Test paper strip for detecting encephalitis virus specificity IgG antibody, method for making same and applications
A Japanese encephalitis virus and antibody detection technology, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of routine use, time-consuming, complicated operation, etc. Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 Japanese encephalitis virus E gene antigen domain III
[0034] (1) Obtaining the target gene
[0035] According to the sequence of the target gene fragment and the characteristics of the pGEX-4T-1 expression vector, design primers containing restriction enzymes BamH1 and HindIII restriction sites at both ends:
[0036] 5'TAAGGATCCCACCTGAAATGTAGGCTG3'
[0037] 5'CGGGAAGCTTGAAGACCCCTCCAATAGA3'
[0038] The total viral RNA was extracted by conventional methods, and then RT-PCR was performed immediately, and the reverse transcription product was identified and recovered by 1% agarose gel electrophoresis.
[0039] (2) Cloning of the target gene and screening of positive recombinants
[0040] Ligate the recovered PCR amplification product with the PMD-18T cloning carrier overnight at 16°C, transform into DH5a competent cells, pick a monoclonal strain, culture overnight at 37°C, extract the plasmid, and use the plasmid as a template to carry ...
Embodiment 2
[0049] The preparation of embodiment 2 Japanese encephalitis virus E gene antigen domain III polyclonal antibody
[0050] (1) Animal immunity:
[0051] New Zealand white rabbits of 1-2 kg were selected, and subcutaneously injected with Japanese encephalitis virus E gene antigen domain III at multiple points on the back, and the immunization dose was 0.5-1 mg / kg. A total of 3 to 5 times of immunization.
[0052] (2) Immunological titer detection:
[0053] Coat the Japanese encephalitis virus E gene antigen domain III protein microtiter plate, 4 μg per well. The titer of immune serum was detected by indirect ELISA method. When the serum titer reaches 1:20000 or more, serum can be collected.
[0054] (3) Antibody purification and verification:
[0055] Purification by conventional octanoic acid method. The purity was checked by non-denaturing PAGE electrophoresis, showing a protein band. The activity is tested by ELISA, and the titer is greater than 1:20000.
Embodiment 3
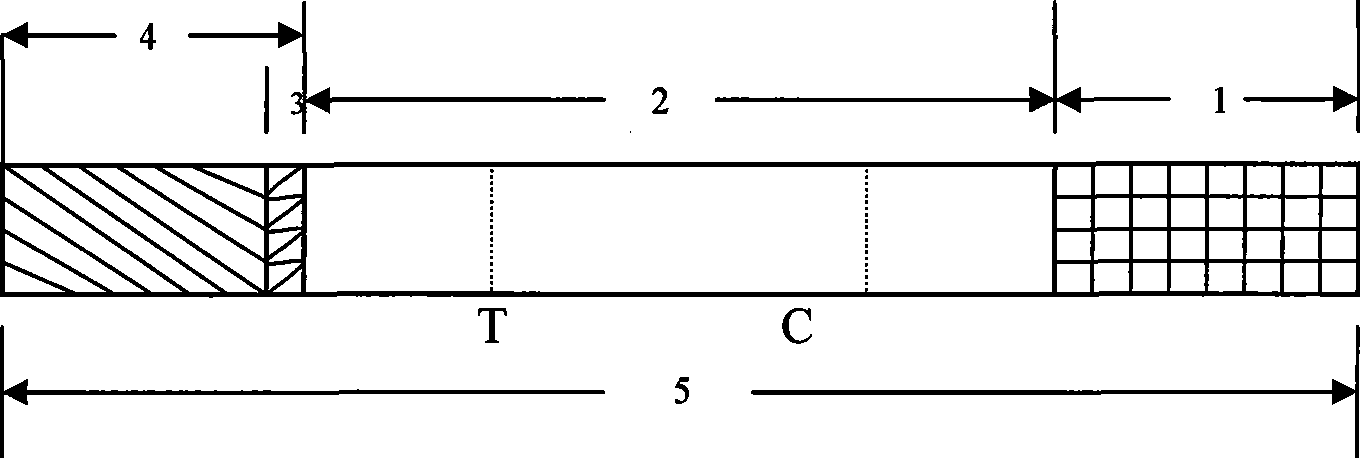
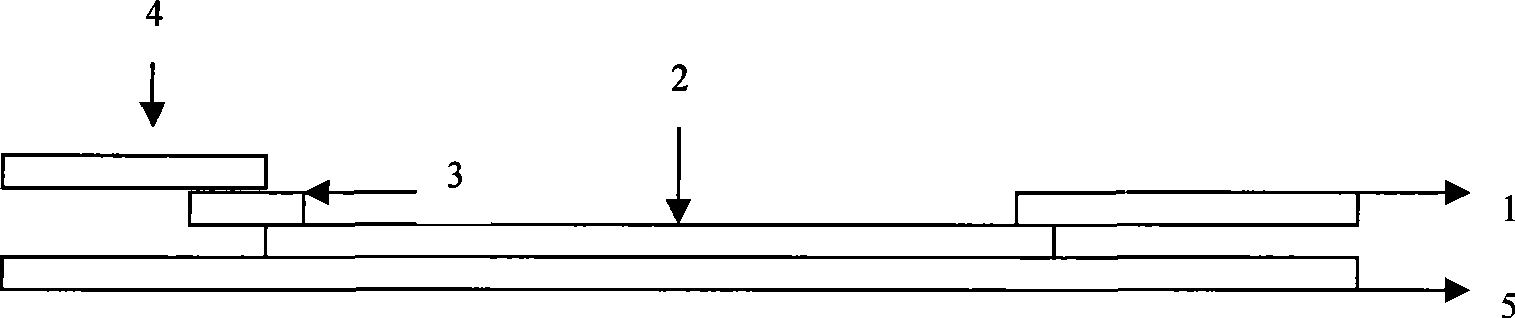
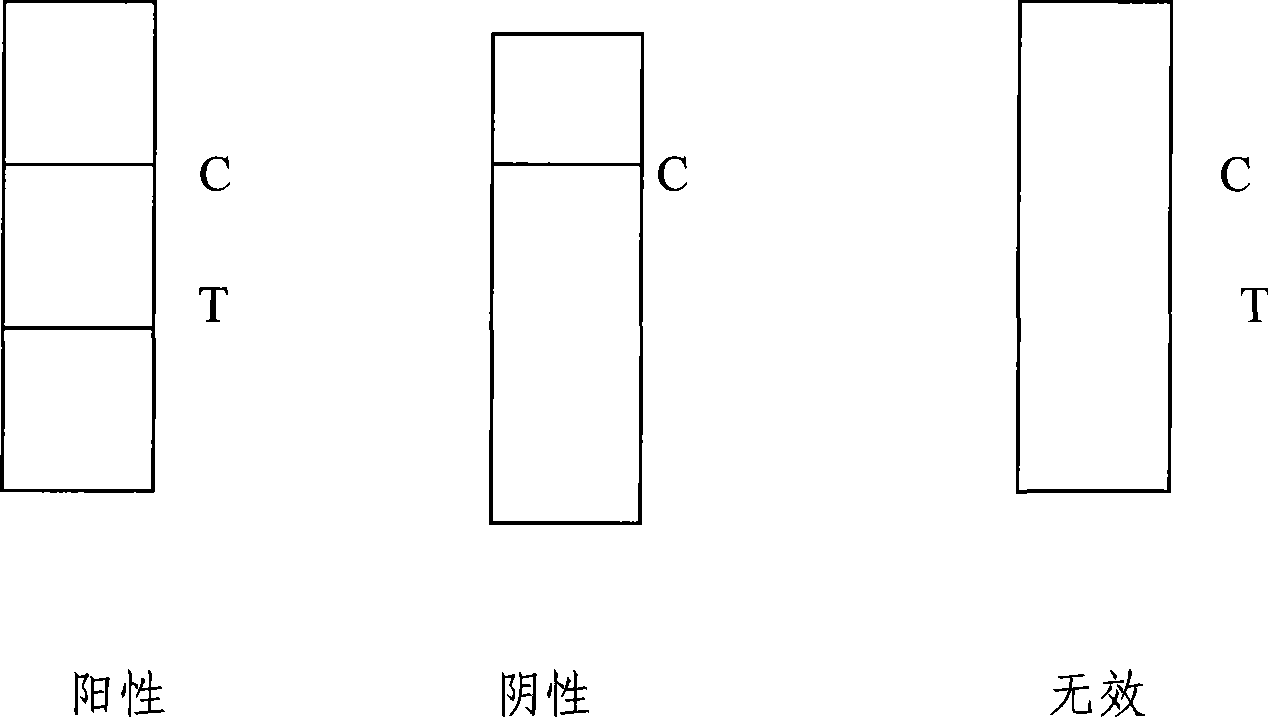
[0056] Example 3 Japanese encephalitis virus-specific IgG antibody colloidal gold rapid detection test strip (see Figure 1)
[0057] (1) Preparation of colloidal gold-antibody conjugates:
[0058] The optimum labeling pH value of JE virus E gene antigen domain III or anti-human IgG monoclonal antibody is 8.0, and the ratio of JE virus E gene antigen domain III and colloidal gold is 18 μg / ml colloidal gold; anti-human IgG monoclonal antibody The optimal ratio of antibody to colloidal gold is 20μg / ml colloidal gold. After being treated with a stabilizer (containing 0.05% BSA, pH 8.0, 0.01 MTris buffer solution), it was evenly adsorbed on a glass fiber membrane in an amount of 65 μl per square centimeter, freeze-dried, and set aside.
[0059] (2) Coating antigen on nitrocellulose membrane:
[0060] Dilute the JE virus E gene antigenic domain III to 3.5 mg / ml, dilute the anti-JE virus polyclonal antibody or anti-mouse IgG antibody to 2 mg / ml, spray on the nitrocellulose membrane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com