Vaccine clinical trial management method and system
A clinical trial and network management technology, applied in data processing applications, instruments, resources, etc., can solve the problems of inability to obtain test data in real time and accurately, low degree of informatization, low degree of intelligence, etc., and achieve network management. , the effect of high degree of informatization and high degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
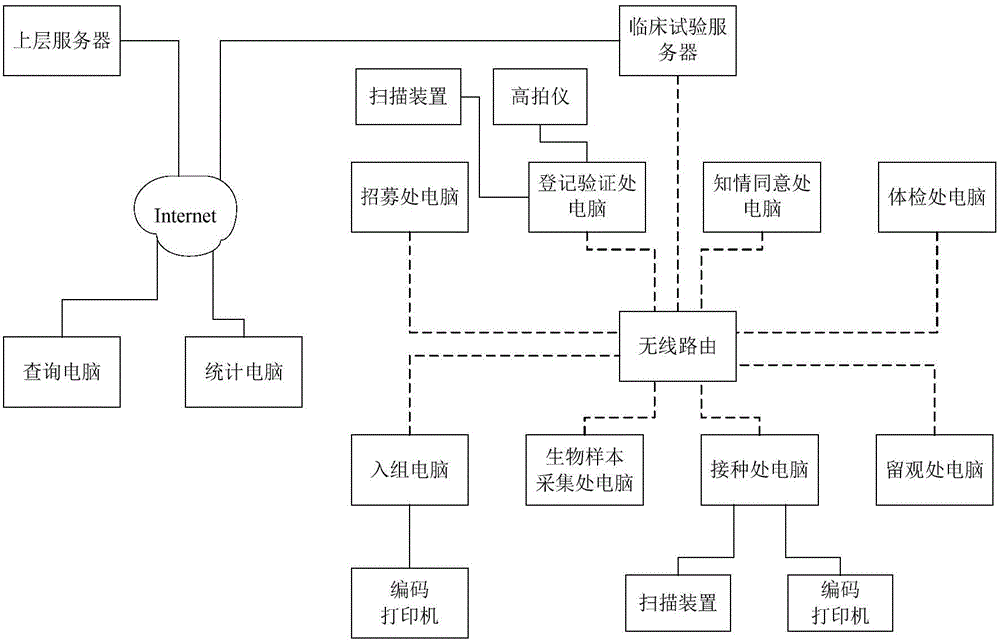
[0085]Network management methods for vaccine clinical trials, including:
[0086] S0. Recruit vaccine clinical trials, screen out candidates who meet the vaccination requirements, store the information of all candidates who meet the vaccination requirements in the clinical trial database, and conduct randomization according to the clinical trial parameters of the subjects The clinical trial code table is formed by grouping and stored in the clinical trial database; clinical trial parameters include any one or more of age, region, immunization history, gender, weight, race, past history, and medical laboratory testing parameters. In addition to any one or more of the above parameters, the clinical trial parameters may also include any one or more of the subject's risk factors, social characteristics or prognostic factors. Medical laboratory testing parameters include physical examination testing parameters such as human biochemical indicators. The specific parameters of the cl...
Embodiment 2
[0109] Vaccine clinical trial network management system, including:
[0110] The initial module is used to recruit vaccine clinical trials, screen out candidates who meet the vaccination requirements, and store the information of all candidates who meet the vaccination requirements in the clinical trial database. Parameters are randomly grouped to form a clinical trial code table and stored in the clinical trial database; clinical trial parameters include any one or more of age, region, immunization history, gender, weight, race, past history, and medical laboratory testing parameters . In addition to any one or more of the above parameters, the clinical trial parameters may also include any one or more of the subject's risk factors, social characteristics or prognostic factors. Medical laboratory testing parameters include physical examination testing parameters such as human biochemical indicators. The specific parameters of the clinical trial parameters can be adjusted ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com