAN UNIVERSITY
Method for improving productivity of broiler chickens
InactiveCN105028316AIncrease production capacityDecreased meat production capacityAnimal husbandryBiotechnologyImmune effects
The invention provides a method for improving productivity of broiler chickens. According to the method, the broiler chickens are fed with a transfer factor solution, the polypeptide content in the transfer factor solution is 1-1.5 mg / ml, the ribose content in the transfer factor solution is 30-50 mcg / ml, and the single feeding dose can be 0.5-2 mL. As an accidental discovery, the productivity of the broiler chickens can be significantly improved by feeding the broiler chickens with transfer factors, and meanwhile meat productivity reduction caused by vaccination of the broiler chickens can be relieved. The technical effects are obtained thanks to the transfer factors, more importantly, the feeding way, the dose and other characteristics determined in the method. The method can be applied to both common broiler chickens and vaccinated broiler chickens, so that while the immune effect is improved through the transfer factors, the meat productivity of the broiler chickens is improved. The outstanding technical effects are achieved through the ingenious technical thought, and meanwhile the method is low in cost and easy to implement and has broad popularization prospects.
Owner:TIANJIN RINGPU BIO TECH
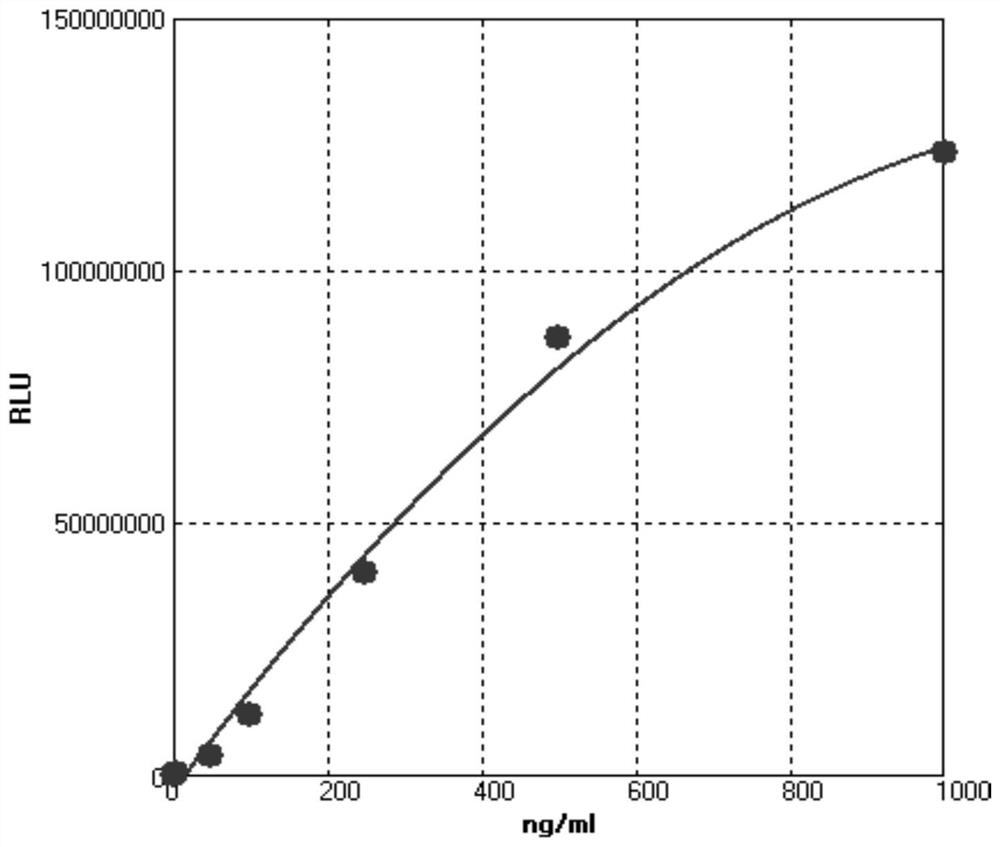
Novel coronavirus neutralizing antibody detection kit based on magnetic particle chemiluminescence and application thereof
PendingCN113009153AImprove stabilityHigh sensitivityChemiluminescene/bioluminescenceBiological testingCoronavirus antibodyNeutralising antibody
The invention discloses a novel coronavirus neutralizing antibody detection kit based on magnetic particle chemiluminescence. The novel coronavirus neutralizing antibody detection kit is composed of magnetic microspheres which are coupled with RBD protein 1 and arranged in a kit body, an RBD neutralizing antibody standard substance solution serving as a positive control, a negative control, a sample diluent, an RBD protein 2 enzyme conjugate, a 20*concentrated washing solution and a luminescent solution. The invention also discloses application of the kit in detection of a biological sample containing the novel coronavirus neutralizing antibody. Experiments prove that the kit disclosed by the invention is high in stability, strong in selectivity, high in detection speed, low in cost and easy to operate, overcomes the defects of high laboratory environment requirement, long operation time and the like when the neutralizing antibody is detected in the prior art, and shortens the operation time from 120 minutes to 35 minutes. The kit not only can be used for judging the antibody neutralizing condition after novel coronavirus vaccine inoculation, but also can be used for screening common novel coronavirus antibodies, and has a great clinical application prospect.
Owner:山东莱博生物科技有限公司
Live Attenuated Antigenically Marked Classical Swine Fever Vaccine
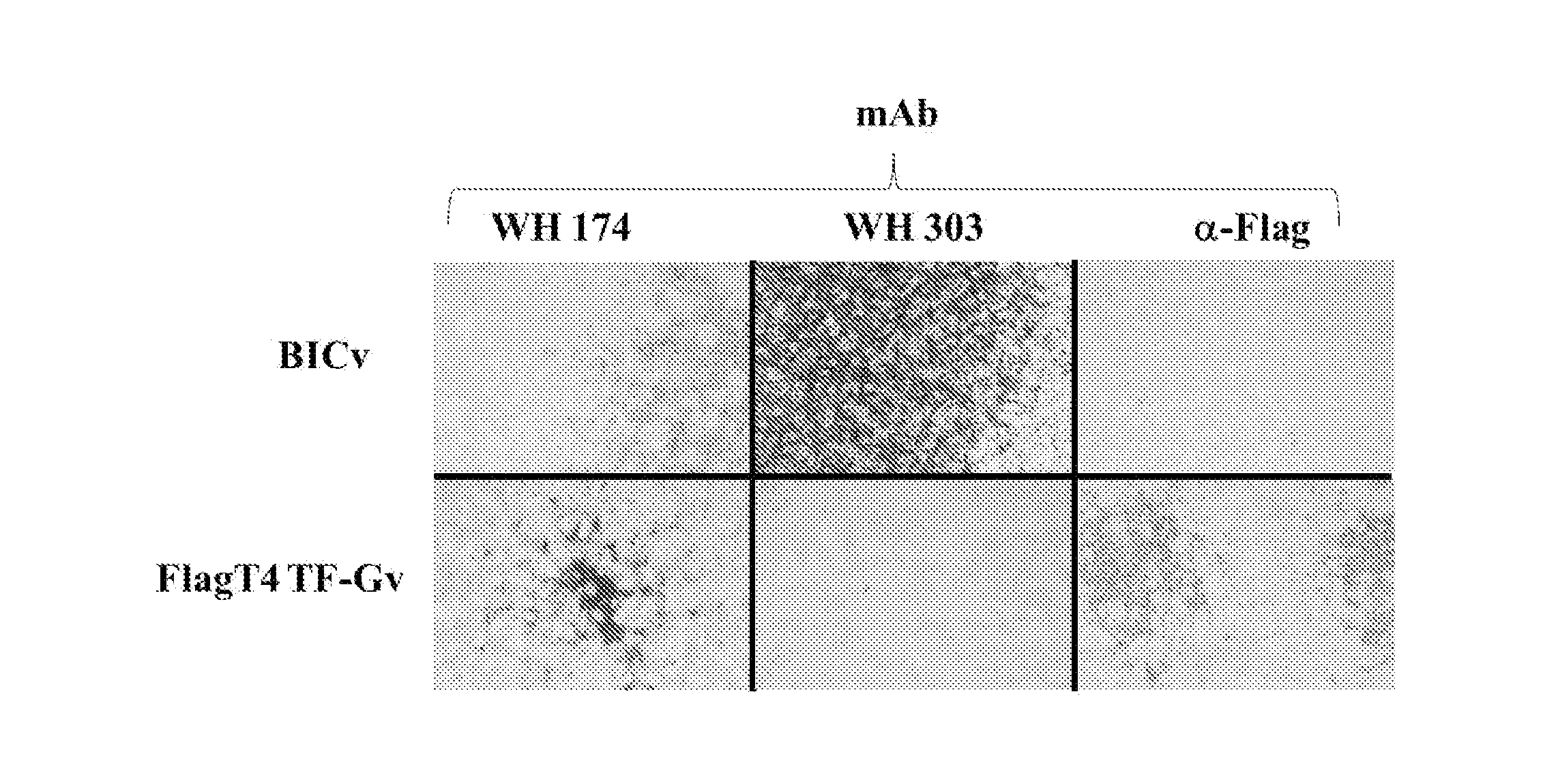
Controlling Classical Swine Fever Virus (CSFV) involves either prophylactic vaccination or non-vaccination and elimination of infected herds depending on the epidemiological situation. Marker vaccines allowing distinction between naturally infected from vaccinated swine could complement “stamping out” measures. Previously, we reported the development of FlagT4v, a double antigenic marker live attenuated CSFV strain. FlagT4v was later shown as not to be completely stable in terms of its attenuation when assessed in a reversion to virulence protocol. We have developed a modified version of the FlagT4v where changes in the codon usage of genomic areas encoding for Flag and T4 were introduced to rectify the reversion to the virulent genotype. The new virus, FlagT4-mFT-Gv, possesses the same amino acid sequence as FlagT4v except for one substitution, Asparagine is replaced by Glycine at position 852 of the CSFV polypeptide. FlagT4-mFT-Gv protected swine against challenge with Brescia virulent virus at 21 days post vaccination.
Owner:UNIV OF CONNECTICUT +1
Hepatitis E virus vaccine and method
InactiveUS20030143241A1Avoid infectionPreventing and treating HEV infectionSsRNA viruses positive-sensePeptide/protein ingredientsAntigenVaccination
Antigen and antibody vaccine composition effective in preventing hepatitis E virus (HEV) infection are disclosed. The antigen composition includes a peptide corresponding to a carboxyl terminal end region of the capsid protein encoded by the second open reading frame 2 of the HEV genome. The composition is effective in preventing HEV infection after vaccination. The antibody composition contains an antibody effective to block HEV infection of human primary hepatocytes in culture.
Owner:GENELABS TECH INC
Cytotoxic T lymphocyte inducing immunogens for prevention treatment and diagnosis of dengue virus infection
Owner:EMERGEX VACCINES HLDG LTD
SARS-CoV-2 specific polypeptides and application thereof
PendingCN113845577AImprove featuresIncreased sensitivitySsRNA viruses positive-senseViral antigen ingredientsActivation cellsT cell
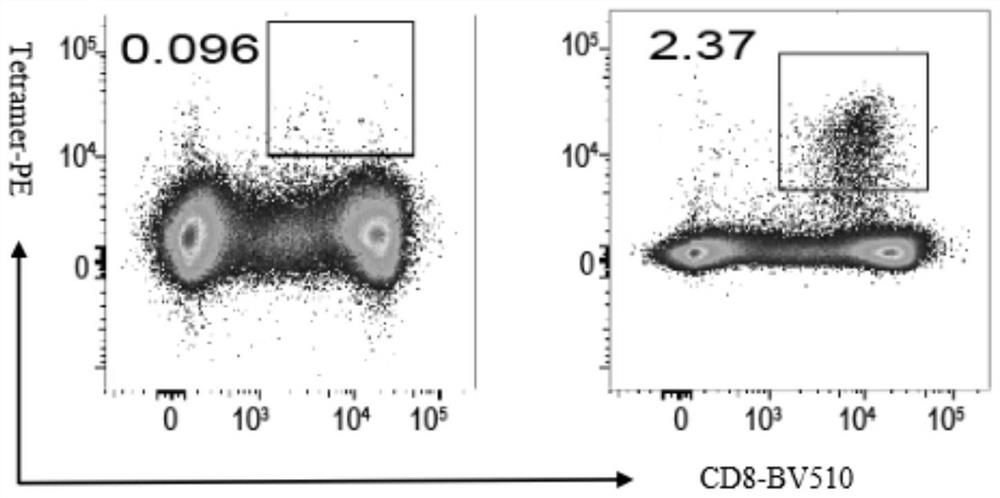
The invention discloses SARS-CoV-2 specific polypeptides and application thereof, and belongs to the field of immunodetection. The invention provides two specific polypeptides aiming at SARS-CoV-2; a polypeptide-MHC tetramer is prepared from the corresponding polypeptides; the polypeptide-MHC tetramer is used for detecting T cells of SARS-CoV-2 infected convalescent patients; the positive rates are 3.63% and 2.37% respectively and are obviously higher than 0.061% and 0.096% of a negative control group; the affinity of the polypeptide-MHC and TCR on the surfaces of specific T cells is effectively increased; the technology can be used as an effective tool for T cell evaluation, can also be used for separating and cloning the specific T cells, is combined with a single cell sequencing technology to separate specific TCR, and can be used as a T cell activation reagent and the like; and the technology has a relatively high application value in the aspect of researching the T cells of people infected with the SARS-CoV-2 or vaccinated with the SARS-CoV-2.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Broad and long-lasting influenza vaccine
InactiveUS20200316188A1SsRNA viruses negative-senseAerosol deliveryPharmaceutical drugFlu immunization
Provided herein are monovalent pharmaceutical compositions (vaccine compositions) and methods for inducing a multi-arm (mucosal, humoral and cell-mediated) immune response and extended seroprotection of at least 12 months post vaccination against influenza virus.
Owner:ALTIMMUNE INC
Diagnostic biomarkers and therapeutic targets for pancreatic cancer
InactiveUS20160327560A1Long-term disease-free survivalBiological material analysisDiagnostic biomarkerWilms' tumor
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com