Patents
Literature
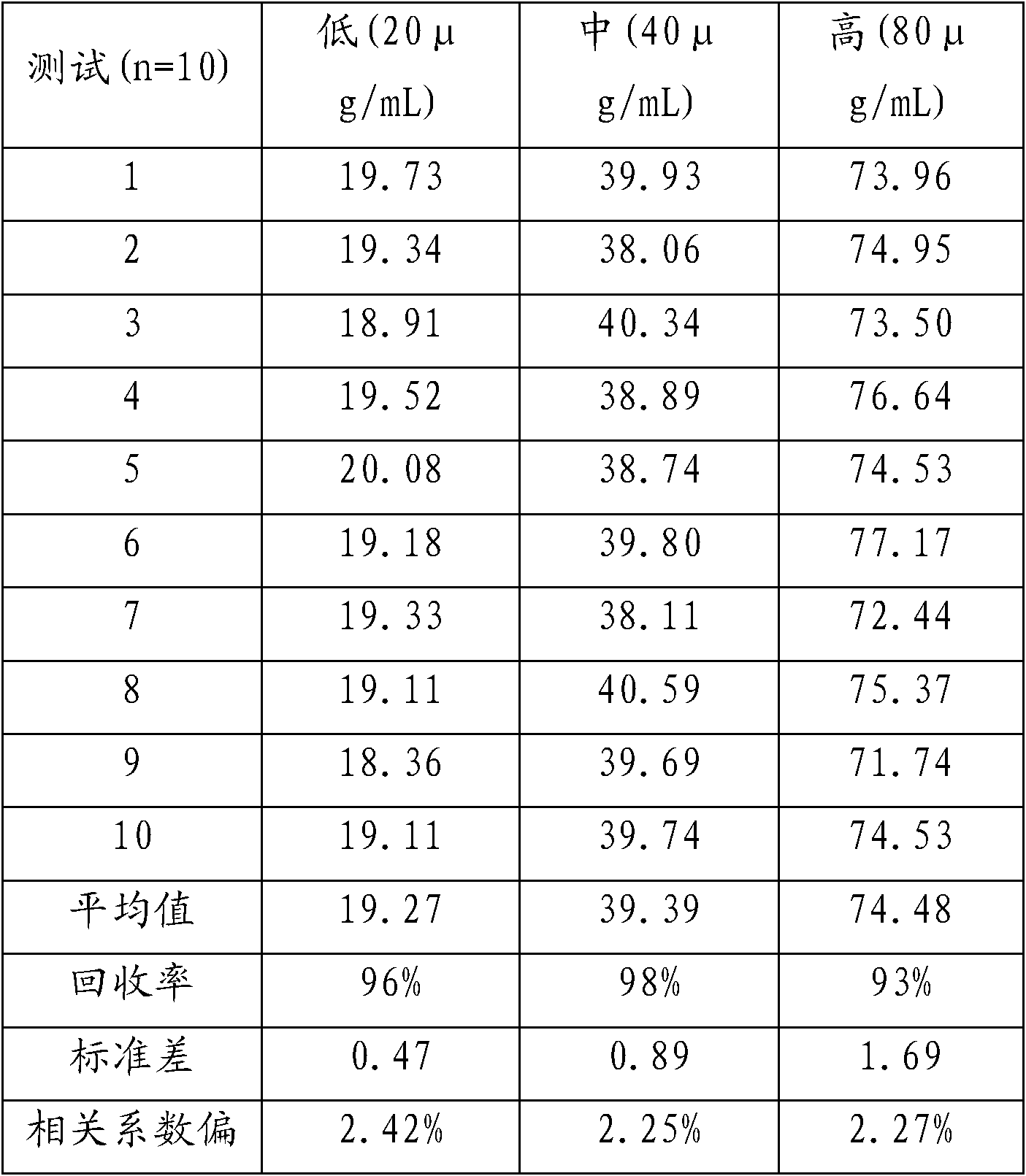
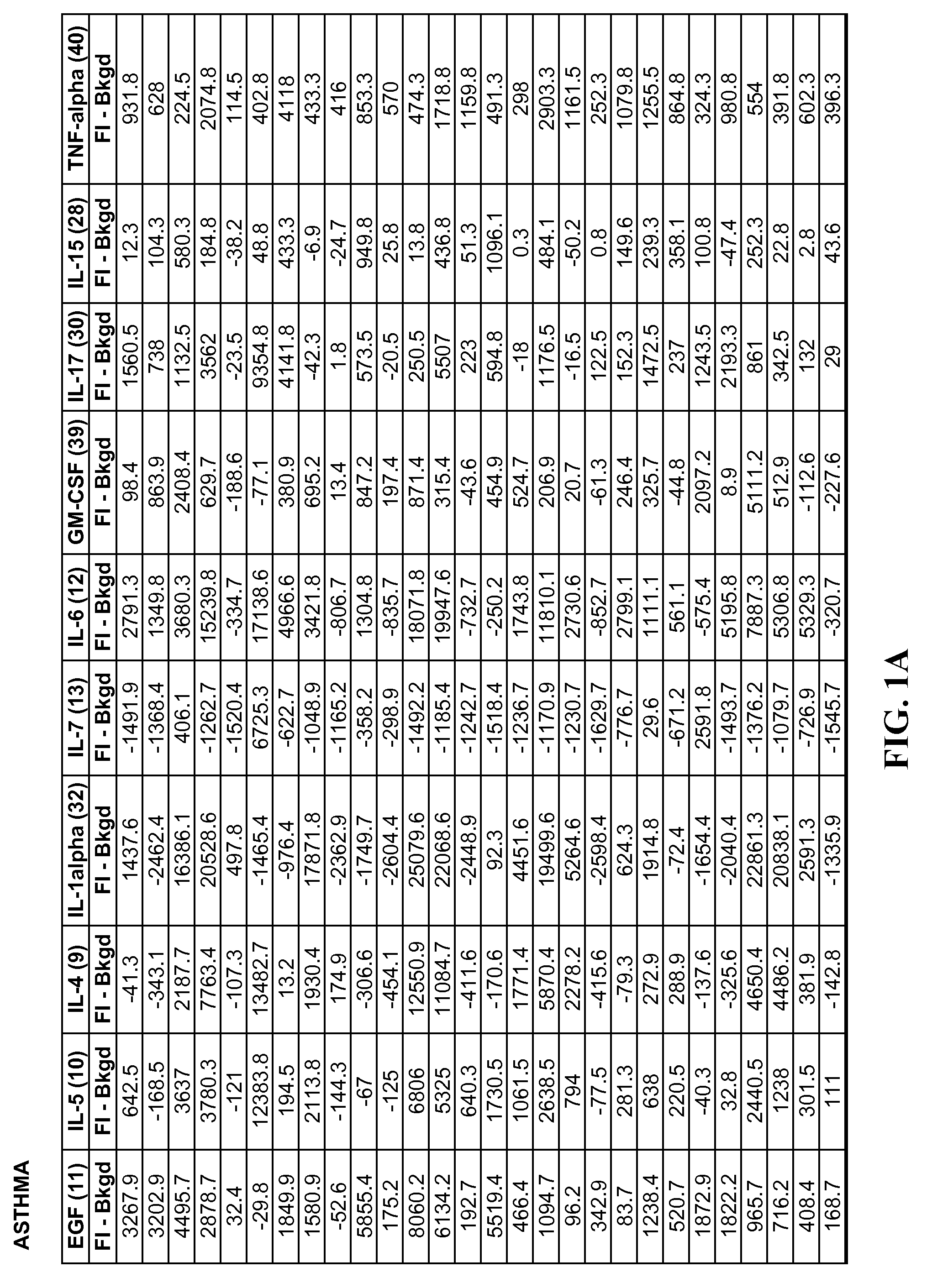
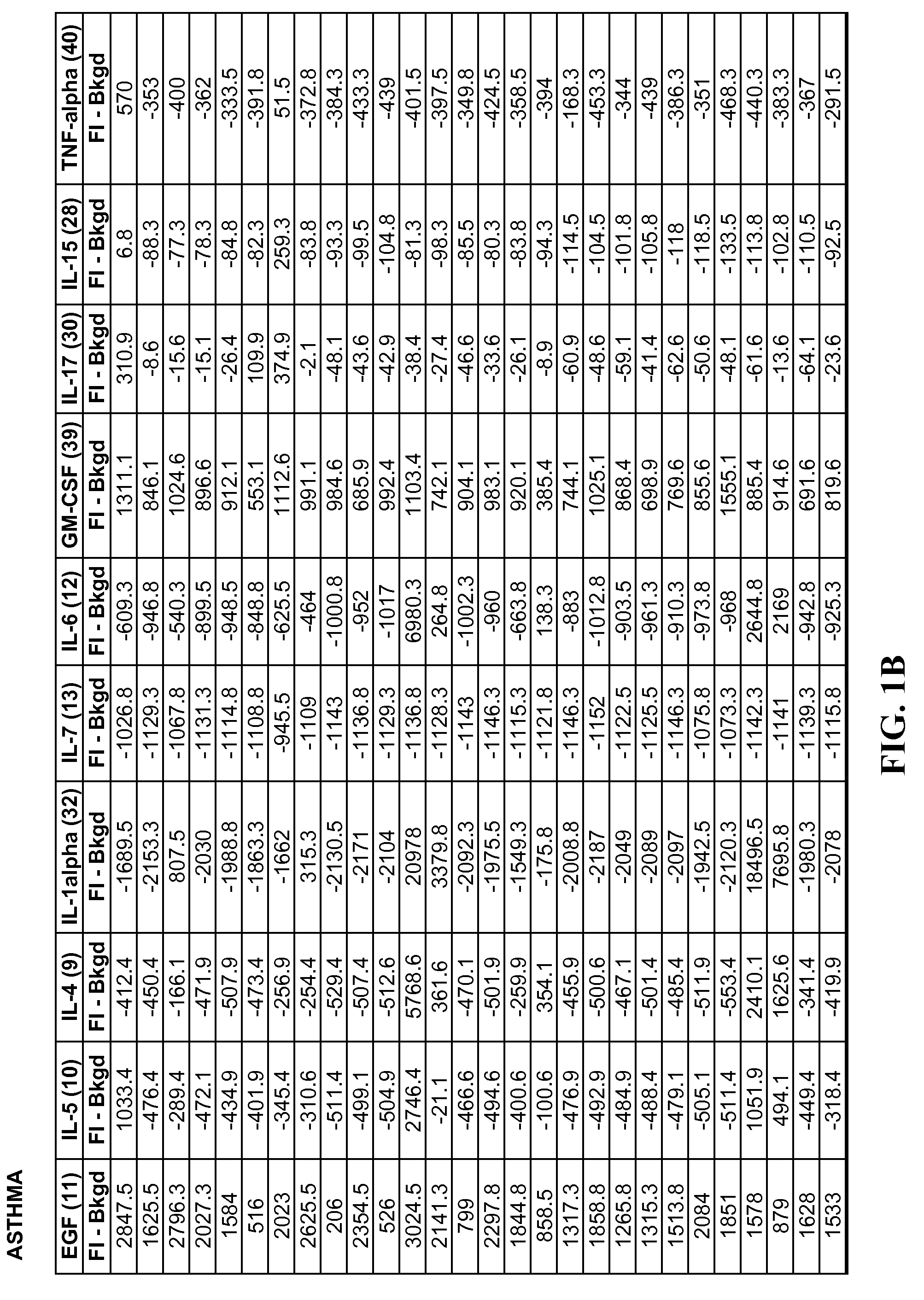
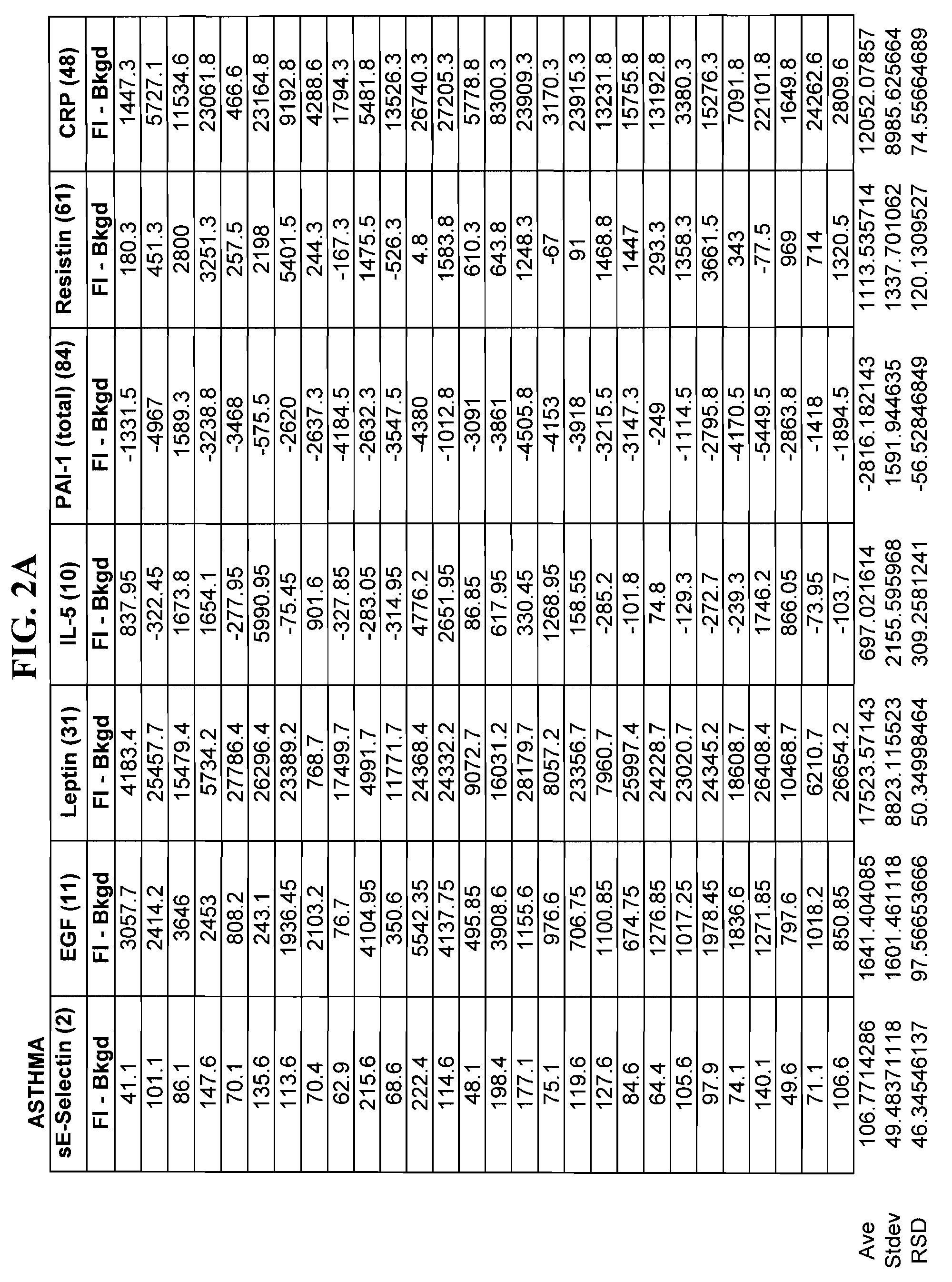
63 results about "Serum specimen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
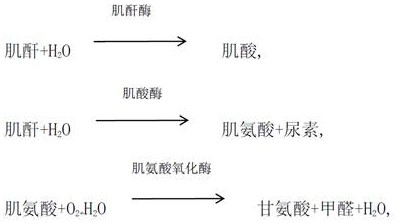
Serum is the supernatant fluid when clotted blood has been centrifuged. It is the best specimen for most clinical chemistry laboratory tests because of its specific characteristics. Here are the reasons why serum is the best specimen.
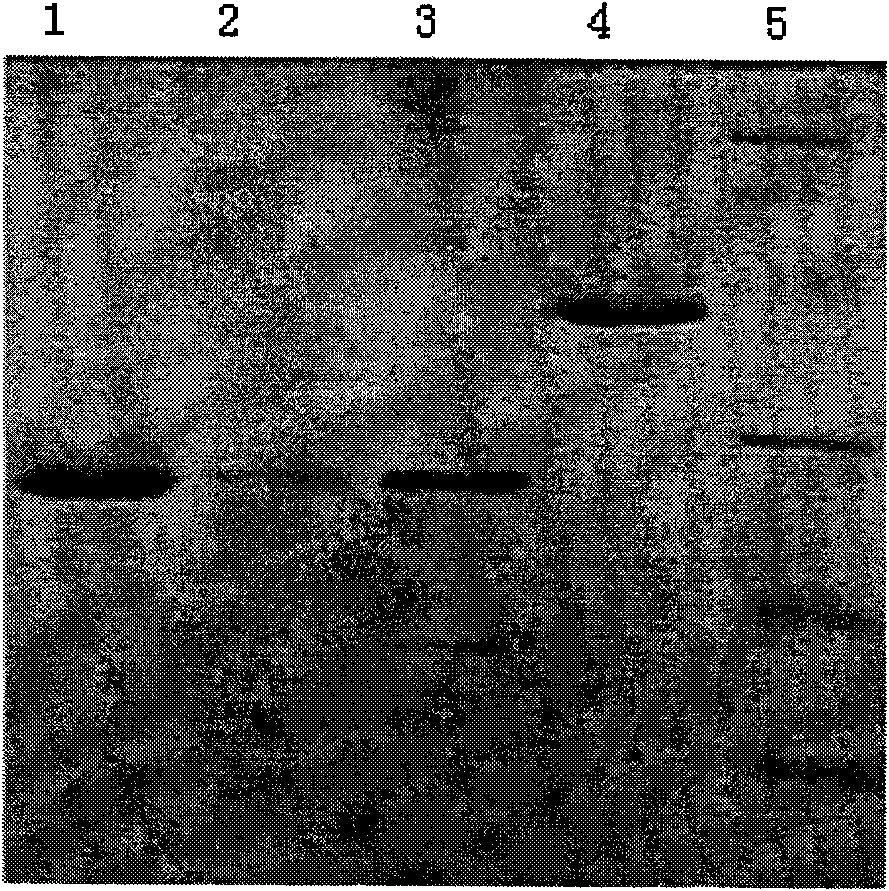
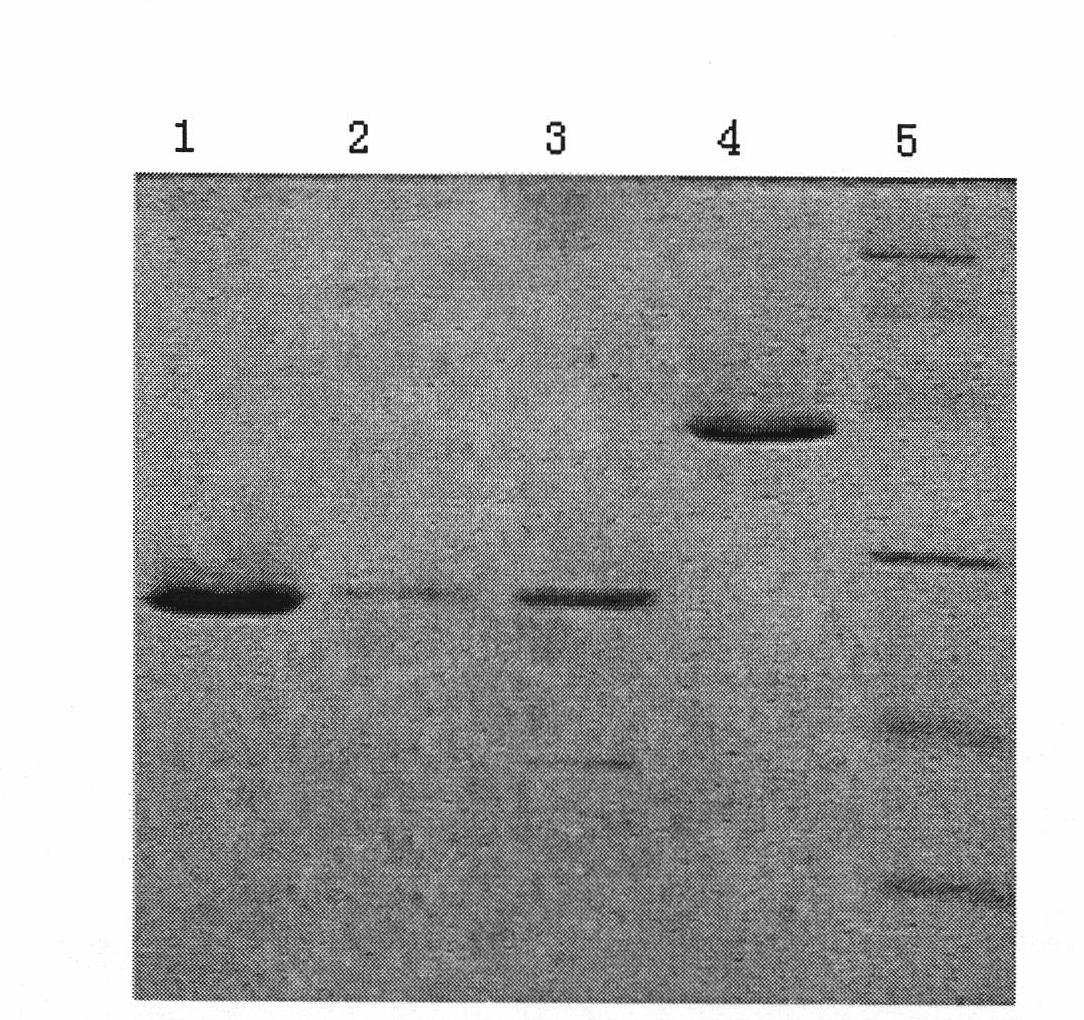
ELISA kit for detecting porcine circovirus antibody II
ActiveCN101629956ASimilar sensitivityStrong specificityMicroorganism based processesBiological testingSerum igeElisa kit
The invention relates to an ELISA kit for detecting porcine circovirus antibodies II, wherein, the kit is developed according to the double antigen sandwich ELISA principle. The ELISA kit comprises a prepacked ELISA antigen plate bar, a cleaning solution, 100 times concentrated enzyme labeled antigen, an enzyme labeled dilution, a zymolyte coloration, a stopping solution, standard PCV2 negative sera, and standard PCV2 positive sera. The kit replaces enzyme labeled anti-antibodies with the enzyme labeled antigen. The enzyme labeled antigen can not combine with antibodies absorbed with ELSA plate without specificity. Meanwhile, the serum specimen to be detected does not need to be pre-diluted, and the serum specimen can be directly mixed with the enzyme labeled antigen with working concentration to be directly used for determination. The ELISA kit has the advantages of simple operation and shorter detecting time.
Owner:北京金诺百泰生物技术有限公司
Preparation method of liquid phase protein chip
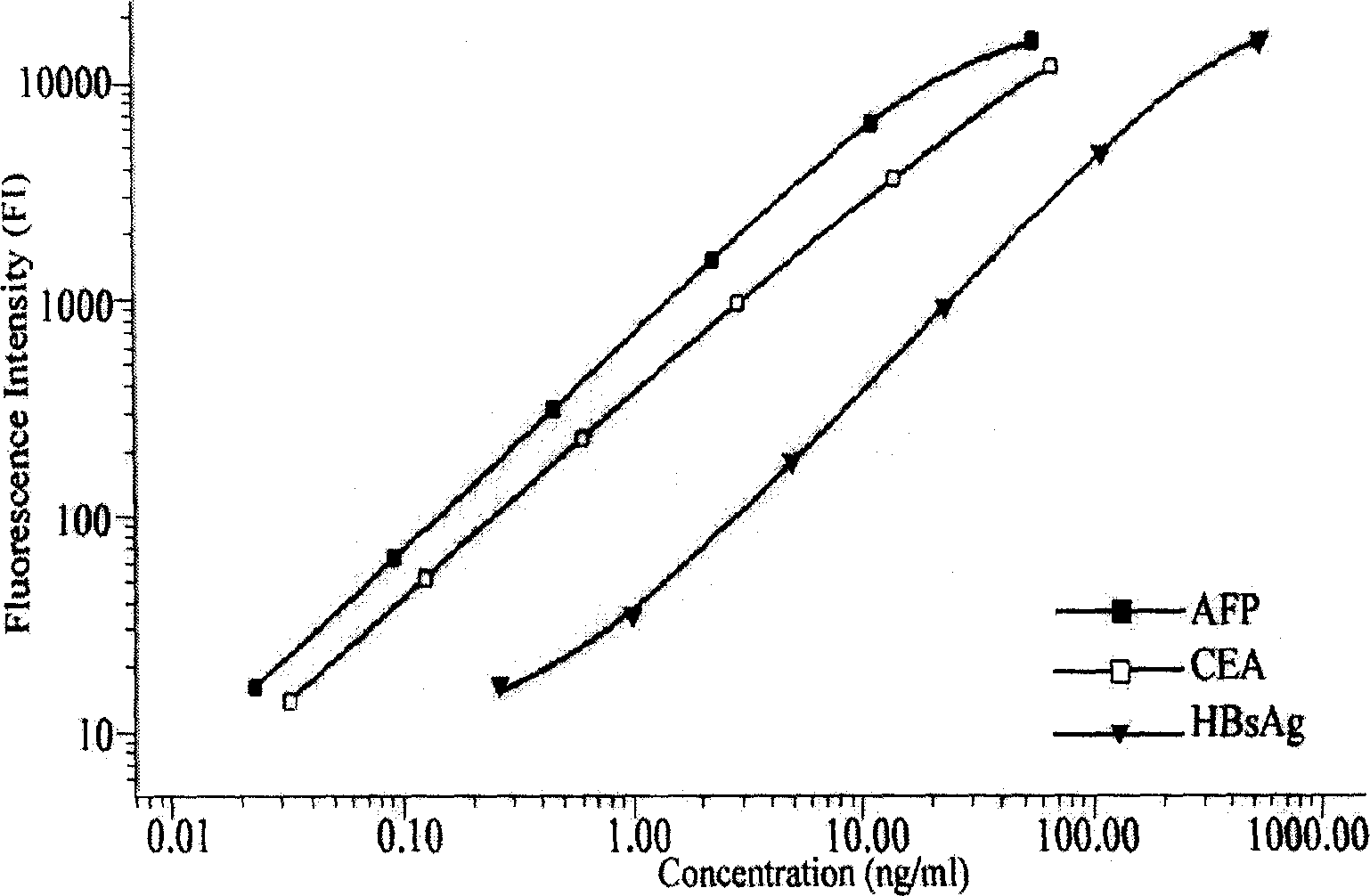
InactiveCN101144815AEarly detectionEarly treatmentMaterial analysisHigh risk populationsFluorescence
The present invention relates to the biologic technology field, and discloses a liquid phase albumen chip and the preparation and the usage method thereof which can simultaneously test human serum carcinoma embryonic antigen (CEA), Alpha fetoprotein (AFP), and hepatitis B surface antigen (HBsAg). The present invention couples the specificity antibody of CEA, AFP, and HBsAg on different fluorescence micro-spheres, and uses the test antibody marked by biotin or phycoerythrin to determine the nature quickly and quantitatively analyze the above three indexes with the double antibody sandwich method. The present invention uses the filtering membrane board when testing, and washes the board 3 times after each reaction is finished to increase the signal and improve the sensitivity. The present invention has high sensitivity, strong specificity, stable result, excellent repeatability, and simple operation; 1 micro liter serum sample can test three indexed simultaneously. The present invention is applicable to the health test and the general examination as well as the clinic test of the high risk population, and can facilitate the early diagnosis and the early treatment of the knub.
Owner:GUANGZHOU DARUI BIOTECH
Quantitative analysis of a biological sample of unknown quantity
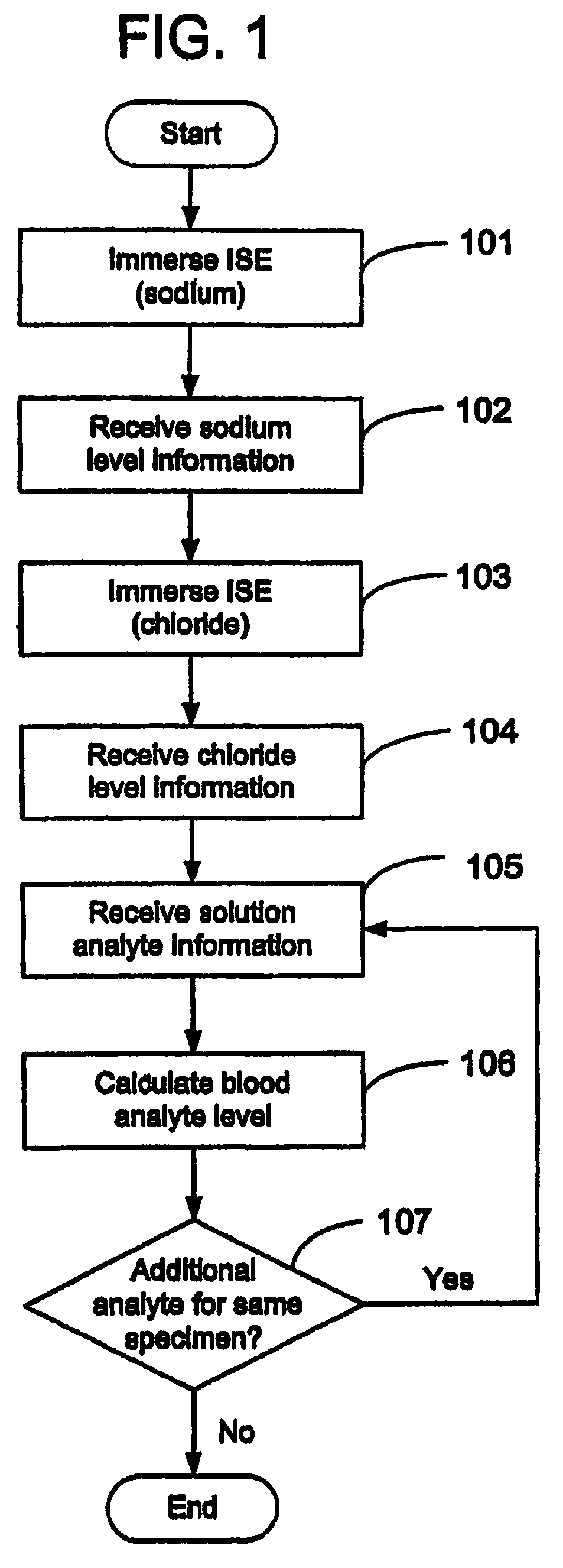
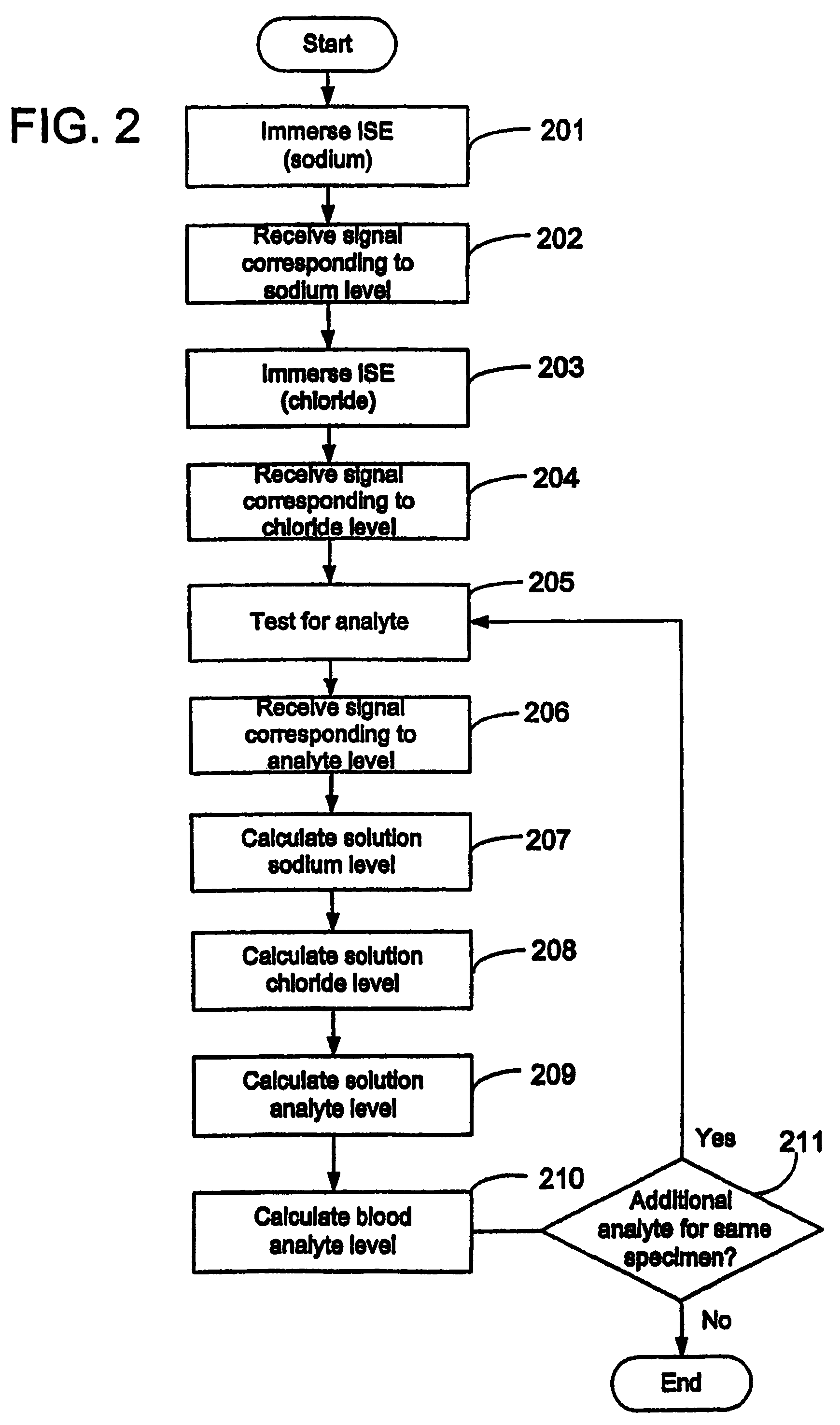
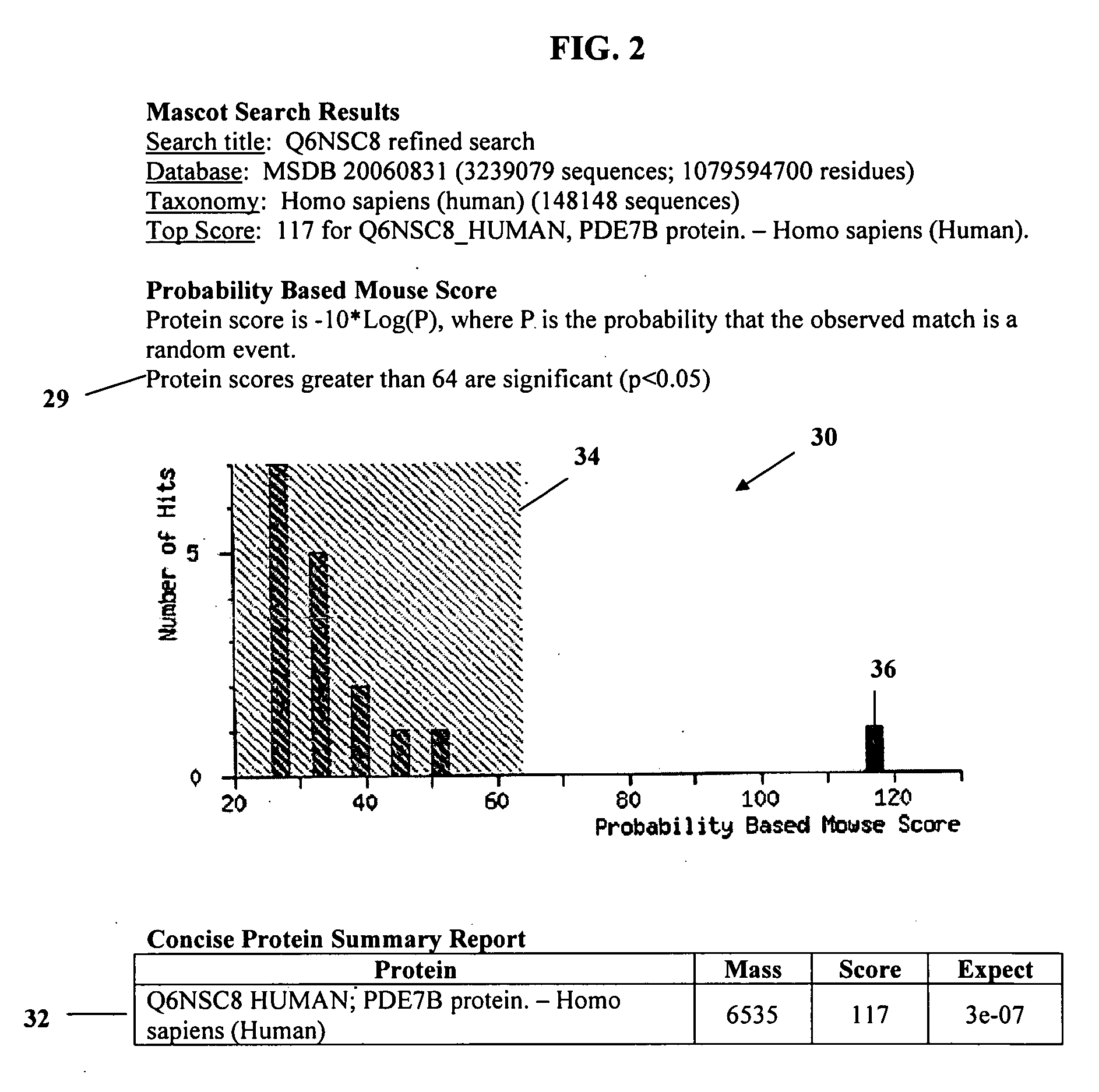
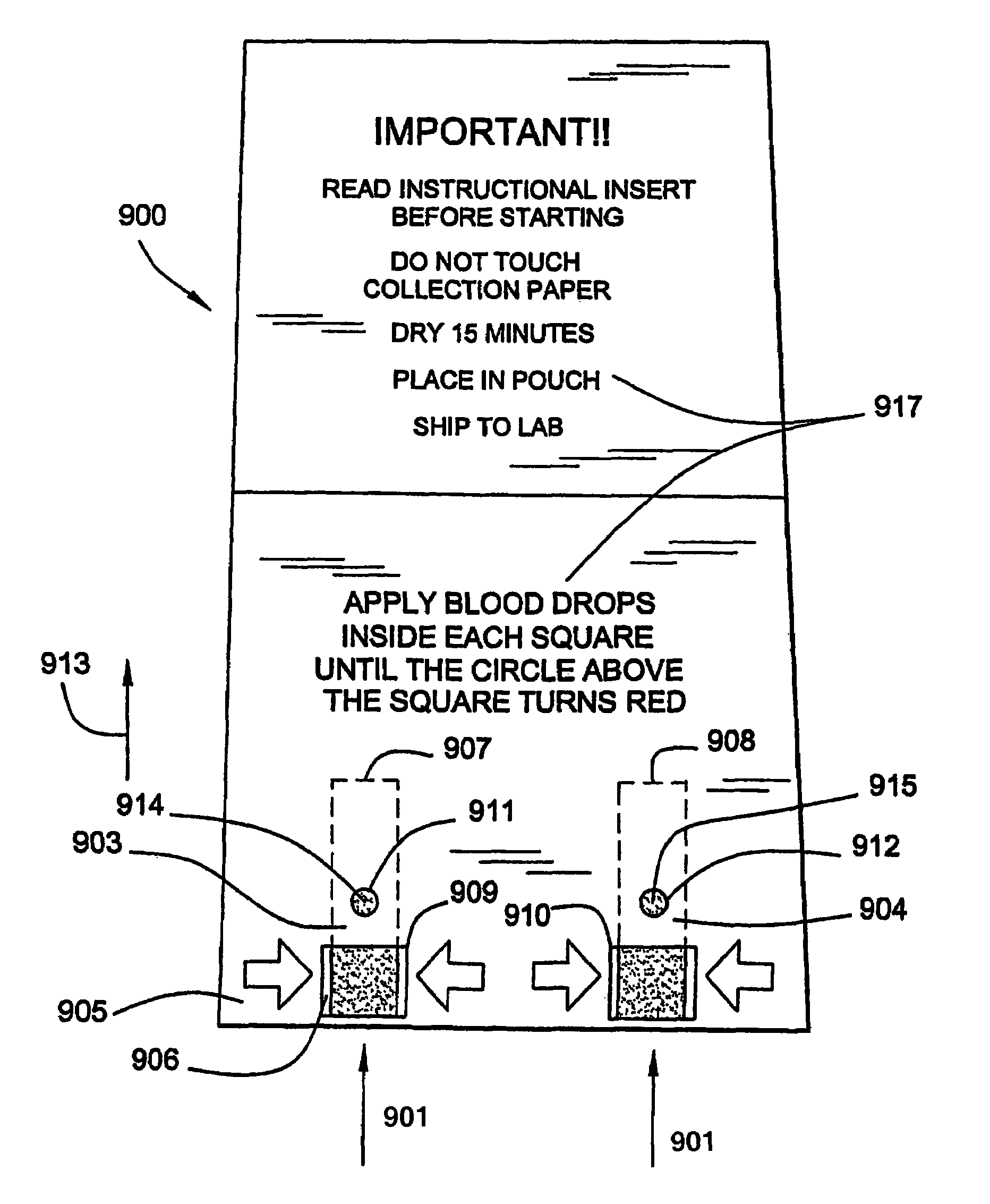
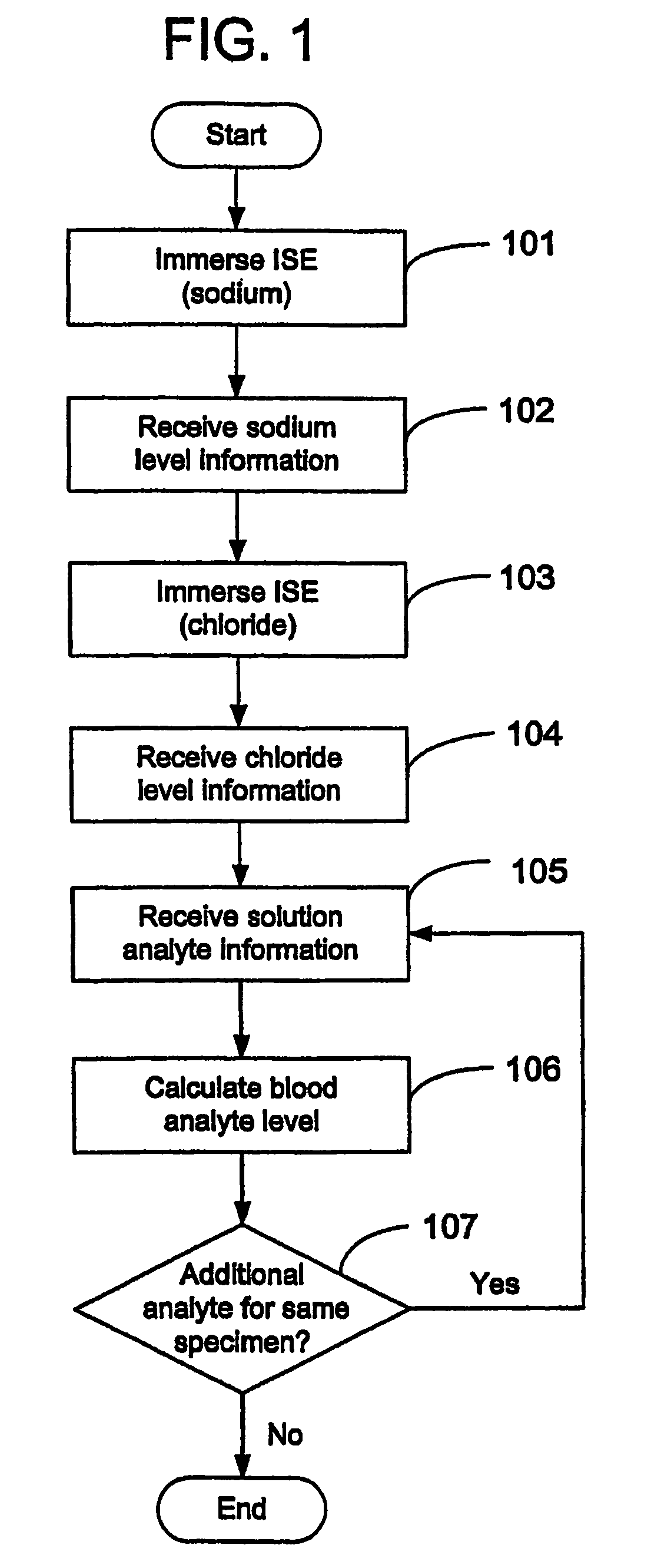
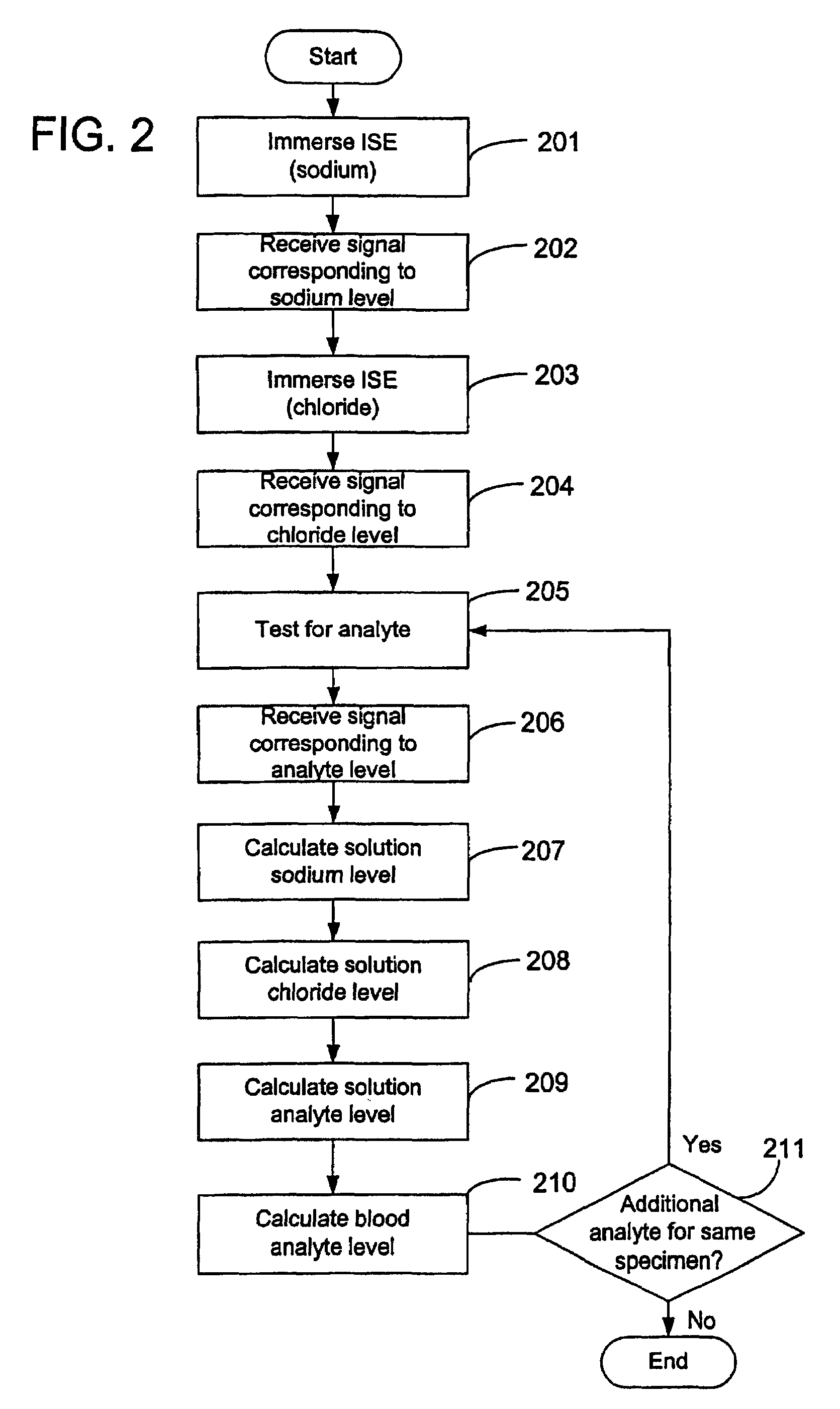
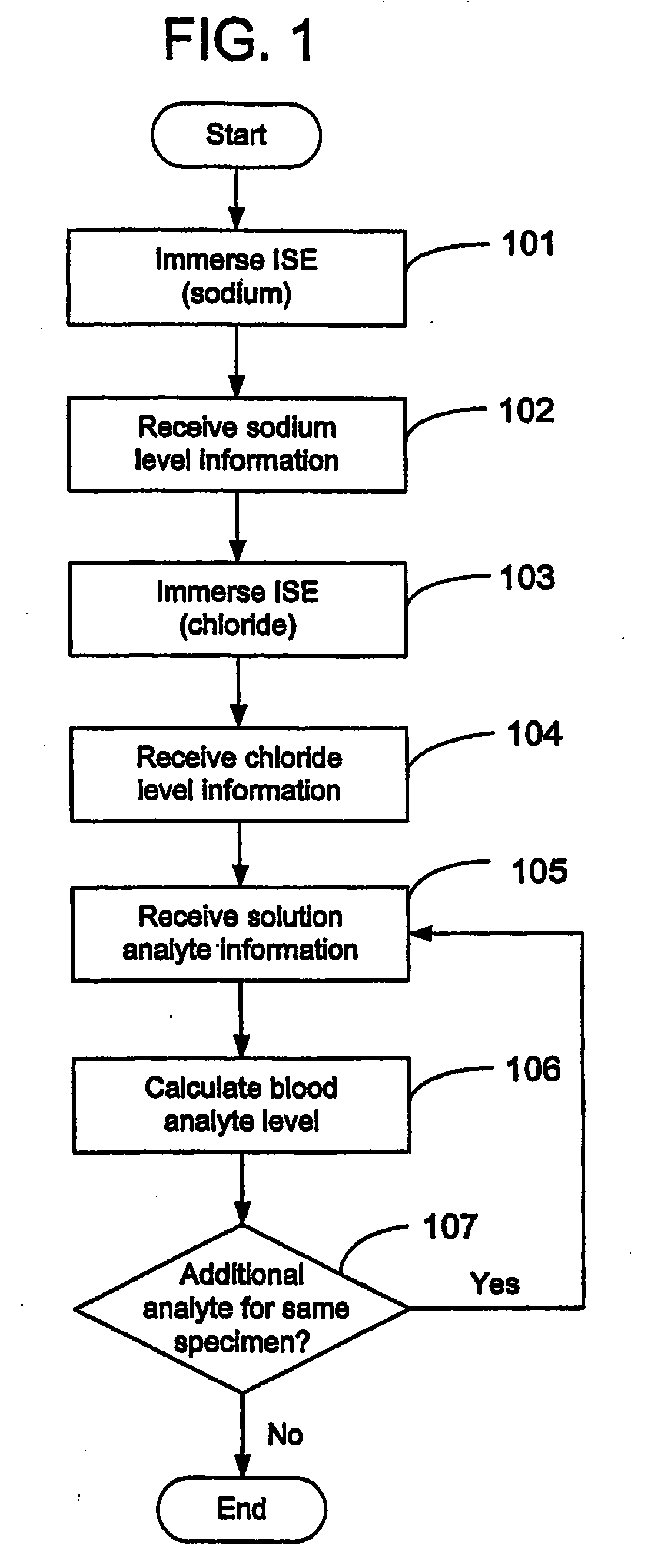
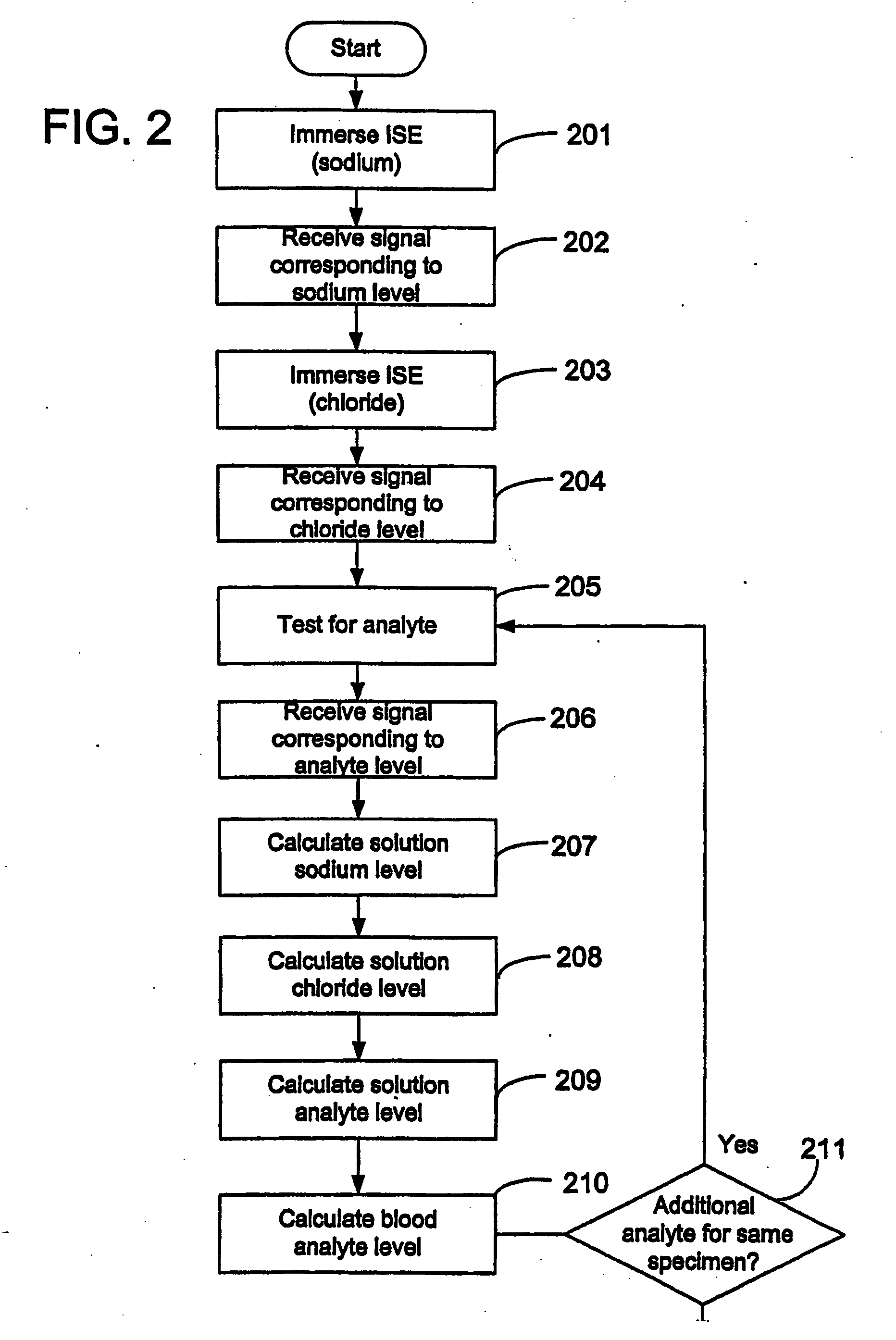
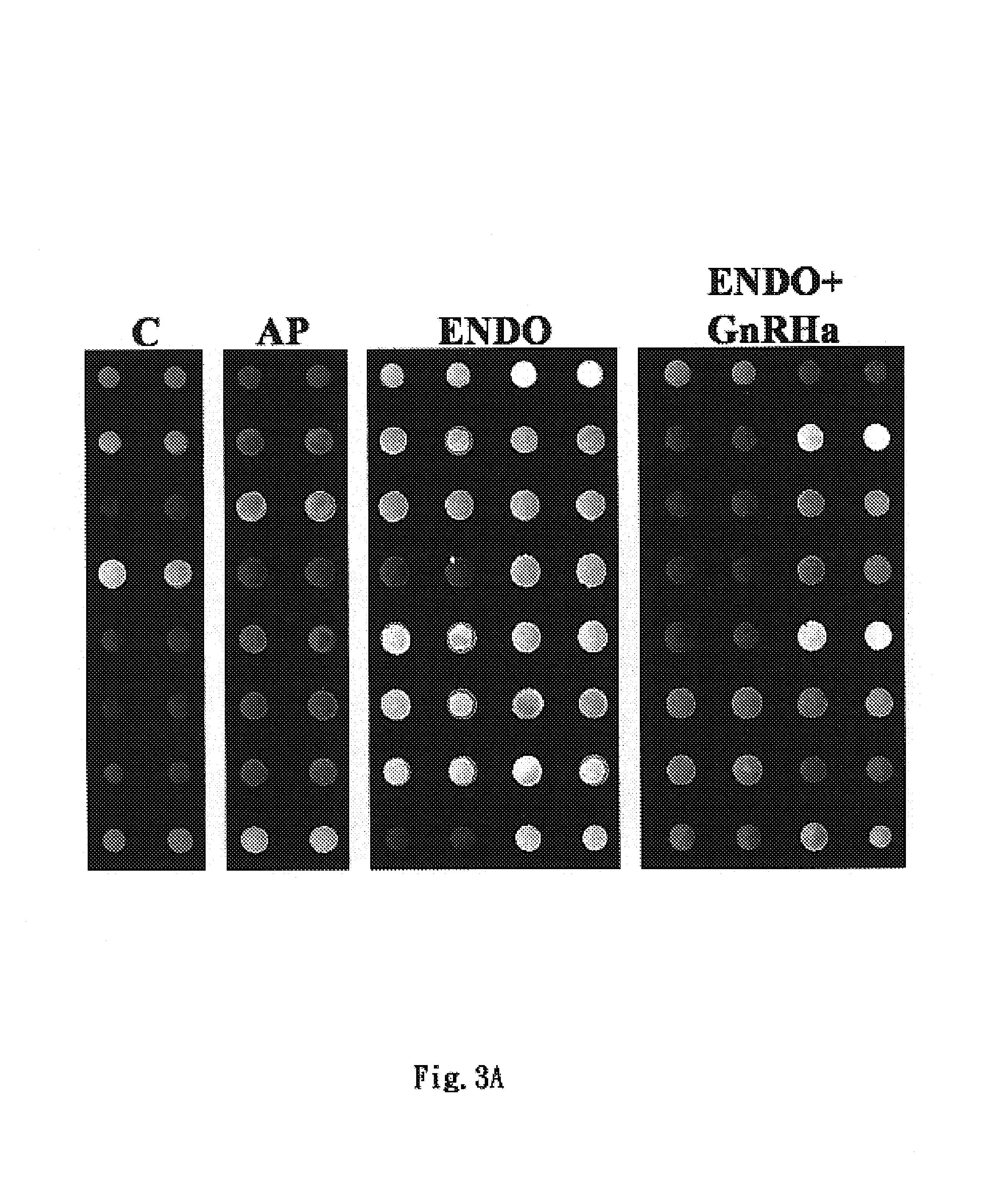
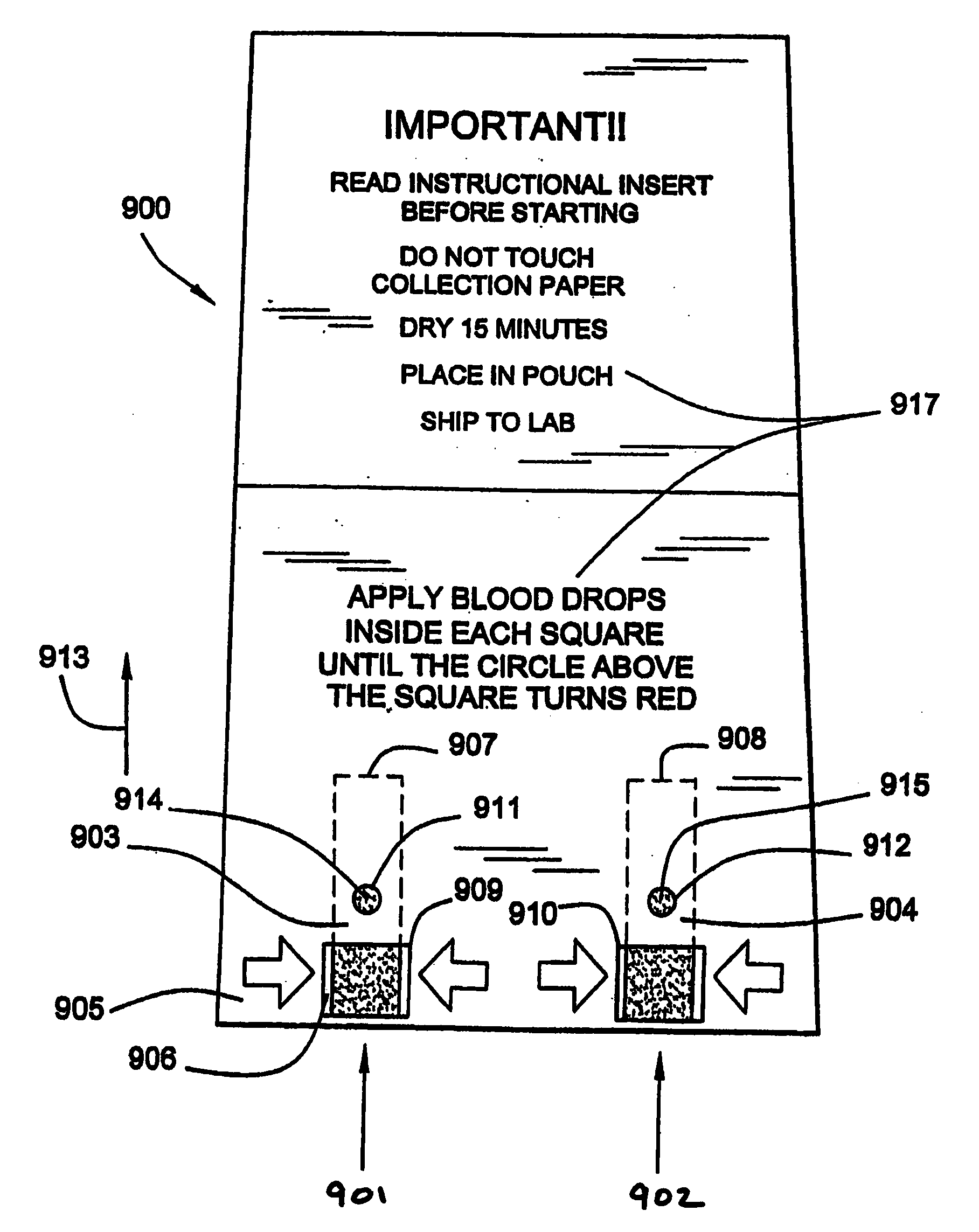
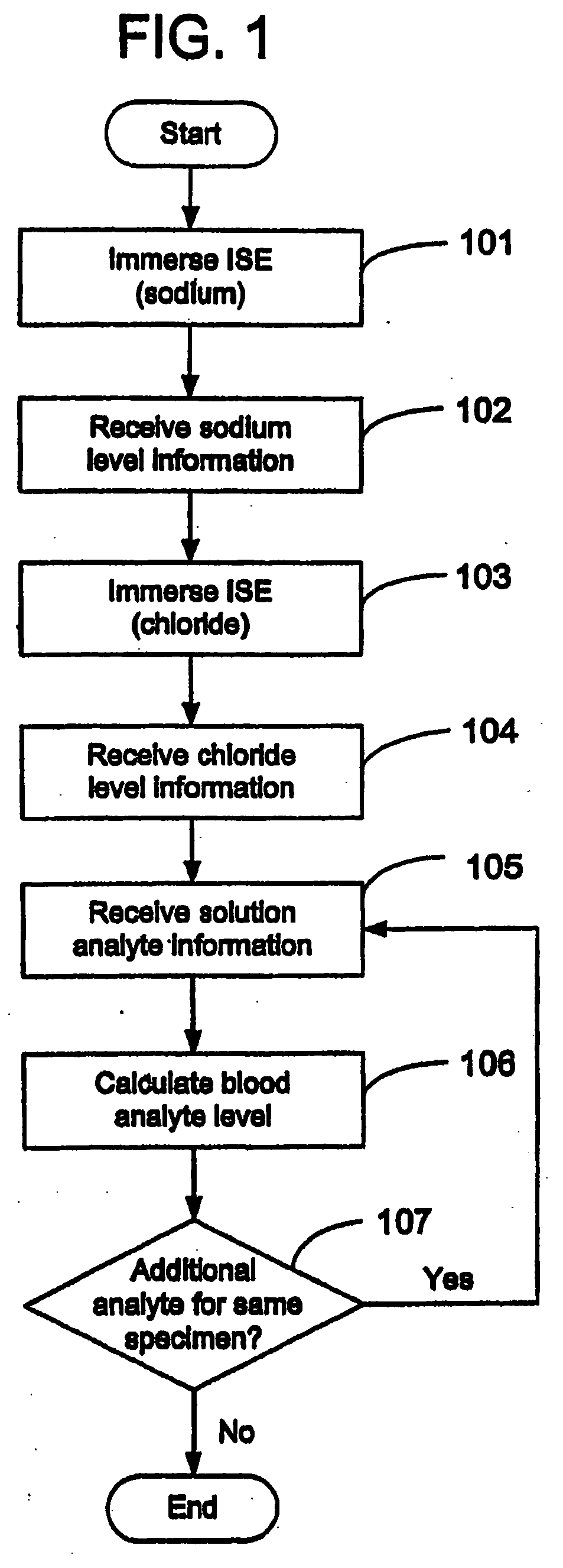
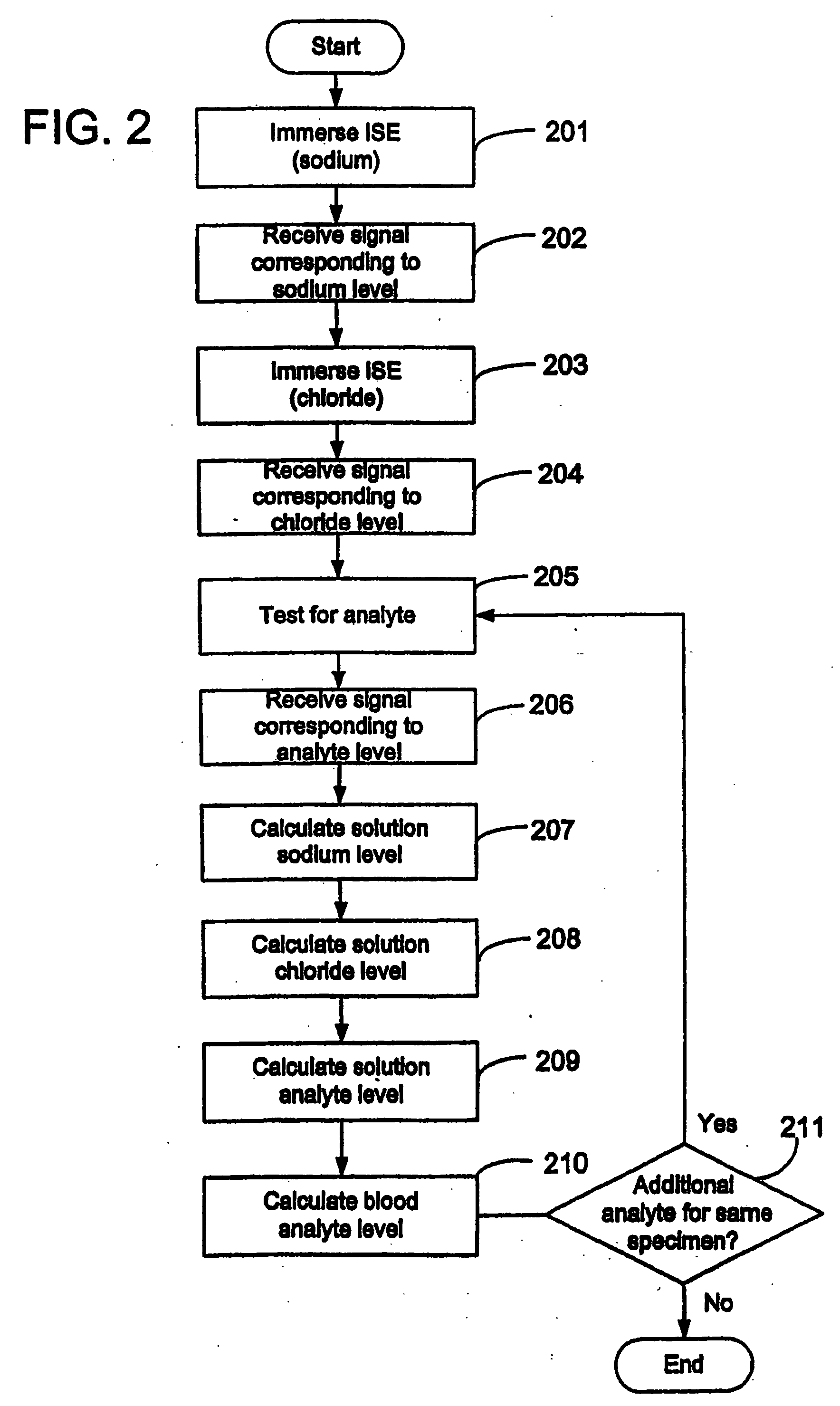
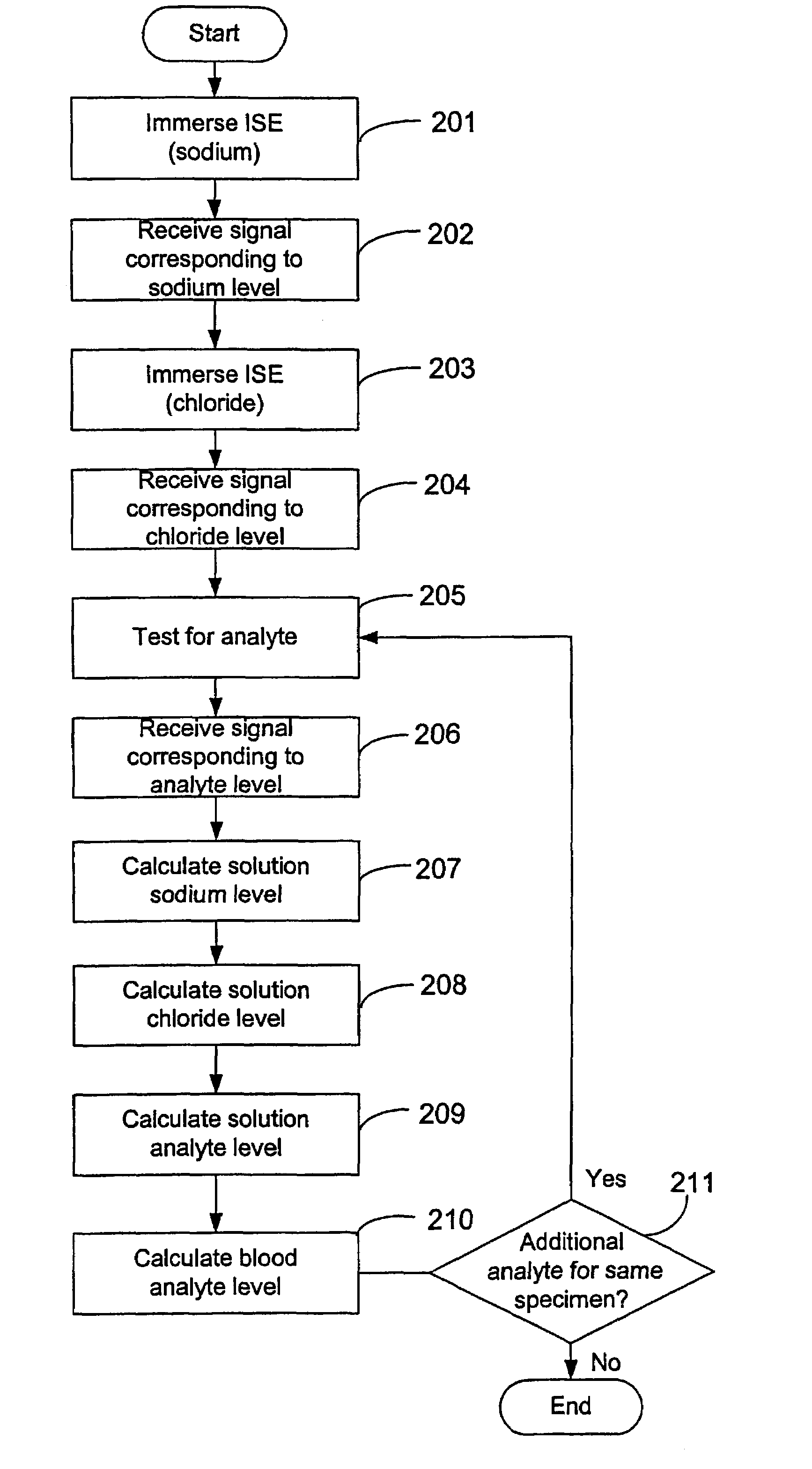
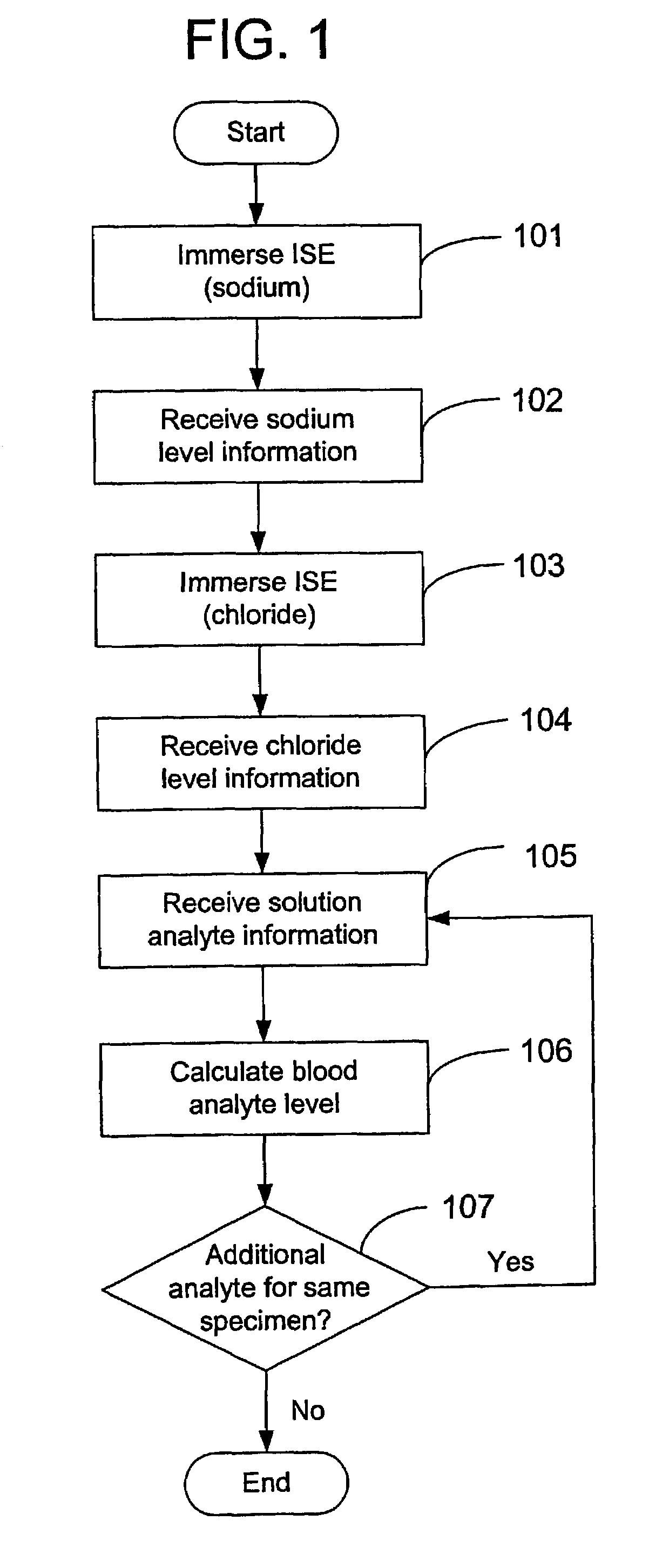
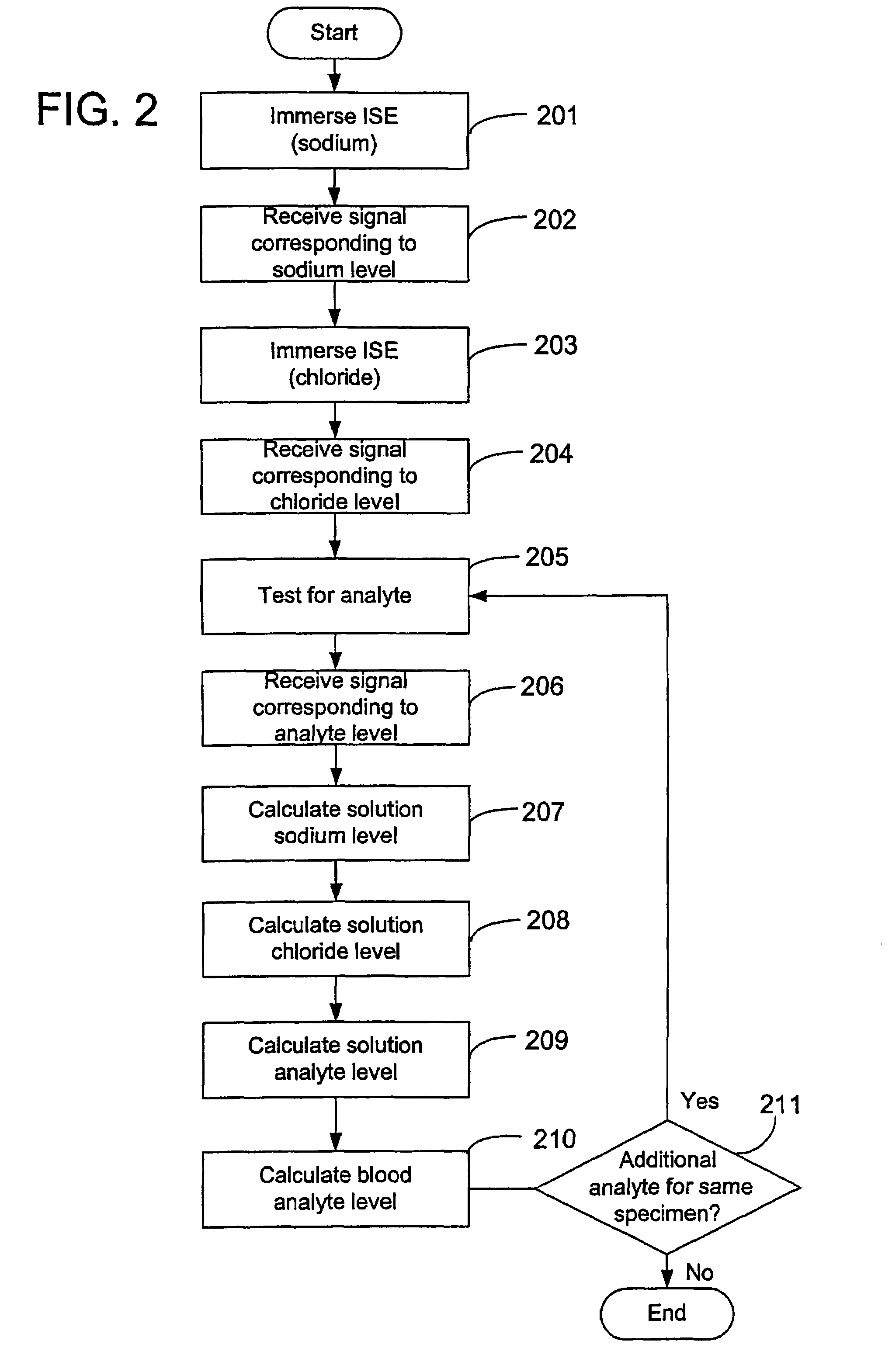
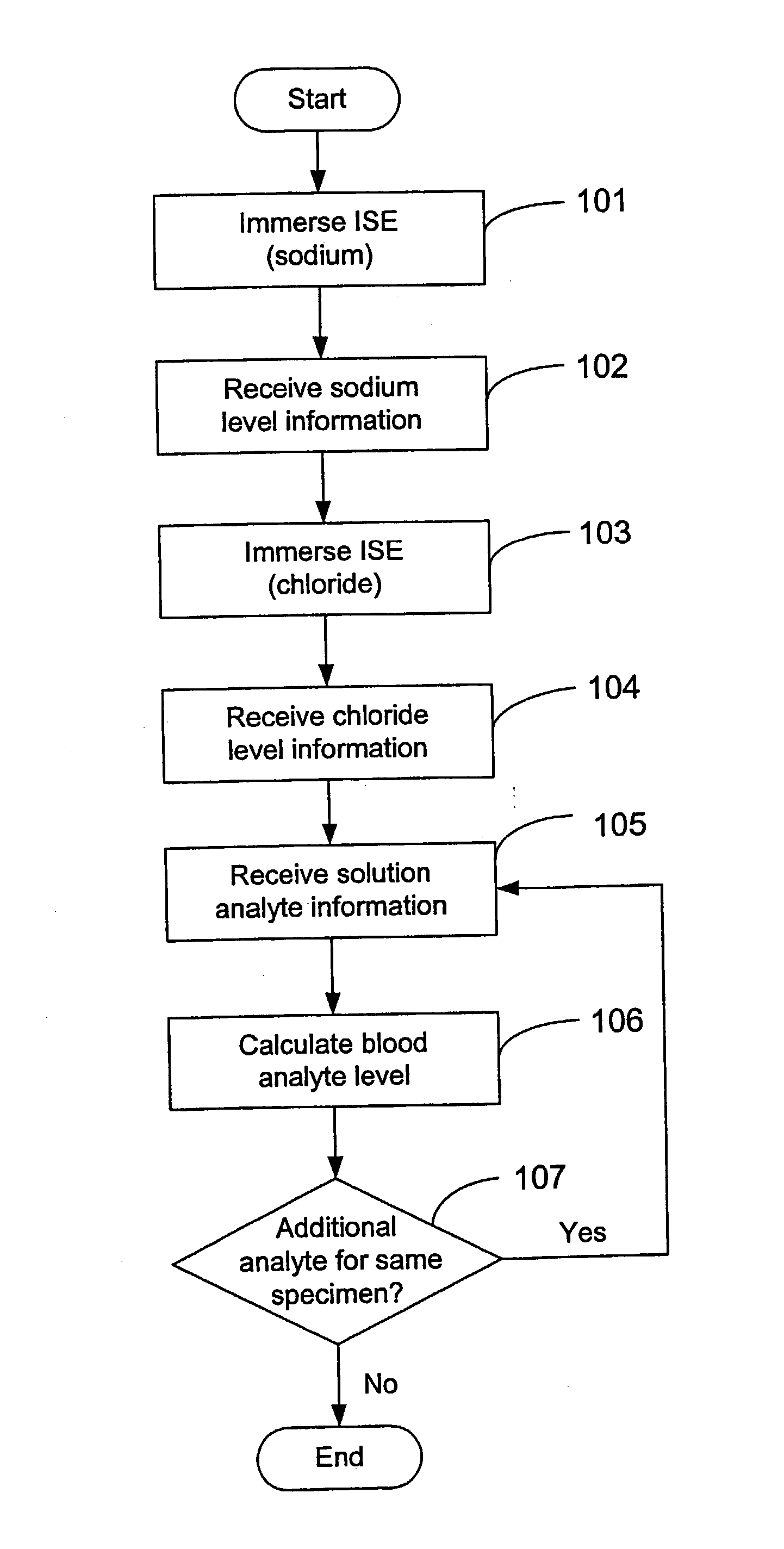
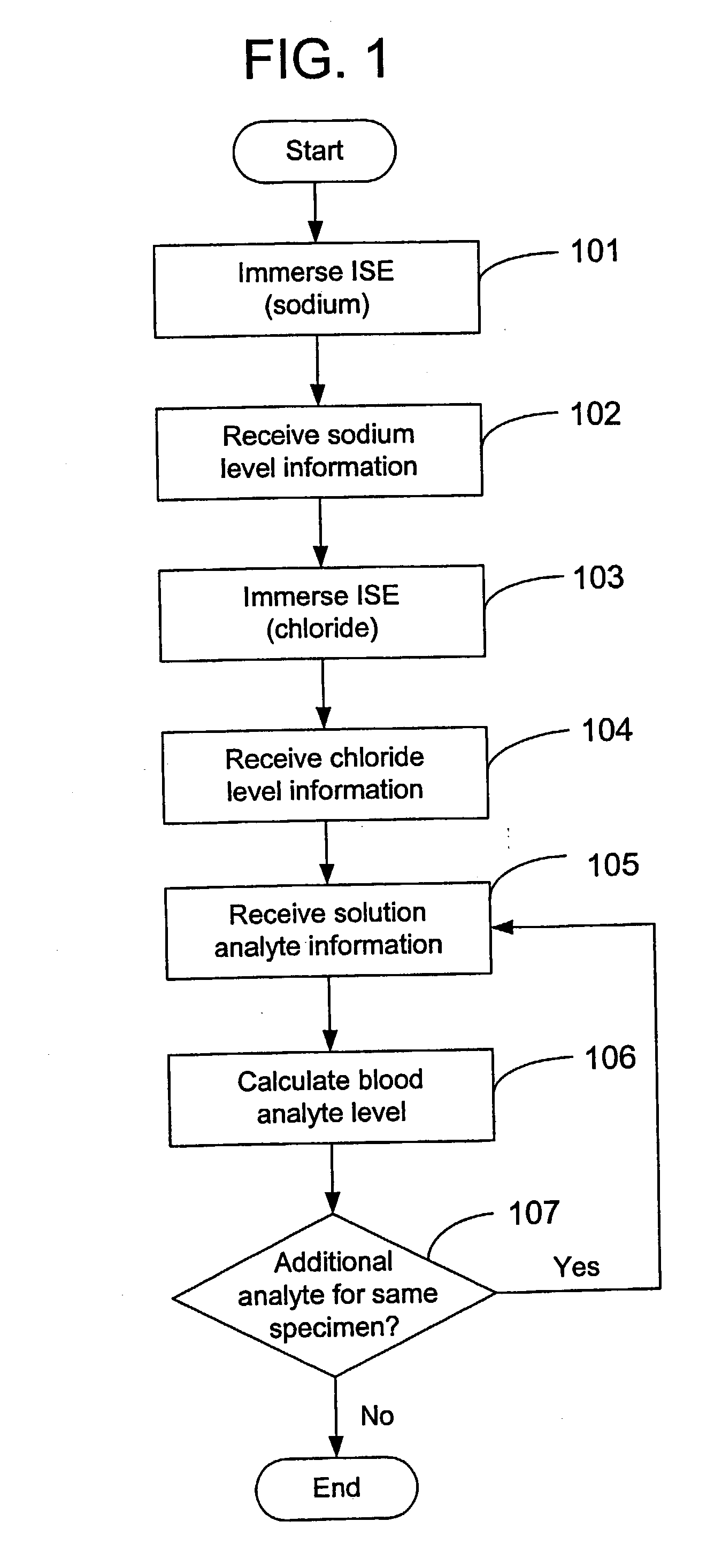
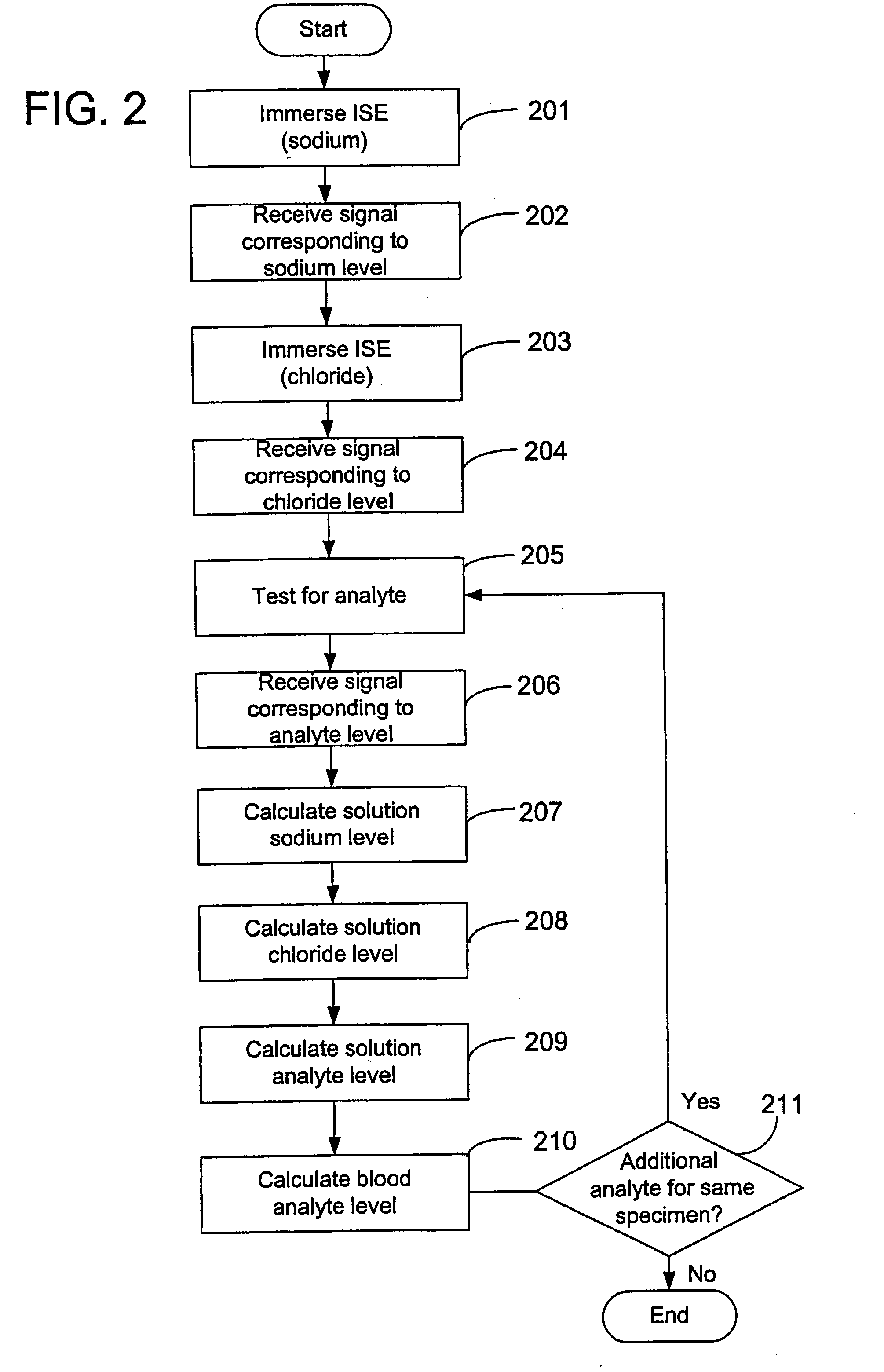
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
Method of identifying proteins in human serum indicative of pathologies of human lung tissues
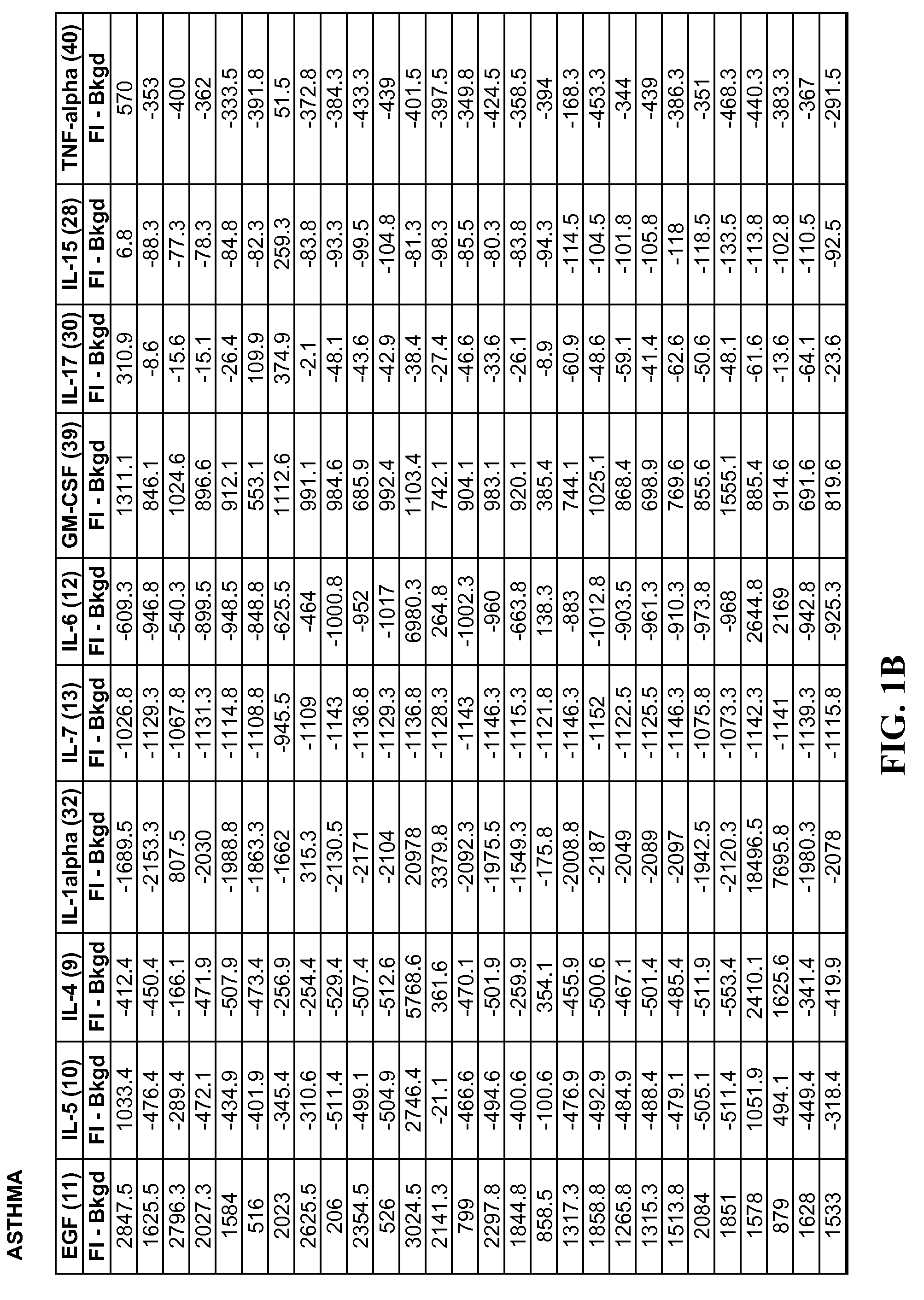
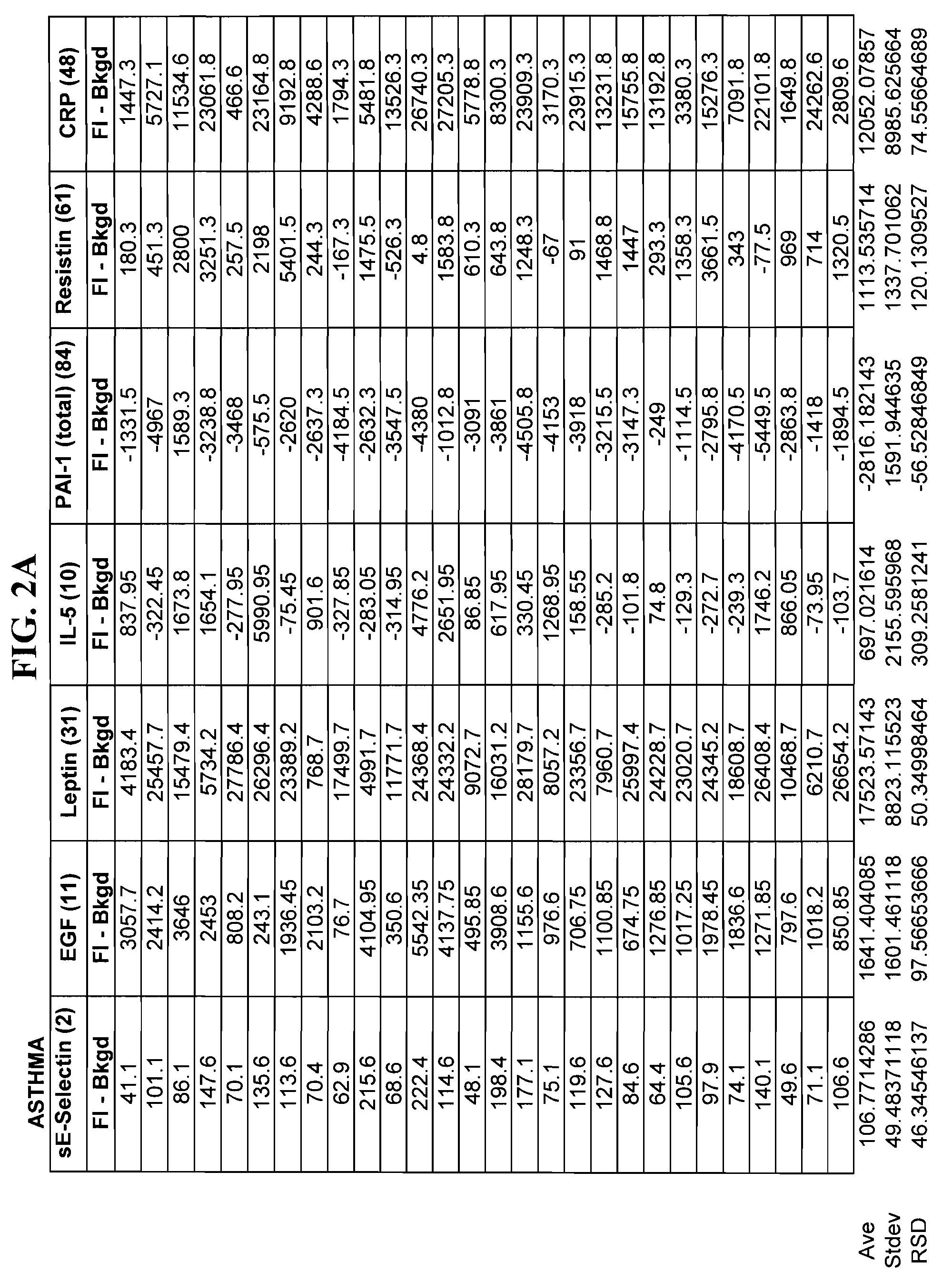
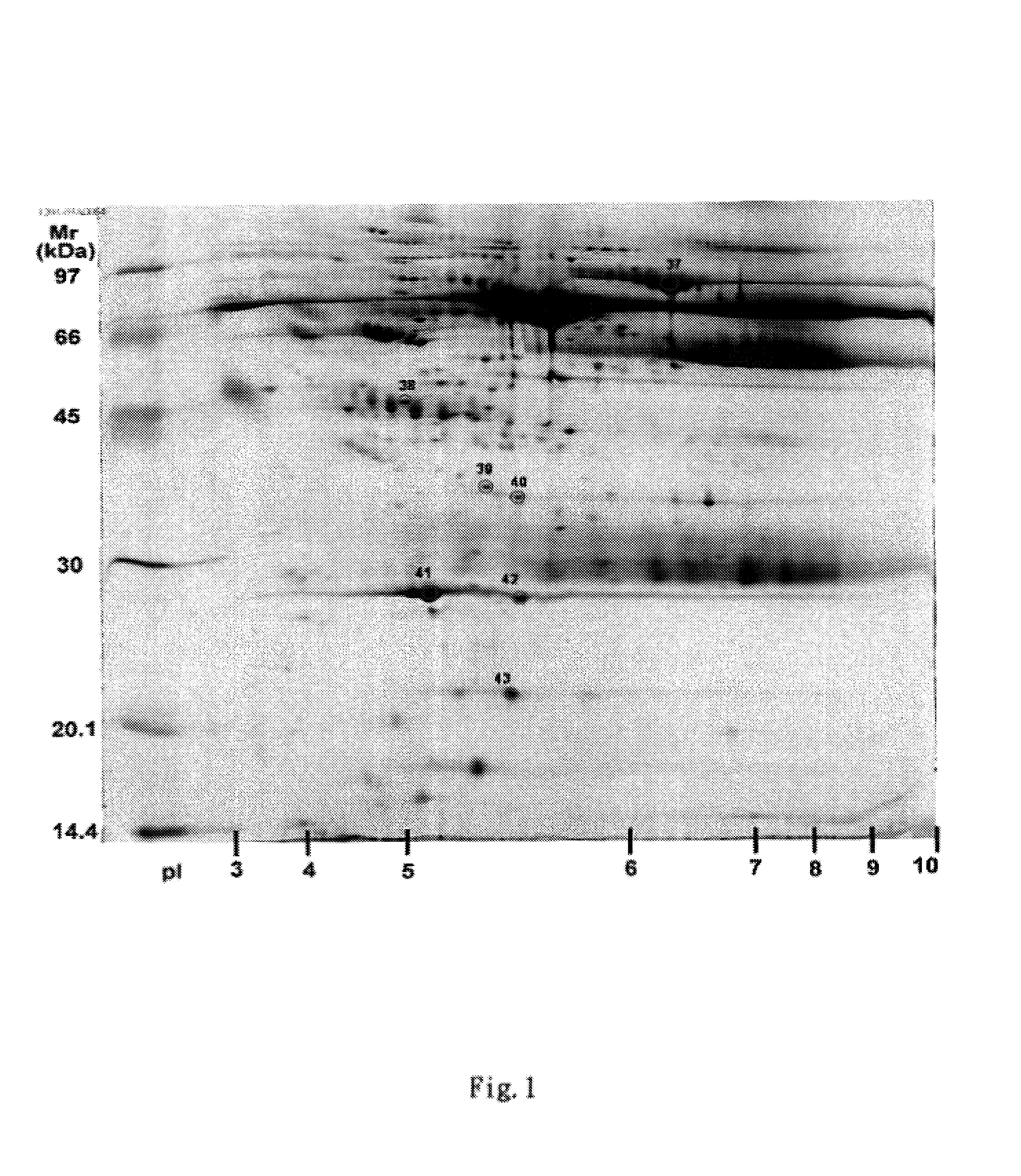
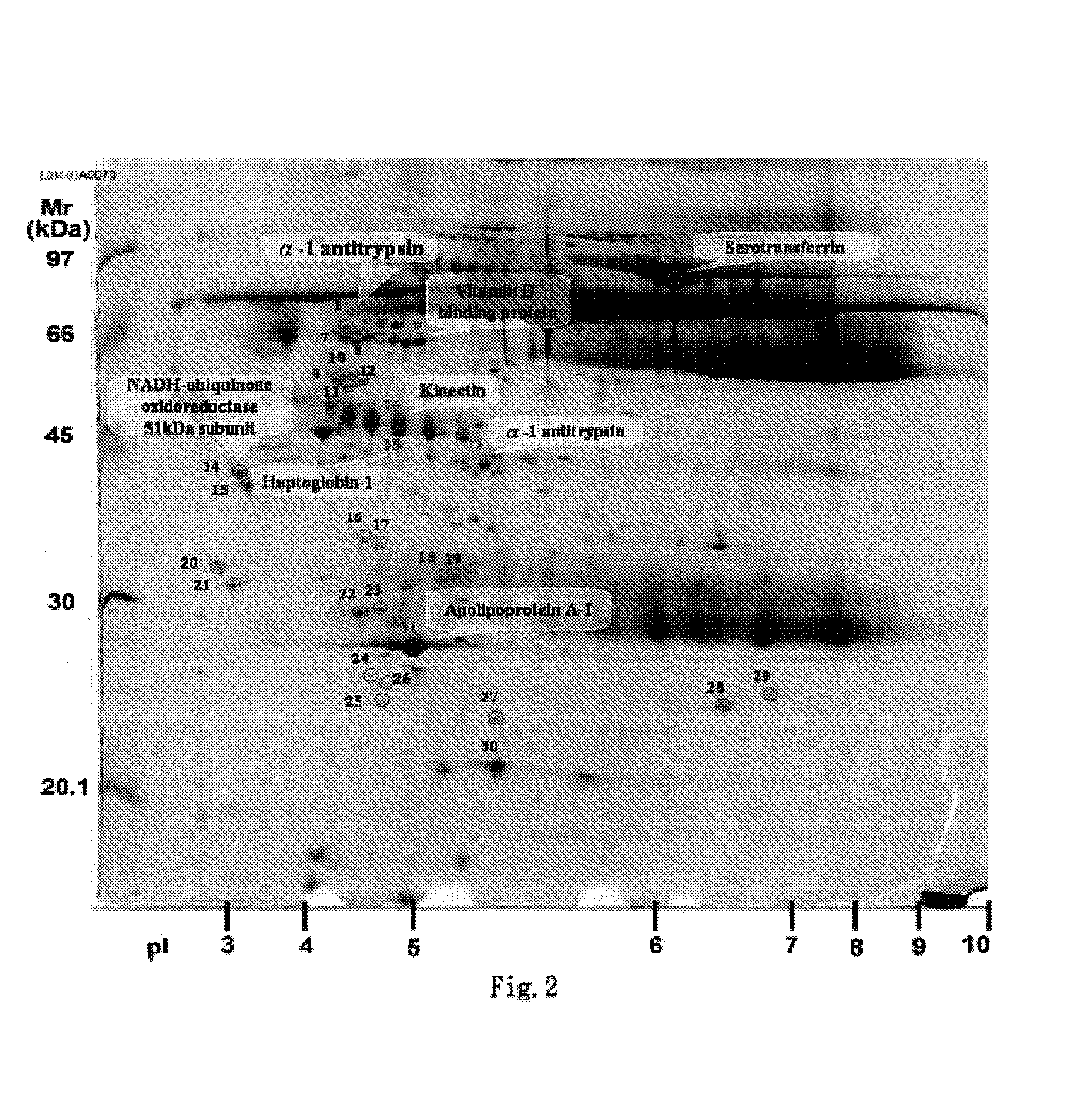
A method of identifying proteins present in human serum which are differentially expressed between normal individuals and patients known to have non-small cell lung cancers and asthma, as diagnosed by a physician. Human serum specimens from each population are digested with trypsin or any other suitable endoproteinase and analyzed using a liquid chromatography electrospray ionization mass spectrometer. Mass spectral data from each population is compared to determine proteins with expression intensities which are significantly differentially expressed between the normal, asthma, and lung cancer populations. Eleven proteins are found to have expression intensities which are significantly differentially expressed between the populations. Finally, the identities of the eleven proteins are obtained by comparing the mass spectral data with known databases having libraries of mass spectral data of known proteins.
Owner:CANCER PREVENTION & CURE
Quantitative analysis of a biological sample of unknown quantity
ActiveUS7611670B2Analysis using chemical indicatorsMicrobiological testing/measurementAnalyteCholesterol
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
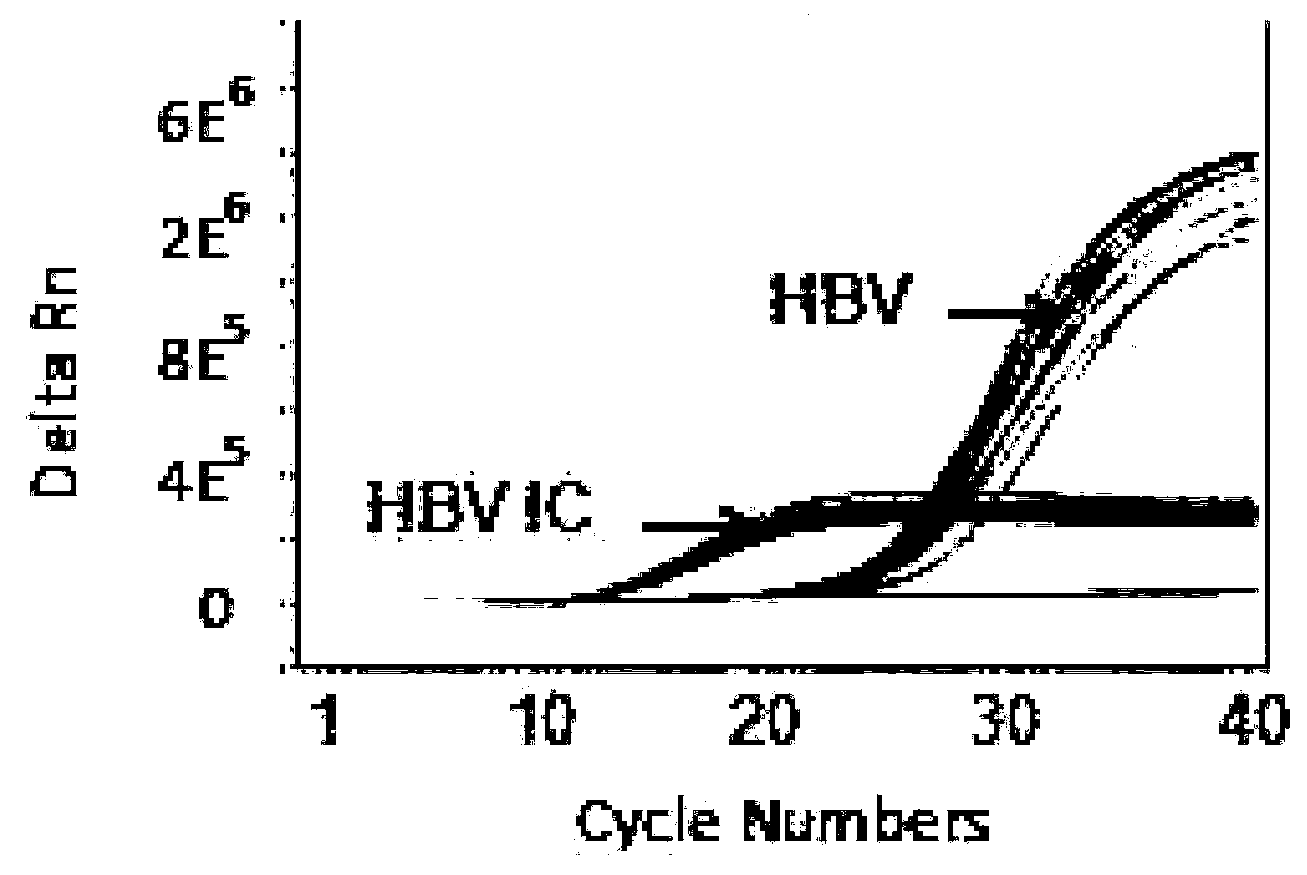
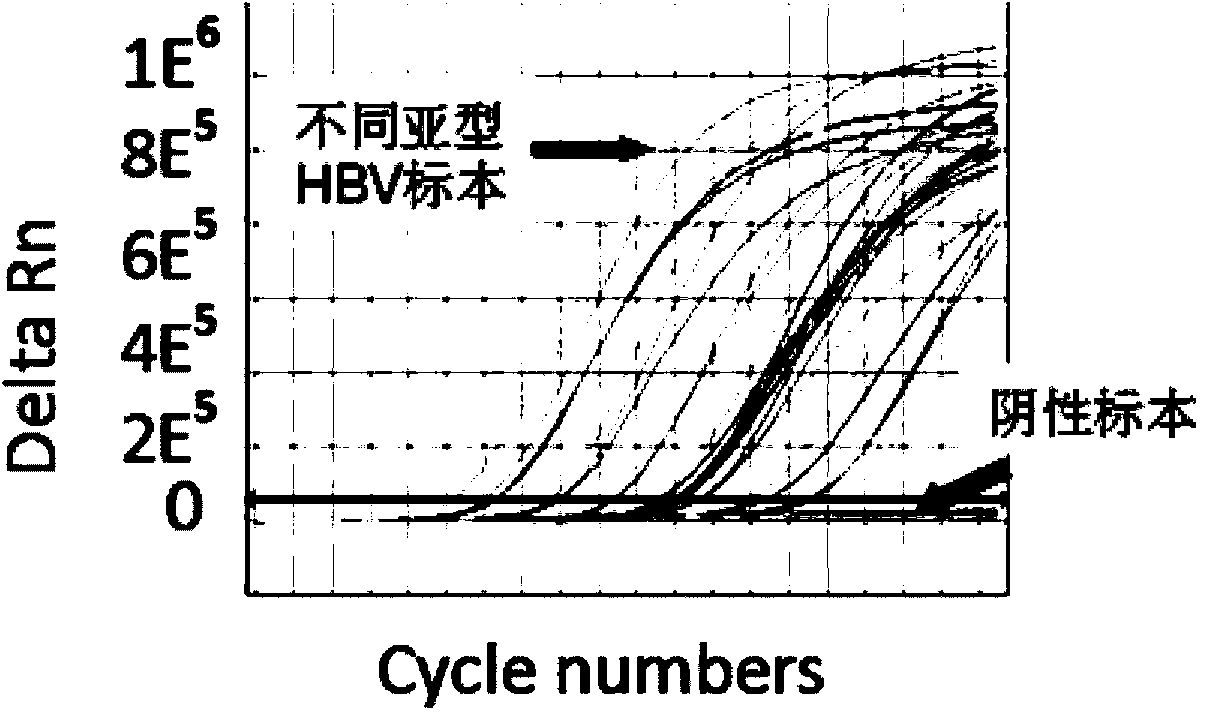
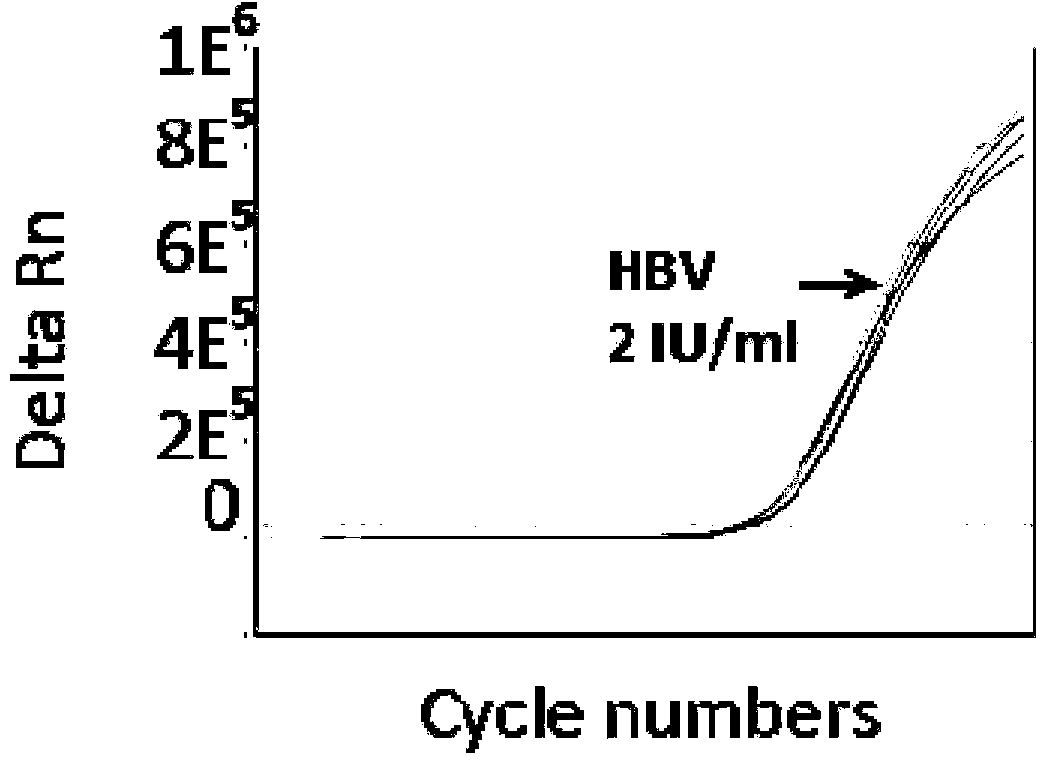
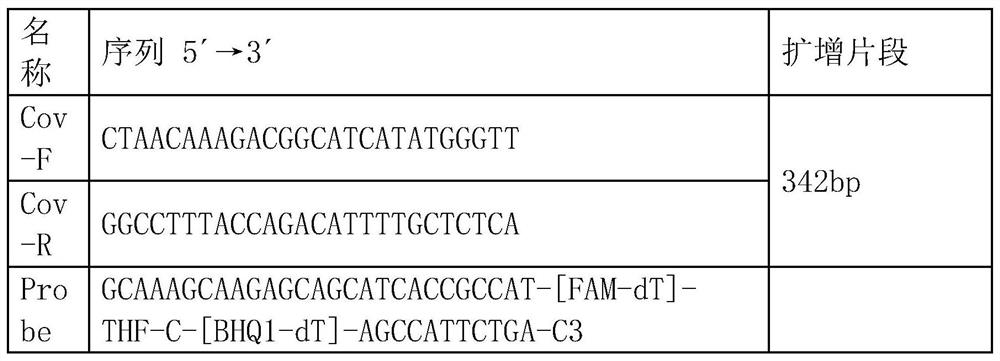
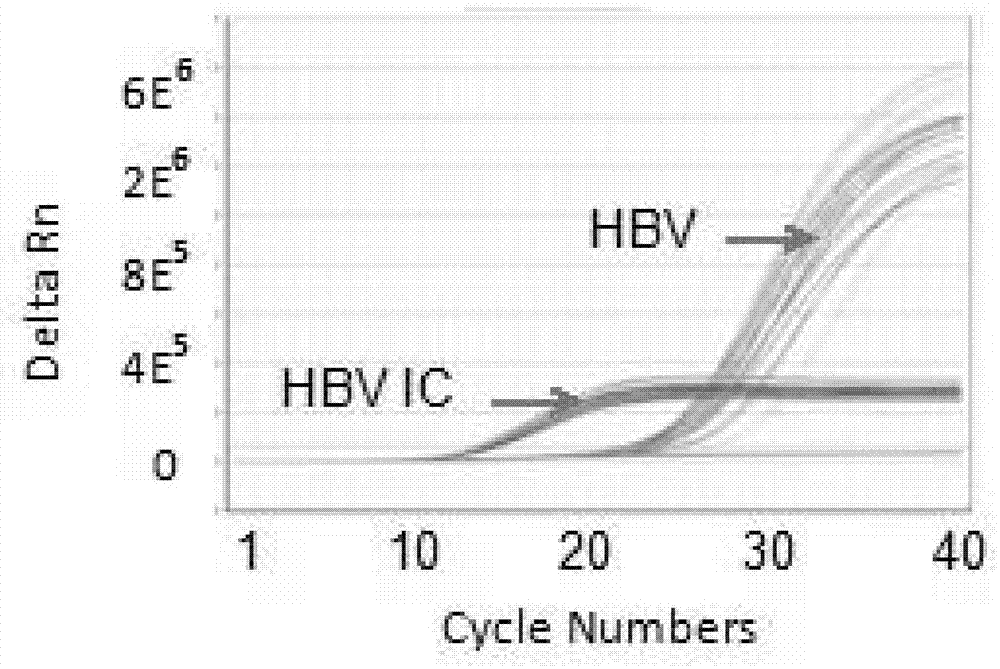
PCR (Polymerase Chain Reaction) primer, PCR primer group, PCR detection probe and PCR detection kit for detecting hepatitis B virus as well as detection method
ActiveCN104004856AAvoid false negativesAvoid pollutionMicrobiological testing/measurementMicroorganism based processesNew medicationsUracil
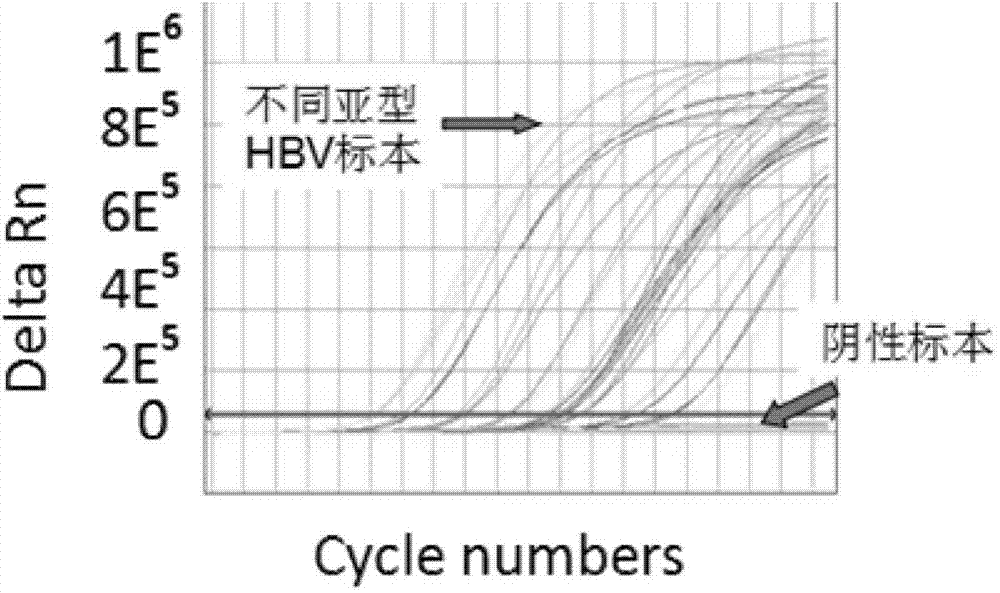
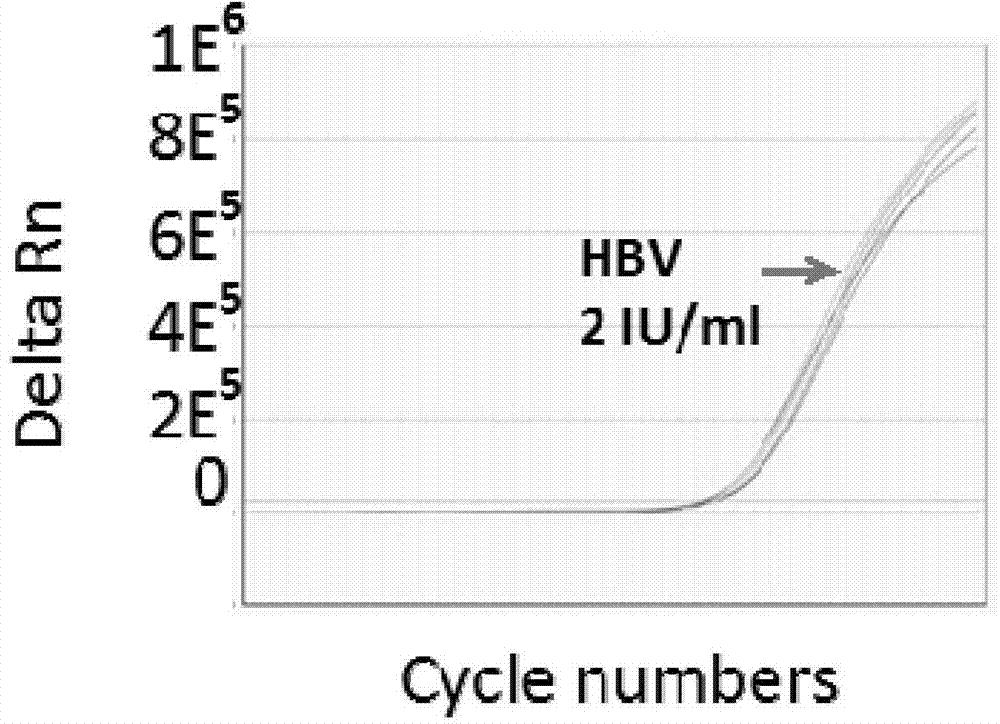
The invention discloses a PCR (Polymerase Chain Reaction) primer, a PCR primer group, a PCR detection probe and a PCR detection kit for detecting hepatitis B virus as well as a detection method. The kit contains the high-sensitivity detection primer group and the high-sensitivity detection probe and can be used for detecting single copy HBVDNA (Hepatitis B virus-Deoxyribonucleic acid), the limit of detection of the kit in a serum specimen can reach 2IU / mL, and compared with an imported reagent Roche COBAS, the kit is relatively sensitive. The detection method disclosed by the invention can be used for detecting different subtypes of hepatitis B pathogens around the world; due to addition of an internal control system in the detection method, false negative can be effectively prevented; due to addition of a UNG (uracil-N-glycosylase) system in the detection method, pollution can be effectively avoided; the kit and the detection method have the beneficial effects that the kit and the detection method can be applied to diagnosis of hepatitis B, evaluation of research, development and screening of anti-hepatitis-B new drugs and evaluation of an anti-hepatitis-B treatment effect and relapsing and have wide clinical application effects, and the popularization and application of the kit and the detection method are facilitated.The invention discloses a PCR (Polymerase Chain Reaction) primer, a PCR primer group, a PCR detection probe and a PCR detection kit for detecting hepatitis B virus as well as a detection method. The kit contains the high-sensitivity detection primer group and the high-sensitivity detection probe and can be used for detecting single copy HBV DNA (Hepatitis B virus-Deoxyribonucleic acid), the limit of detection of the kit in a serum specimen can reach 2IU / mL, and compared with an imported reagent Roche COBAS, the kit is relatively sensitive. The detection method disclosed by the invention can be used for detecting different subtypes of hepatitis B pathogens around the world; due to addition of an internal control system in the detection method, false negative can be effectively prevented; due to addition of a UNG (uracil-N-glycosylase) system in the detection method, pollution can be effectively avoided; the kit and the detection method have the beneficial effects that the kit and the detection method can be applied to diagnosis of hepatitis B, evaluation of research, development and screening of anti-hepatitis-B new drugs and evaluation of an anti-hepatitis-B treatment effect and relapsing and have wide clinical application effects, and the popularization and application of the kit and the detection method are facilitated.
Owner:GUANGZHOU SUPBIO BIO TECH & SCI
Quantitative analysis of a biological sample of unknown quantity
ActiveUS20050130310A1Simple methodAnalysis using chemical indicatorsMicrobiological testing/measurementAnalyteCholesterol
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
Phenobarbital homogeneous-phase enzyme immunoassay reagent kit and preparation method thereof
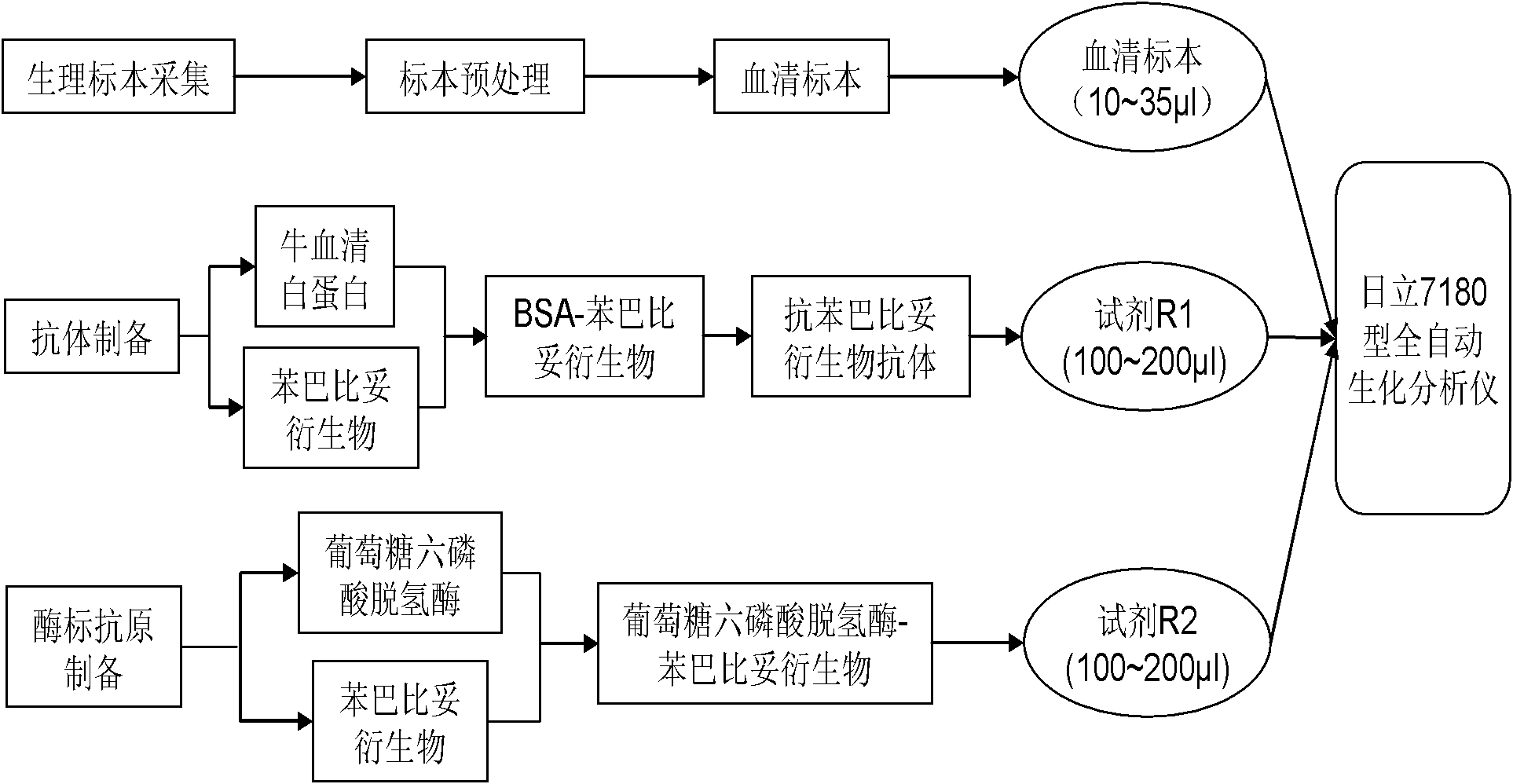
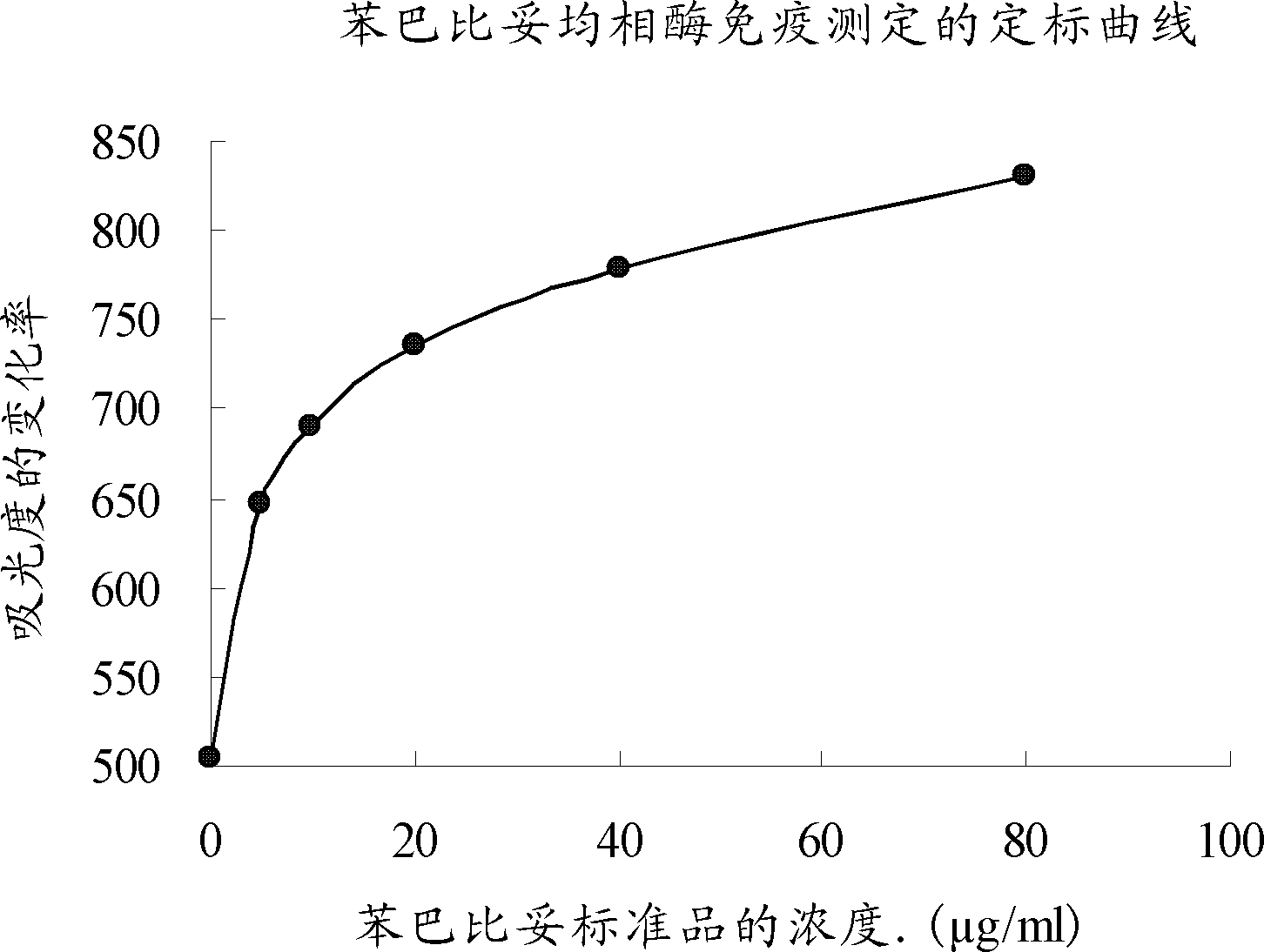
ActiveCN102323414AImprove accuracyHigh precisionImmunoglobulinsMaterial analysisDrug interactionEnzyme immunoassays
Owner:西安金域医学检验所有限公司
Method of Identifying Biomarkers in Human Serum Indicative of Pathologies of Human Lung Tissues
A method of identifying altered biomarker expression levels in a human serum specimen to diagnose asthma or non-small celled lung cancers in humans. The existence of asthma or non-small celled lung cancers in a patient can be determined by subjecting a blood sample from the patient to a simple blood test to determine the expression levels of certain specific biomarkers. The expression levels of the specific biomarkers are compared to ranges of expression levels for the same biomarkers which are indicative of individuals known to have asthma, non-small cell lung cancers, or neither. Comparing the expression levels will determine the existence or non-existence of asthma or non-small cell lung cancer.
Owner:LUNG CANCER PROTEOMICS LLC
Special primer, kit and method for testing minRNA-128 in colorectal cancer serum
ActiveCN103074431AOmit the extraction processEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationReverse transcription polymerase chain reactionColorectal cancer
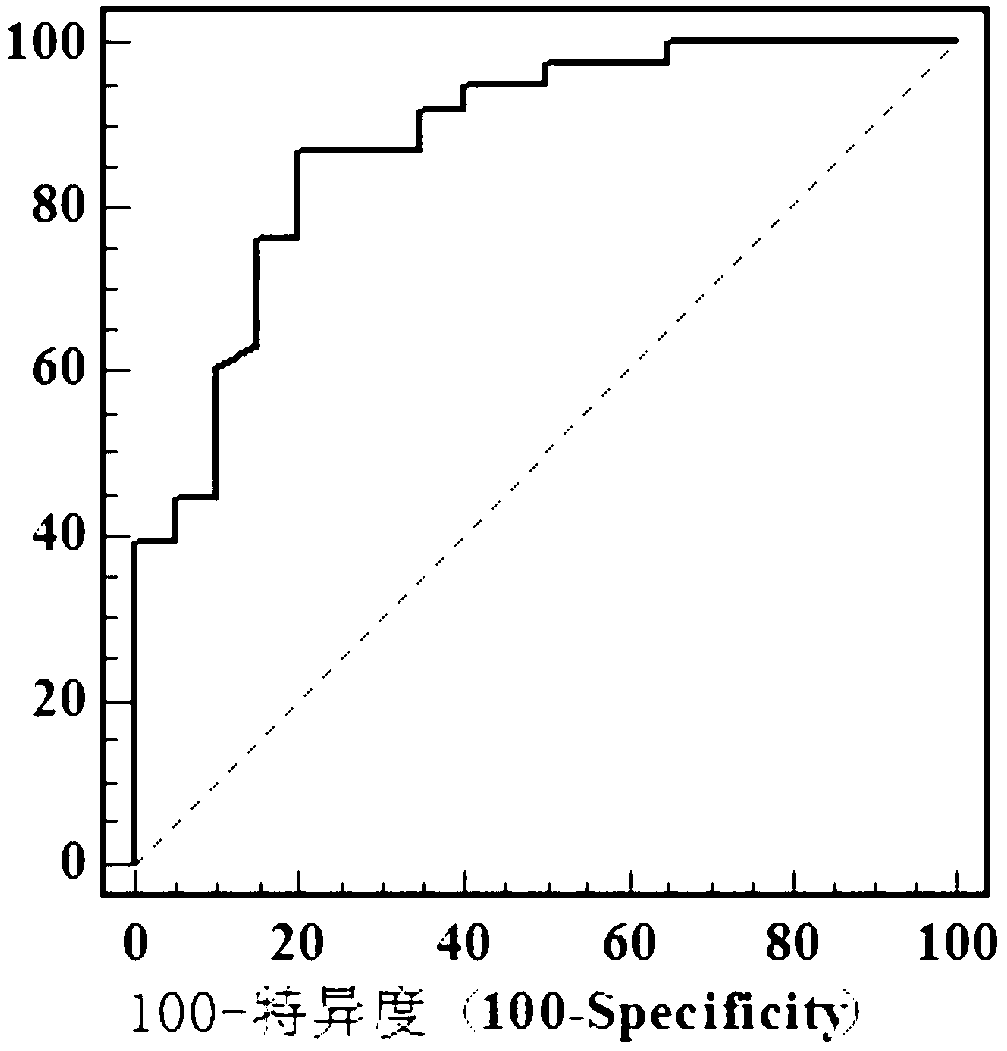
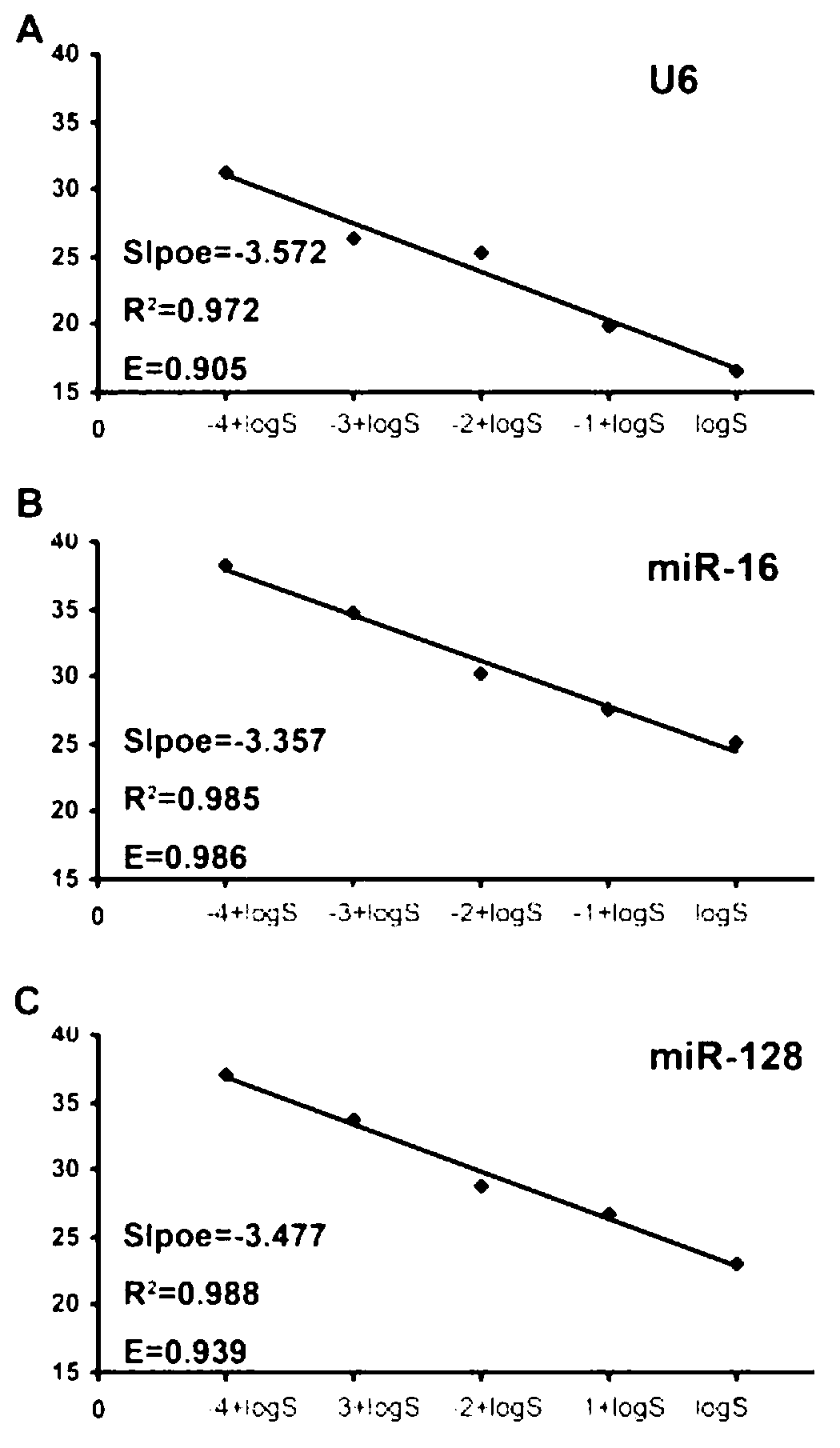
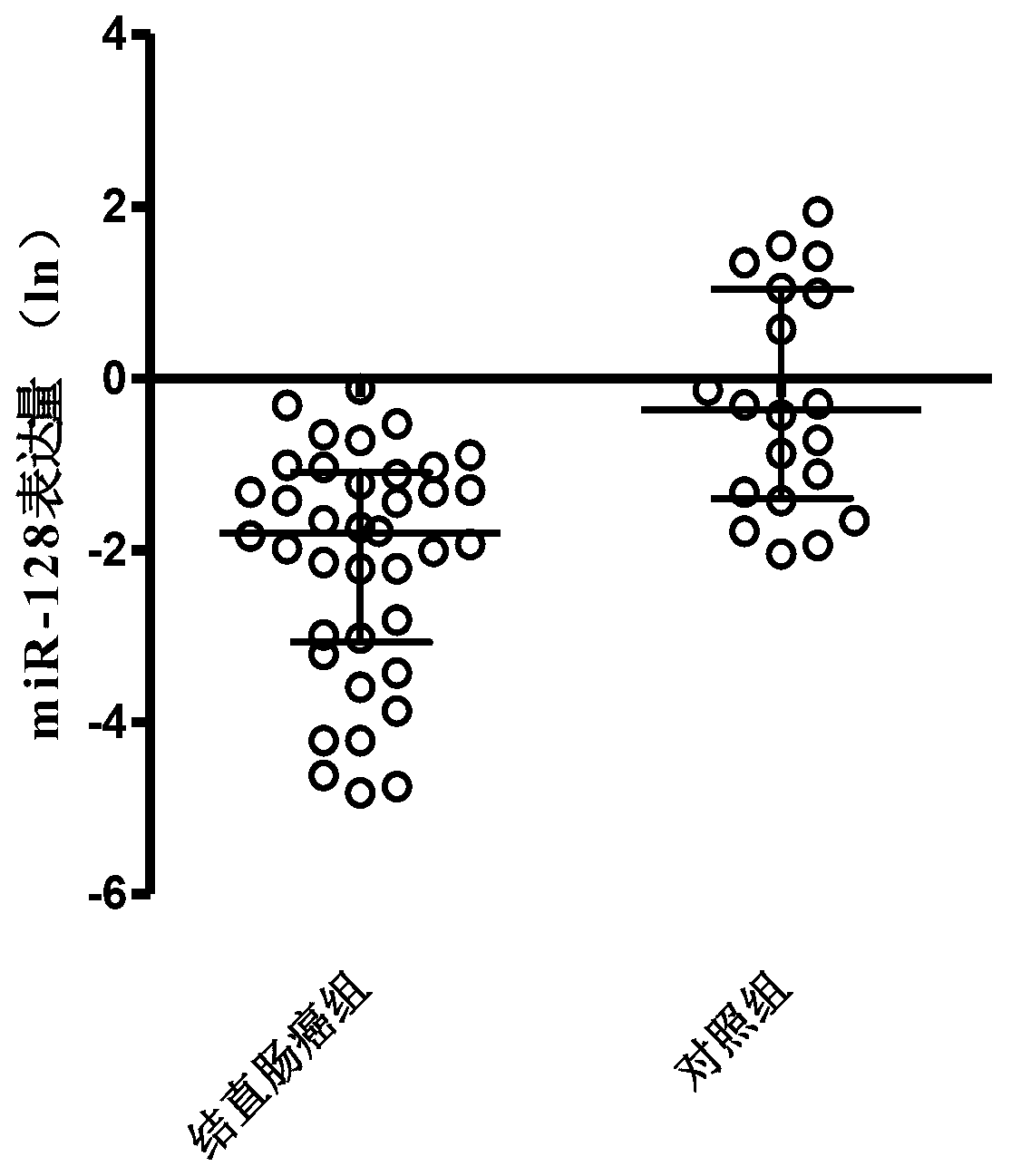
The invention discloses a special primer for testing the minRNA-128 in colorectal cancer serum. The special primer comprises a reverse transcription primer and a test primer of the miR-128, a reverse transcription primer and a test primer of U6 and a reverse transcription primer and a test primer of miRNA-16, as shown by SEQ IDNO: 1-9. A special kit for testing colorectal cancer serum minRNA-128 comprises the primers and a RNA separation solution. A method for testing the expression amount of the colorectal cancer serum minRNA-128 comprises steps: mixing a serum specimen to be tested with an isovolumetric RNA separation solution, centrifuging 16000g of the mixture for 10 minutes, and separating supernate; performing RT-PCR (reverse transcription-polymerase chain reaction); manufacturing a standard curve; and performing calculation to obtain a gene calibration initial copy number Q, and comparing the Q value of the miR-128 and the geometrical mean of Q values of reference gene U6 and miR-16 to obtain a relative expression amount of the minRNA-128. According to the method for testing the minRNA-128 in colorectal cancer serum, important references are provided for early discovery and early treatment of colorectal cancer.
Owner:SHANDONG UNIV QILU HOSPITAL
Method of identifying biomarkers in human serum indicative of pathologies of human lung tissues
A method of identifying altered biomarker expression levels in a human serum specimen to diagnose asthma or non-small celled lung cancers in humans. The existence of asthma or non-small celled lung cancers in a patient can be determined by subjecting a blood sample from the patient to a simple blood test to determine the expression levels of certain specific biomarkers. The expression levels of the specific biomarkers are compared to ranges of expression levels for the same biomarkers which are indicative of individuals known to have asthma, non-small cell lung cancers, or neither. Comparing the expression levels will determine the existence or non-existence of asthma or non-small cell lung cancer.
Owner:LUNG CANCER PROTEOMICS LLC
Diagnosis method of endometriosis by detecting biochemical markers and usage of these biochemical markers
ActiveUS7399598B2Fast expressionFast quantitiesDisease diagnosisBiological testingGuidelinePeritoneal fluid
The present invention relates to a non-invasive diagnosis method of endometriosis by detecting biochemical marker in serum or peritoneal fluid, in particular alpha 1-antitrypsin, fragments of alpha 1-antitrypsin, or a combination of both. The diagnosis of endometriosis is performed with observing in serum specimens of a patient the concentration and change of the biochemical marker, in particular molecules related to alpha 1-antitrypsin, and comparing with a predetermined baseline level of the biochemical marker contained in serum. Statistical analysis can be performed to evaluate the baseline level indicating the occurrence of endometriosis. Therefore, the present invention can provide an auxiliary guideline for the diagnosis of endometriosis. The present invention also relates to usage of the biochemical marker.
Owner:TAIPEI MEDICAL UNIV
Quantitative analysis of a biological sample of unknown quantity
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
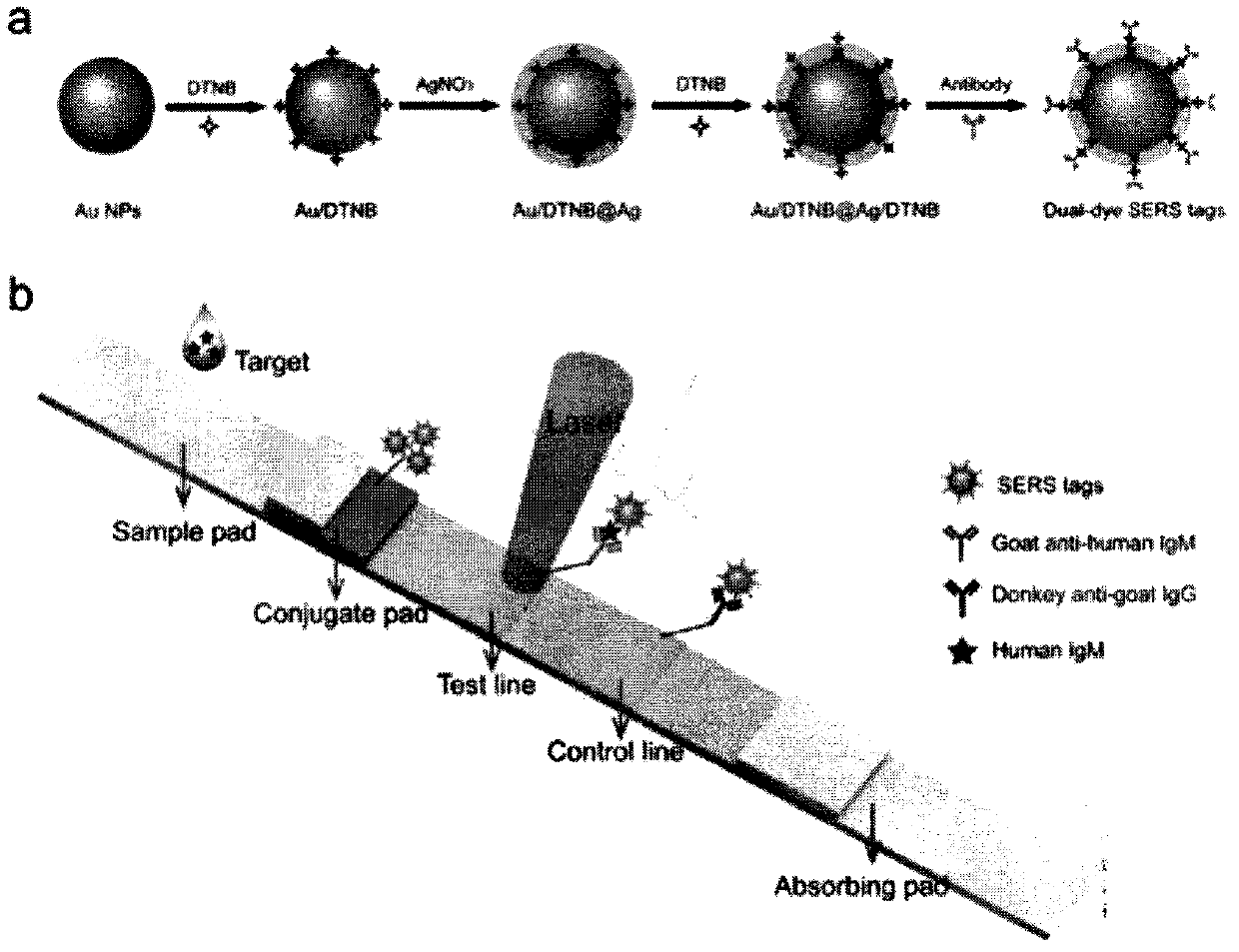
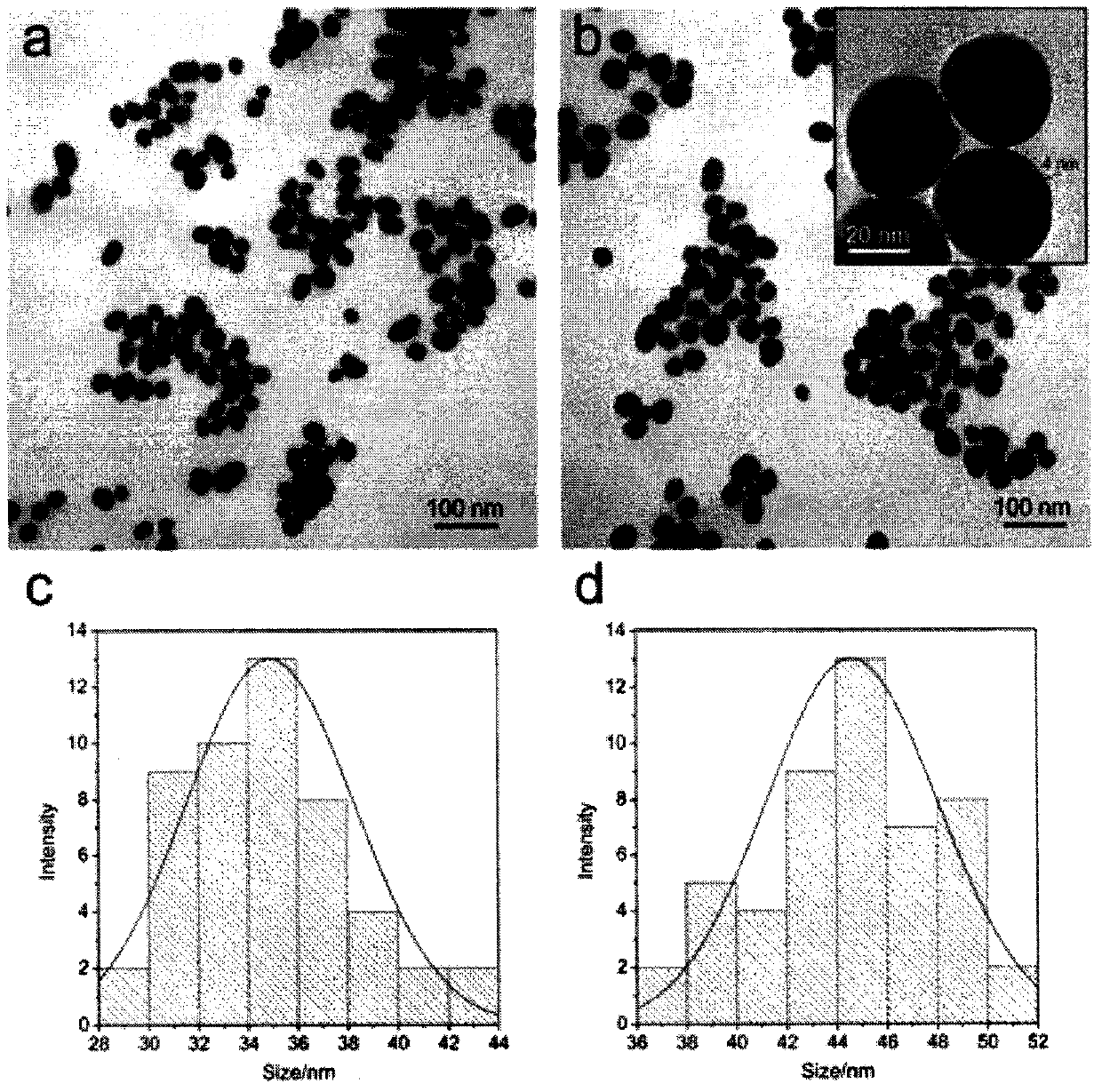
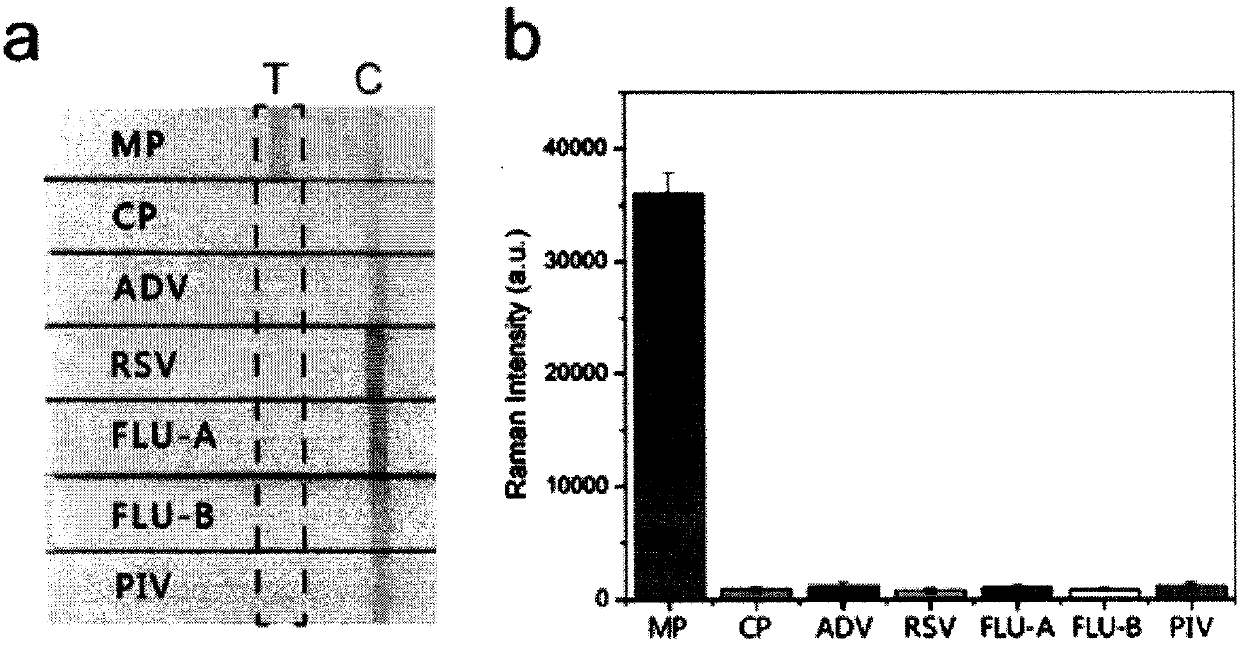
SERS-immunochromatography detection method for rapidly and highly sensitively detecting Mycoplasma pneumoniae infection
InactiveCN110133255AHigh detection sensitivityImprove the detection rateBiological testingSurface-enhanced Raman spectroscopyDTNB
The invention relates to a SERS-immunochromatography detection method for rapidly and highly sensitively detecting Mycoplasma pneumoniae infection. The detection principle is that Nitrocellulose membranes (NC) are used as a carrier, a double-layer dye 5,5'-dithiobis (2-nitrobenzoic acid){5,5'-dithiobis- (2-nitrozoic acid), DTNB} labeled Au@Ag nano material coupling detection antibody is used as anSERS probe, a novel surface enhanced Raman spectroscopy (SERS-ICA)-based immunochromatography technology is established by combining the traditional Immunochromatography assay (ICA), and the technology is used for detecting human IgM and Mycoplasma pneumoniae (MP) specific IgM positive serum samples. The detection comprises the processes of sample dilution, SERS probe release, antigen-antibody reaction, result analysis and the like. The SERS-immunochromatography detection method for rapidly and highly sensitively detecting Mycoplasma pneumoniae infection can improve the detection sensitivityof human IgM and the detection rate of the lung branch positive serum specimen, and has important significance for clinical treatment.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Serum CENPF antibody quantitative detection kit
The invention relates to a serum CENPF antibody quantitative detection kit which can be used for specifically and quantitatively detecting the level of a CENPF antibody in a serum specimen. According to the serum CENPF antibody quantitative detection kit, recombinant CENPF antigen protein is coated in a 96-pore enzyme label board, the level of the CENPF antibody in the serum specimen can be quantitatively tested by using an enzyme linked immunosorbent assay, and the disease state of a liver cell cancer patient or high risk groups can be evaluated according to the level of the CENPF antibody in the serum. By adopting the serum CENPF antibody quantitative detection kit, a novel liver cell cancer screening and early diagnosis method is provided, and the sensitivity of the detected serum CENPF self-antibody is remarkably superior to that of a conventional clinical serum marker AFP when being used for diagnosing liver cell cancer.
Owner:北京博清科创生物技术有限公司
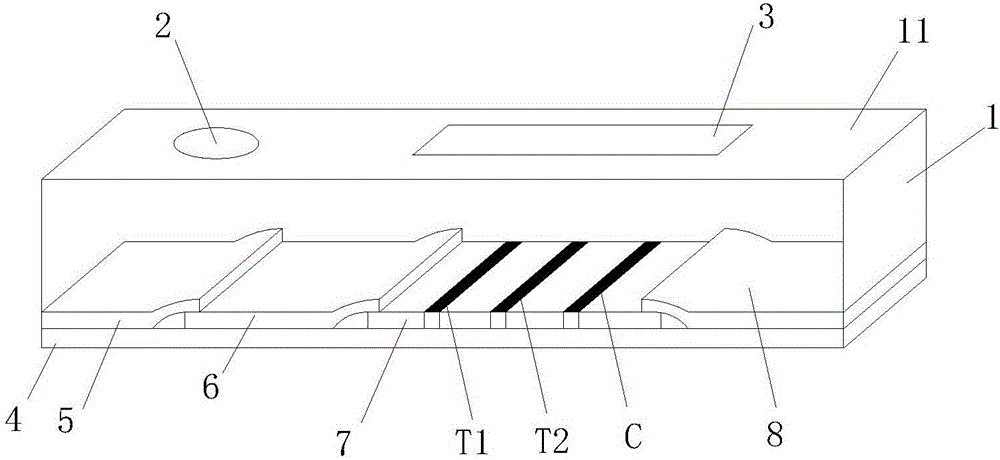
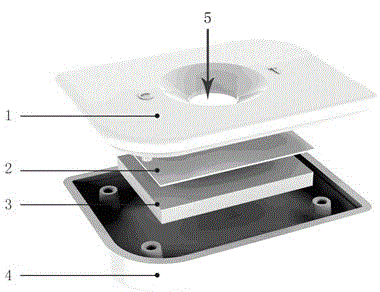
Mycobacterium bovis and brucella abortus dual detection card and preparation method thereof
InactiveCN106706906AHigh compliance rateStrong specificityMaterial analysisBrucella abortusQuality control
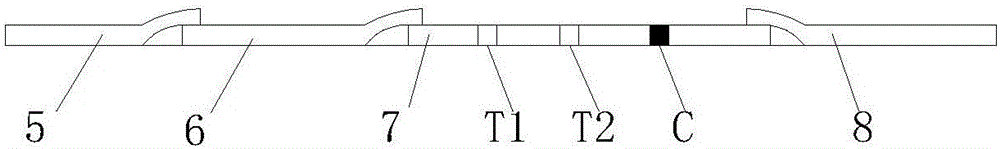
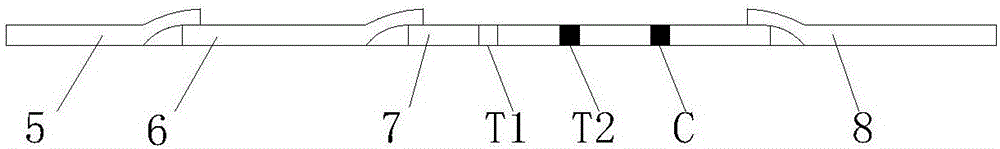
The invention discloses a mycobacterium bovis and brucella abortus dual detection card and a preparation method thereof and aims to provide a detection card which is capable of simultaneously detecting mycobacterium bovis and brucella abortus in a bovine serum specimen, is short in detection time, high in stability, simple in operation and intuitive and reliable in result judgment and does not need other instruments or professional personnel. According to the technical key points, the detection card comprises a shell (1), wherein a sample adding hole (2) and an observation window (3) are formed in the shell (1); and a colloidal gold test strip is arranged in the shell (1). The detection card is characterized in that the colloidal gold test strip comprises a bottom plate (4); a sample pad (5), a gold label pad (6), a coating membrane (7) and an absorbent pad (8) are sequentially connected onto the bottom plate (4); a mycobacterium bovis membrane protein MPB70-MPB83 detection line T1, a brucella abortus LPS detection line T2 and a goat anti-rabbit polyclonal antibody quality control line C are formed in the coating membrane (7); and the three lines are arranged in parallel. The detection card belongs to the technical field of biology.
Owner:深圳市绿诗源生物技术有限公司
Quantitative analysis of a biological sample of unknown quantity
ActiveUS7479392B2Analysis using chemical indicatorsMicrobiological testing/measurementAnalyteCholesterol
Owner:PWNHEALTH CHICAGO LLC
Method for constructing hepatoma protein group fingerprint model and serum detection application thereof
InactiveCN1654953AReduce fatality rateHigh cure rateSamplingComponent separationProtein insertionMortality rate
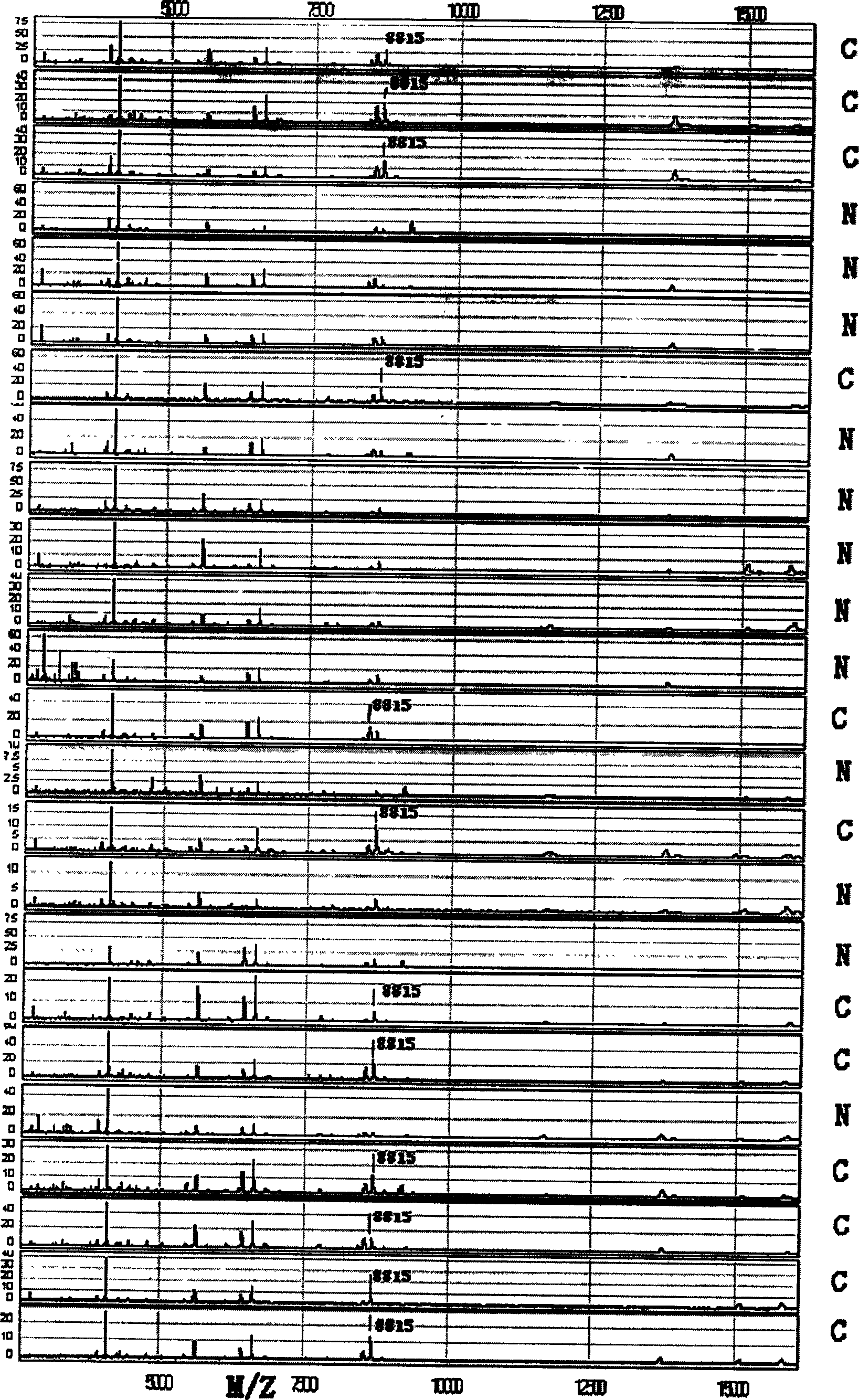
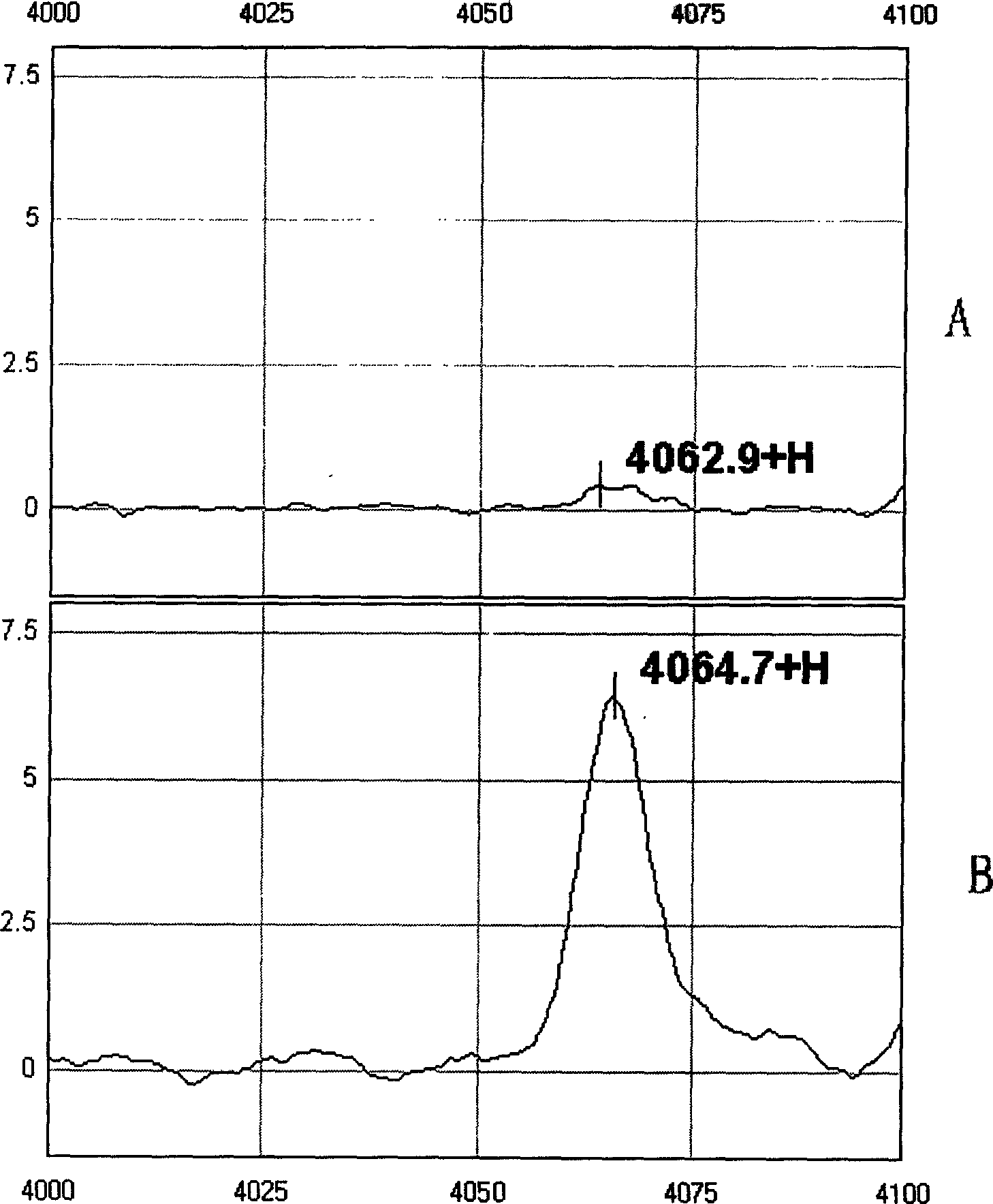
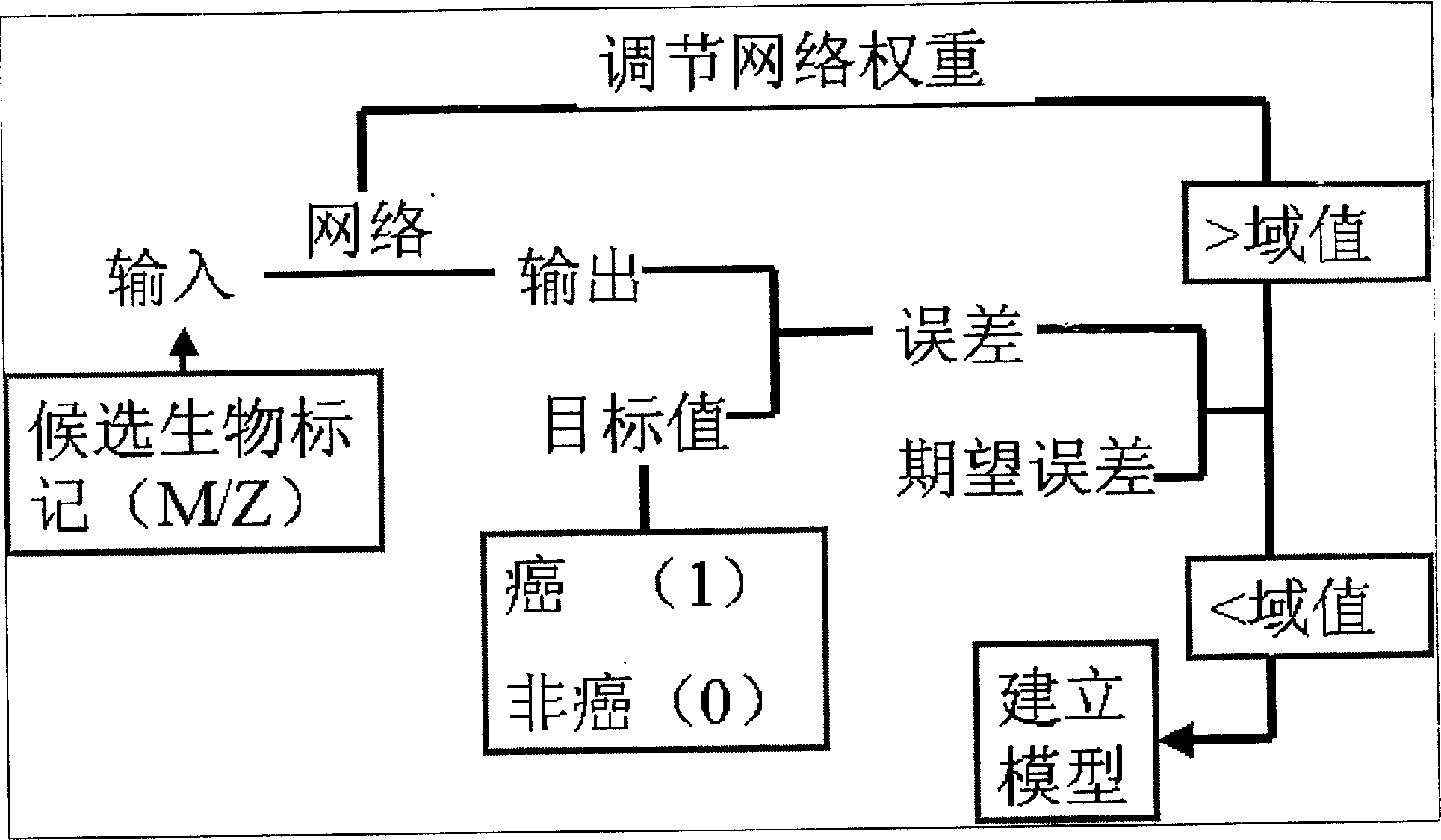
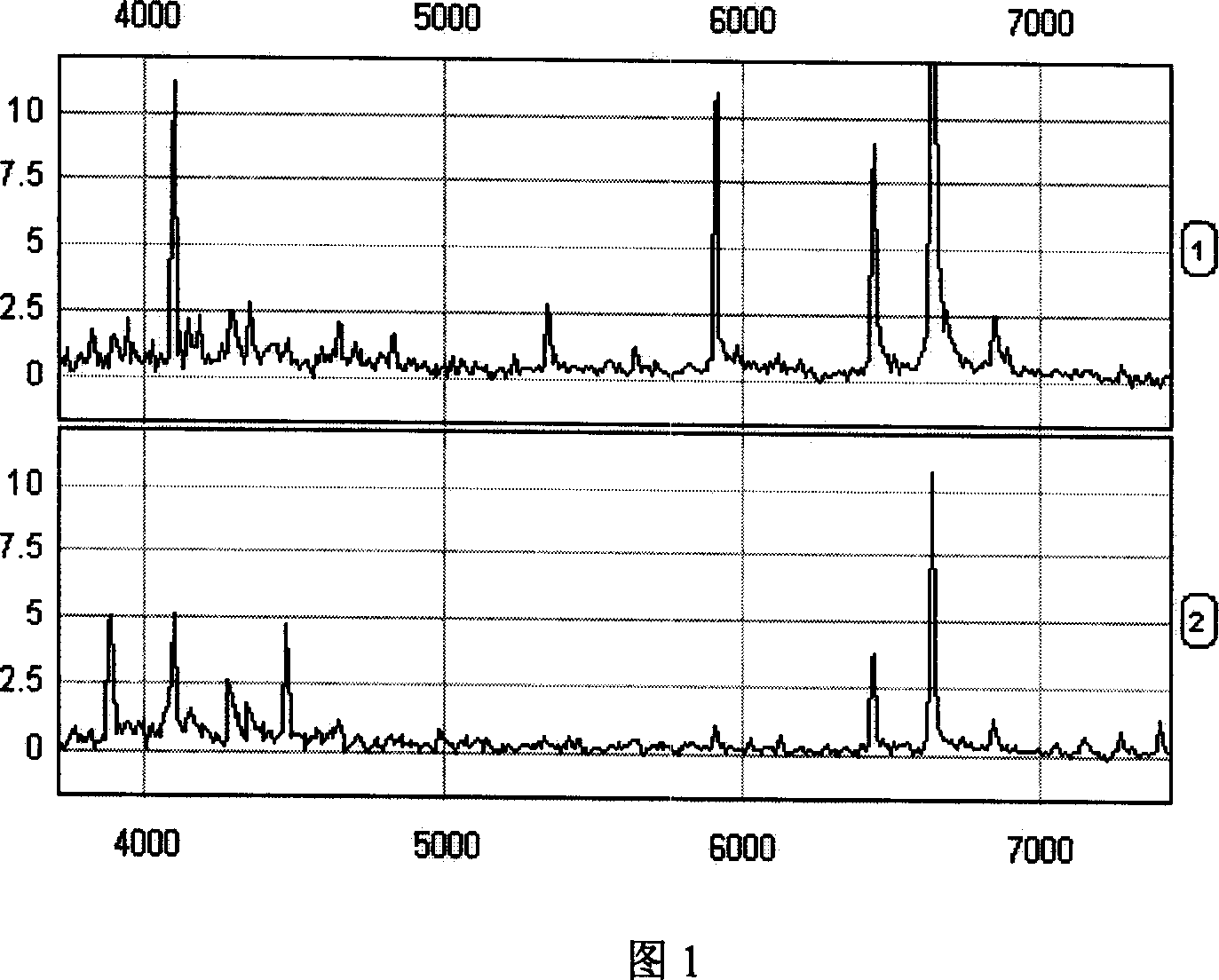
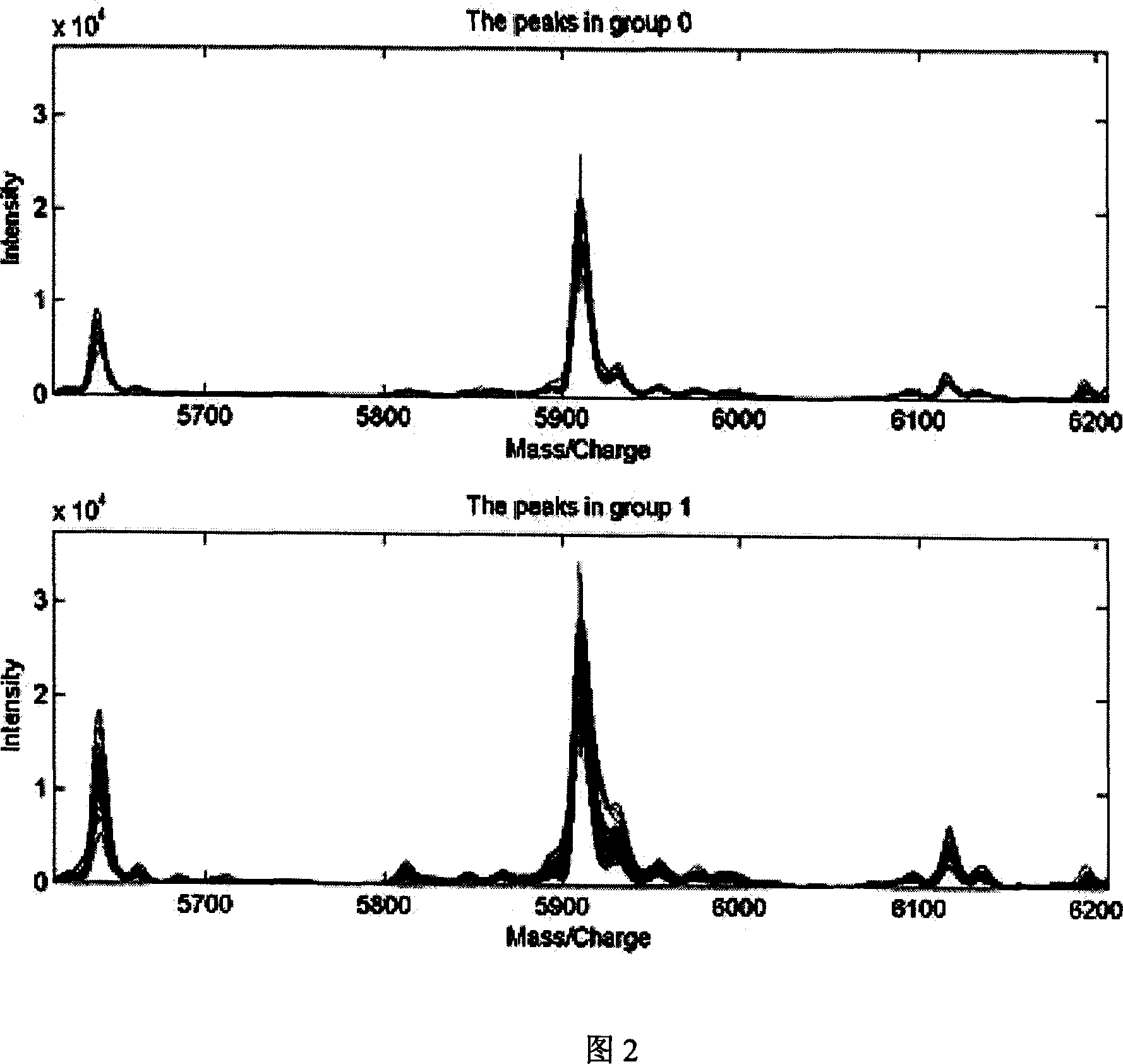
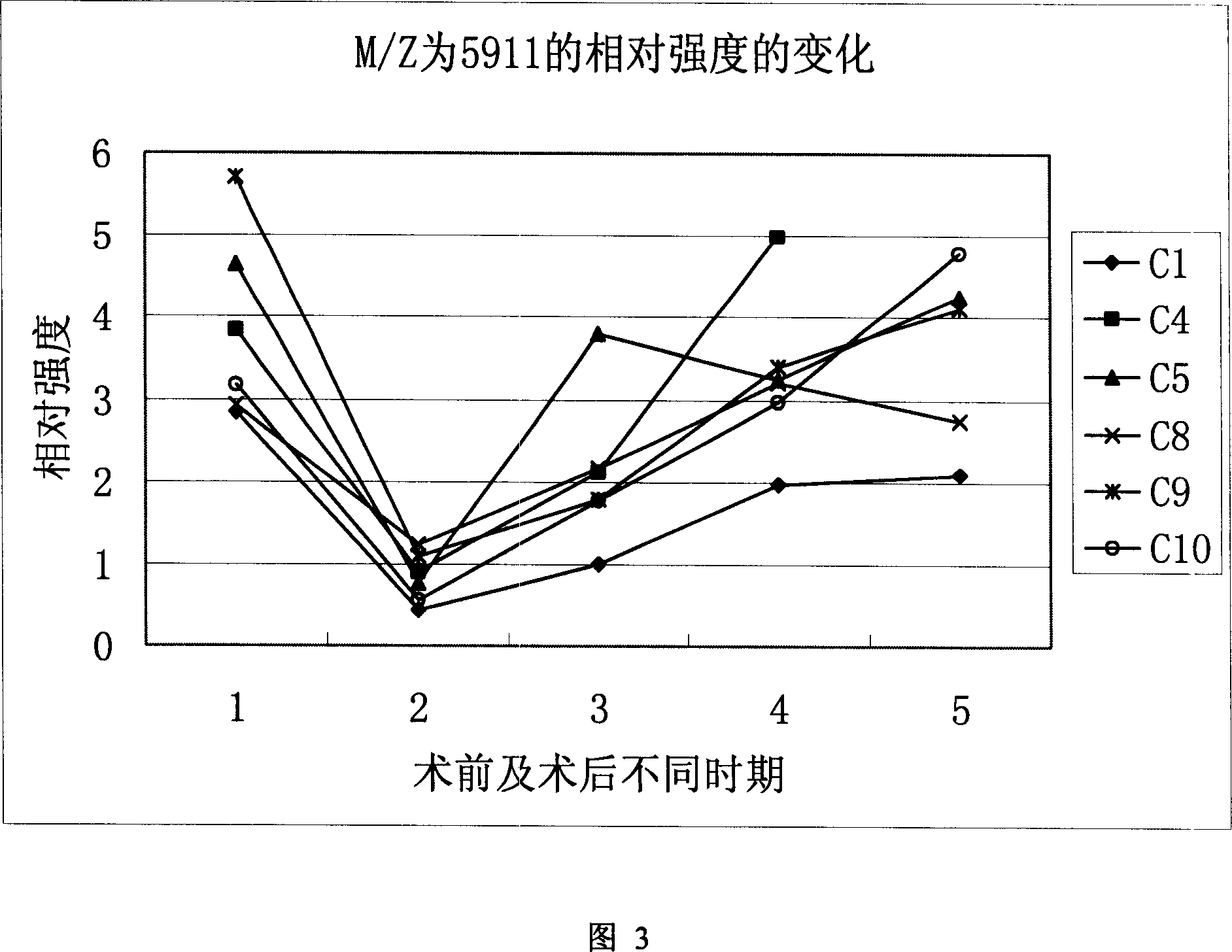
This invention provides a non-invasion for testing liver cancer, which sets up a liver cancer protein set fingerprint atlas model used in the serum test. Said model is composed of a HAS set mass spectrum atlas and an artificial nerve net analysis method used in early serology test. The atlas is composed of the protein with five M / Z. The method for setting up the atlas model includes: 1, preparation of serum, 2, mass spectrum test and collection of data for the serum specimen, 3, artificial nerve net analysis of the spectrum data, which can test the liver cancer before there is not any pathologic change appearing to the cell.
Owner:郭爱林
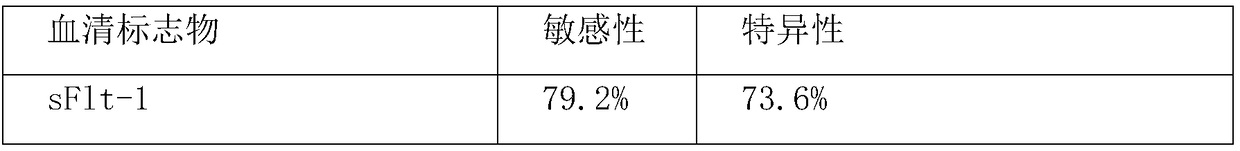
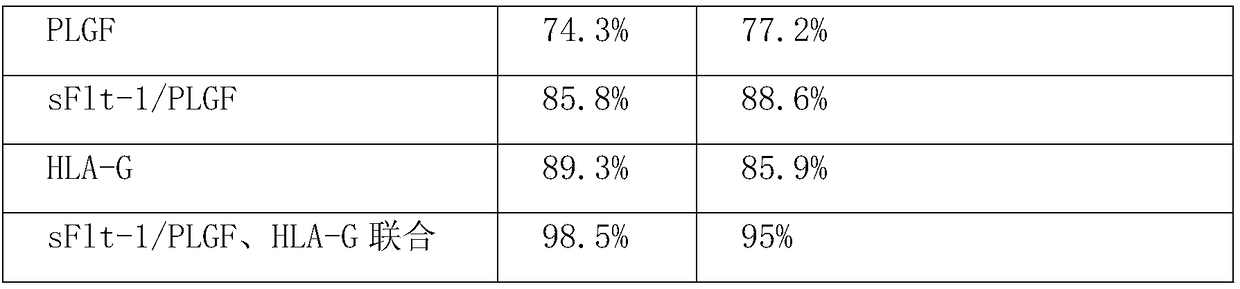
Method for jointly detecting sFlt-1/PLGF and HLA-G for detecting pre-eclampsia
InactiveCN108717123AIncreased sensitivityImprove featuresDisease diagnosisBiological testingWater bathsPositive control
The invention relates to a method for jointly detecting sFlt-1 / PLGF and HLA-G for predicting pre-eclampsia. The method comprises the following steps: collecting serum specimen: respectively collecting5-10 ml of venous blood from pregnant women in a pre-eclampsia group and normal pregnant women with same gestational weeks, centrifuging all specimens in a low temperature centrifuge at 3000 rpm for10 min within 4 h, taking the serum and subpackaging in EP tubes, and storing in a refrigerator at minus 80 DEG C for testing; coating: using a 0.05 M PH9.0 carbonate coated buffer solution to diluteantibodies to the protein content of 1-10[mu]g / ml, adding 0.1ml of the diluted antibodies into reaction holes of each polystyrene board, staying overnight at 4 DEG C, discarding the solution in the holes the next day, and washing for 3 times; setting up standard holes and sample holes, washing, and setting up blank holes, negative control holes and positive control holes at the same time; adding enzyme-labeled antibodies; adding 100 [mu]L of horseradish peroxidase-labeled detection antibodies into each of the standard holes and the sample holes except for the blank holes, sealing the reactionholes with microplate sealers, and incubating in a water bath kettle or an incubator at 37 DEG C for 60 min; performing board-washing; adding 50 [mu]L of a substrate A and a substrate B into each of the holes, and measuring OD values of each hole. The method is high in detection specificity and sensitivity.
Owner:卢英
Immunocapture molecule detection method for complete HBV particles
PendingCN113322302AEasy to operateImprove compatibilityMicrobiological testing/measurementMicroorganism based processesSerum markersMagnetic bead
The invention provides an immunocapture molecular detection method for complete HBV virus particles. The immunocapture molecular detection method comprises the following steps that a specific antibody is coupled with carboxyl magnetic beads serving as a medium, virus particles are captured and separated, and then QPCR detection is carried out. Experiments show that the virus particles in the sample can be successfully captured and separated, and the virus particles with different components in the sample can be distinguished due to the difference of the magnetic bead coupling antibodies. By means of the immunocapture molecular detection method for the complete HBV virus particles, it is accidentally found that virion components in cell supernatant and serum are different, HBV DNA in the cell supernatant is mainly derived from capsid viruses, and the content of complete virion is small; and the capsid virus content in the serum specimen is lower than that of the complete virus particles. Along with the increase of the HBV DNA copy number in the serum, the content of the complete virus particles in the serum is obviously increased, which prompts that the detection of the complete virus particles can be used as a new serum marker.
Owner:CHONGQING MEDICAL UNIVERSITY
Test paper strip for detecting encephalitis virus specificity IgG antibody, method for making same and applications
ActiveCN101363864ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseSpecific igg
The invention provides a colloidal gold test strip for the detection of Japanese encephalitis virus specific IgG antibody. A Japanese encephalitis virus E gene antigen domain III and an anti III polyclonal antibody are coated on a nitrate cellulose film (NC film), and a membrane chromatography double antigen sandwich method is adopted to detect the Japanese encephalitis virus specific IgG antibody in an animal or human body serum specimen in combination with a colloidal gold labeled Japanese encephalitis virus E gene antigen domain III. Or the Japanese encephalitis virus E gene antigen domain III and an anti-mouse IgG are coated on the nitrate cellulose film (NC film), and a capture method is adopted to detect the Japanese encephalitis virus specific IgG antibody in the human body serum specimen in combination with a colloidal gold labeled antihuman monoclonal antibody. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, has auxiliary effect on the diagnosis of Japanese encephalitis virus infection, and can be used for the effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
Creatinine kit for eliminating calcium dobesilate and etamsylate and preparation method thereof
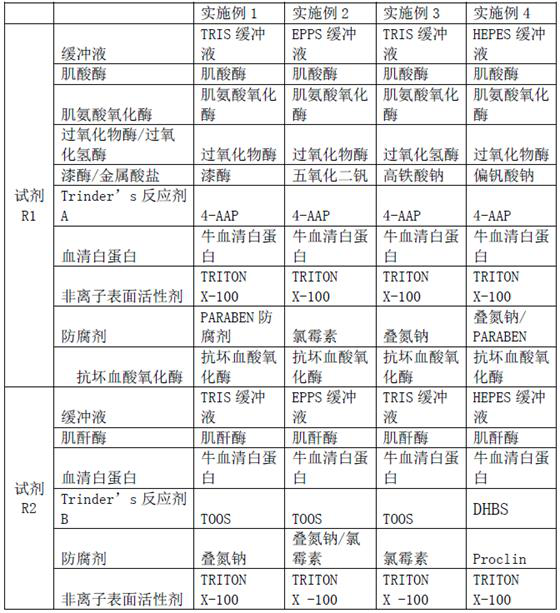
ActiveCN111733208AImprove stabilityEasy to operateMicrobiological testing/measurementBiological material analysisActive agentPeroxidase
The invention relates to a creatinine kit capable of eliminating negative deviation interference in a creatinine determination caused by calcium dobesilate and etamsylate drugs in serum and a preparation method thereof. Key points of a technical scheme of the invention are that the creatinine kit includes a reagent R1 and a reagent R2, wherein the reagent R1 includes buffer, creatinase, sarcosineoxidase, ascorbic acid oxidase, peroxidase or catalase, serum albumin, nonionic surfactant, preservative, Trinder's reagent A, laccase or metalic acid salt; and the reagent R2 includes buffer, serum albumin, creatinine enzyme, preservative and Trinder's reagent B. The invention overcomes a defect of inaccurate creatinine determination due to the presence of the negative deviation caused by calciumdobesilate and etamsylat in serum samples when the clinical tests of creatinine determination are being carried out in various hospitals, and the invention is suitable for the detection of serum creatinine by an automatic biochemical analyzer.
Owner:ZHONGSHAN BGH BIOTECH CO LTD
Quantitative analysis of a biological sample of unknown quantity
ActiveUS20040018484A1Effective recoveryAnalysis using chemical indicatorsMicrobiological testing/measurementAnalyteCholesterol
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
Application of polypeptide tagging object in detecting tumor in large intestine, and monitoring after operation
InactiveCN101004420AImprove the level of diagnosis and treatmentImprove postoperative survivalMaterial analysis using wave/particle radiationBiological testingLarge Intestine CancerLarge intestine
An application of polypeptide mark object in large intestine cancer detection includes knowing that correlation polypeptide content in serum specimen of large intestine cancer is obviously raised, using surface intensified laser de-absorption ionization flying time mass analyzer to determine correlation polypeptide content in serum specimen of patient for confirming whether detected patient is large intestine cancer patient or not.
Owner:ZHEJIANG UNIV
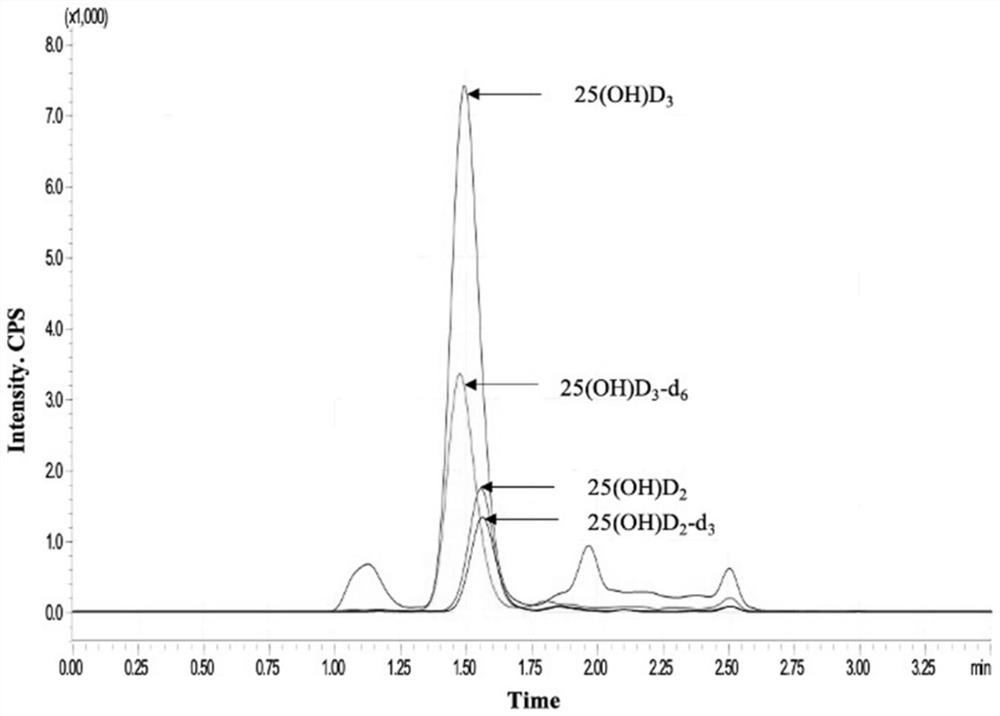
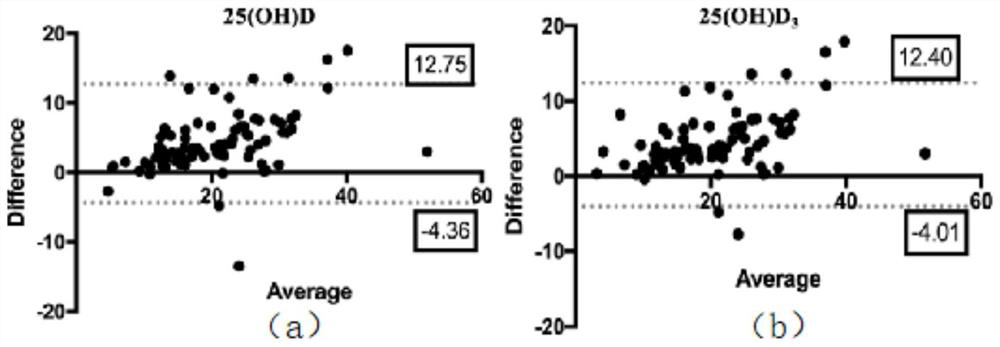
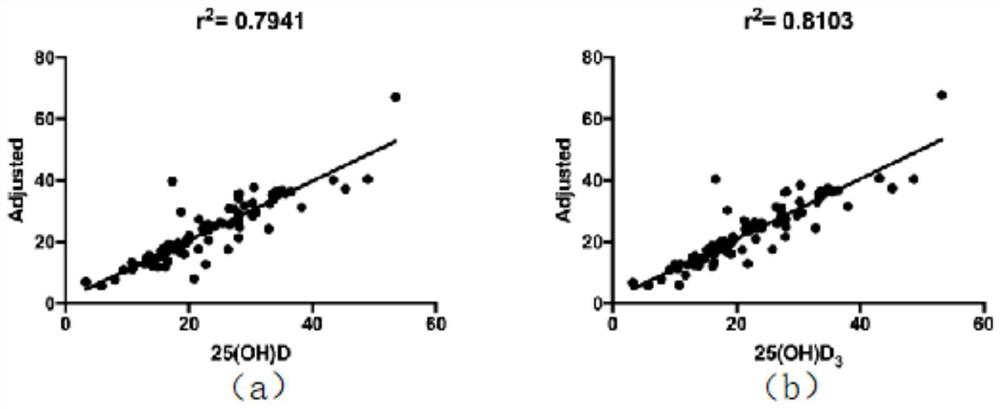
Mass spectrometric detection method for vitamin D in peripheral trace blood
The invention relates to a mass spectrometric detection method for vitamin D in peripheral trace blood. The method comprises the steps of serum specimen collection, preparing a detection material anda chemical product; calibration standard, venous blood cleaner, a trace serum 25-hydroxyvitamin D detection method, analyzing data, evaluating the analysis performance of an HPLC-MS / MS method, verifying the result of a vein and trace blood HPLC-MS / MS analysis method, comparing the level of 25-hydroxyvitamin D in vein and trace blood, and comparing the concentration result of 25-hydroxyvitamin D invein and trace blood specimens. The method has the advantages that trace blood and constant blood of the same object are collected at the same time, the 25-hydroxyvitamin D levels of the constant blood and the trace blood are detected at the same time through the high performance liquid chromatography-tandem mass spectrometry, the correlation and conversion coefficient of 25-hydroxyvitamin D of the constant blood and the trace blood are analyzed through the linear regression analysis method, and therefore a conversion formula is obtained; the concentrations of 25-hydroxyvitamin D in constantblood and trace blood can be mutually converted through a formula; and a reliable basis is provided for clinical VitD detection by replacing constant blood with trace blood.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
ELISA percolation method for rapidly detecting pathogen antibody, kit for detection, and preparation method of kit
The present invention provides an ELISA percolation method for rapidly detecting pathogen antibody, a kit for detection, and a preparation method of the kit. According to the present invention, an antigen-antibody reaction is used to make antigen or second antibody be immobilized on a nitrocellulose membrane, the pathogen IgM (IgG) antibody in a serum sample and the corresponding solid-phase antigen or second antibody on the membrane produce specific binding so as to form a complex when the serum sample passes through the nitrocellulose membrane due to a percolation effect while other unrelated substances are filtered, enzyme-labeled antibody or antigen is added and is combined with the antigen-antibody complex on the membrane during the filtering, and a coloration liquid is added to carry out coloration so as to produce the purple blot conveniently observed by naked eyes; and the time consuming of the detection process is short, the sensitivity is high, the accuracy is strong, the shelf life of the kit can achieve more than or equal to 12 months with the stable dilution buffer solution and the coloration prepared through the method, and the whole detection has characteristics of simple and rapid operation process, no requirement of special equipment, high sensitivity, and high accuracy.
Owner:QINGDAO HIGHTOP BIOTECH
ELISA kit for detecting porcine circovirus antibody II
ActiveCN101629956BSimilar sensitivityStrong specificityMicroorganism based processesBiological testingSerum igeElisa kit
The invention relates to an ELISA kit for detecting porcine circovirus antibodies II, wherein, the kit is developed according to the double antigen sandwich ELISA principle. The ELISA kit comprises a prepacked ELISA antigen plate bar, a cleaning solution, 100 times concentrated enzyme labeled antigen, an enzyme labeled dilution, a zymolyte coloration, a stopping solution, standard PCV2 negative sera, and standard PCV2 positive sera. The kit replaces enzyme labeled anti-antibodies with the enzyme labeled antigen. The enzyme labeled antigen can not combine with antibodies absorbed with ELSA plate without specificity. Meanwhile, the serum specimen to be detected does not need to be pre-diluted, and the serum specimen can be directly mixed with the enzyme labeled antigen with working concentration to be directly used for determination. The ELISA kit has the advantages of simple operation and shorter detecting time.
Owner:北京金诺百泰生物技术有限公司
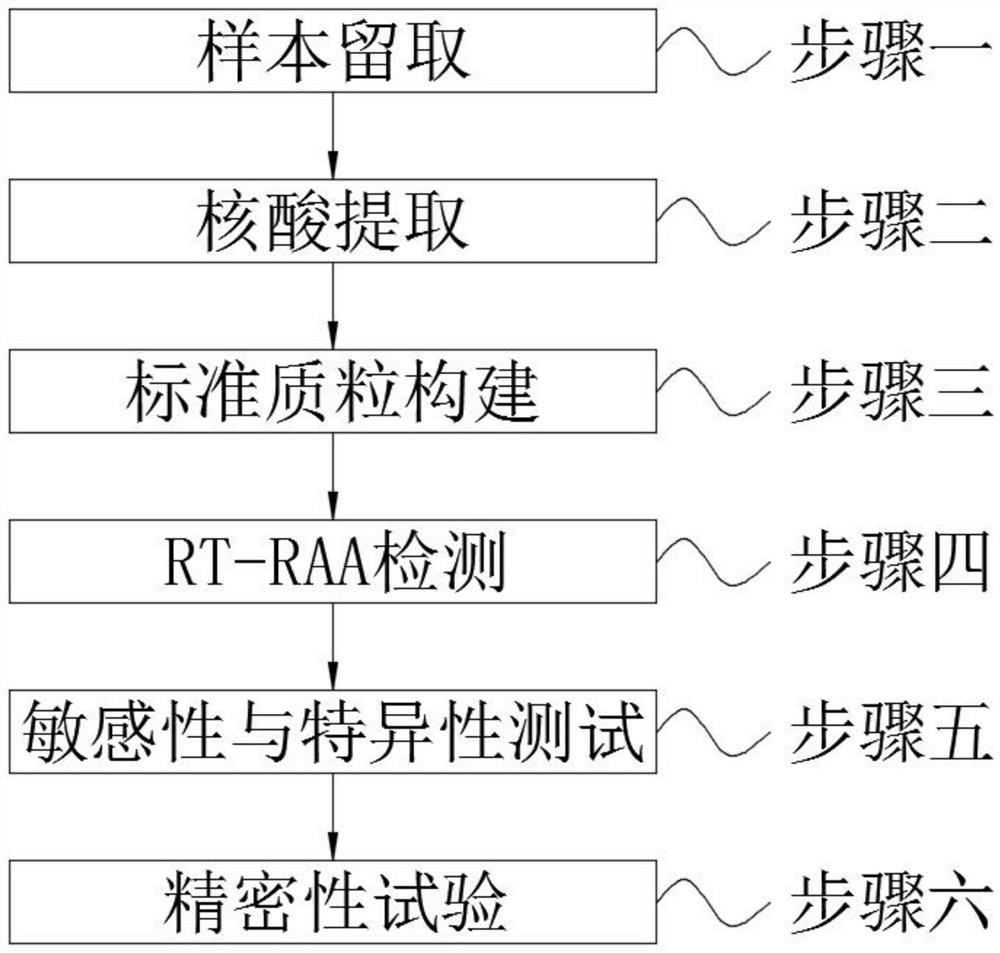
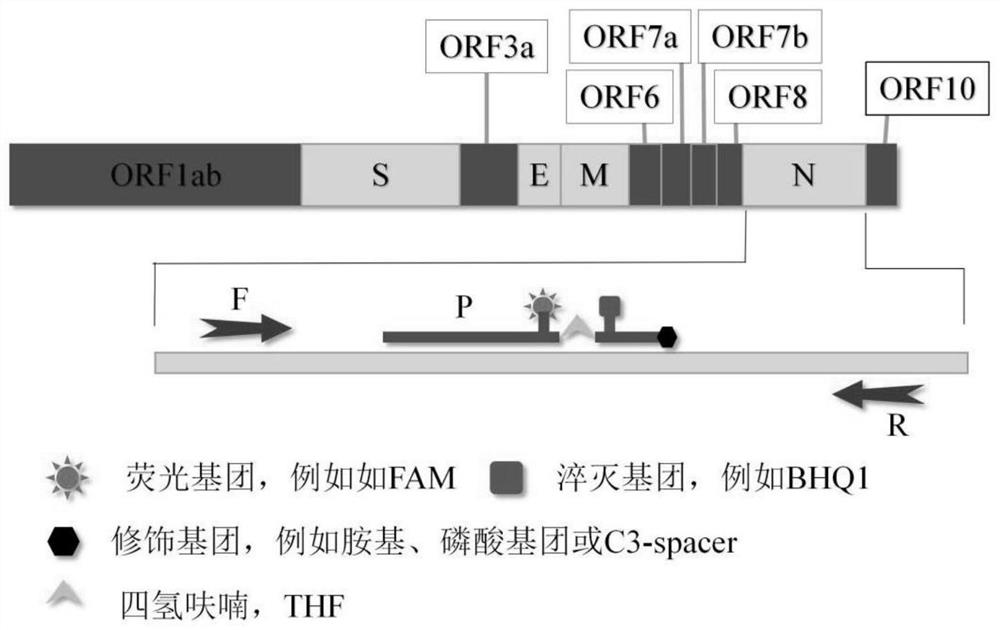
Method for detecting novel coronavirus virus based on real-time fluorescence RT-RAA
PendingCN111979304AMeet detectionHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesBronchial LavageBronchial epithelium
The invention discloses a method for detecting novel coronavirus virus based on real-time fluorescence RT-RAA. The method comprises the following steps: step 1, reserving a sample; 2, extracting nucleic acid; step 3, constructing standard plasmids; step 4, carrying out RT-RAA detection; step 5, testing sensitivity and specificity; step 6, carrying out a precision test, wherein in the step 1, a throat swab, a nasopharynx extract or a respiratory tract extract, a deep expectoration solution, a bronchial lavage solution, an alveolar lavage solution, a blood specimen, a serum specimen and an eye conjunctival swab are manually collected. According to the method for detecting the novel coronavirus virus based on the real-time fluorescence RT-RAA, a novel real-time fluorescence RT-RAA method fordetecting SARS-CoV-2 is constructed based on a recombinase mediated isothermal nucleic acid amplification technology, and compared with other molecular diagnosis methods, the method is high in detection sensitivity, easy, convenient and rapid to operate and suitable for large-scale industrial production. No special equipment is needed, the result is accurate, clinical sample detection can be met,and the method is particularly suitable for mobile emergency detection of resource-deficient areas or airports, schools and other units.
Owner:HANSHAN NORMAL UNIV +1
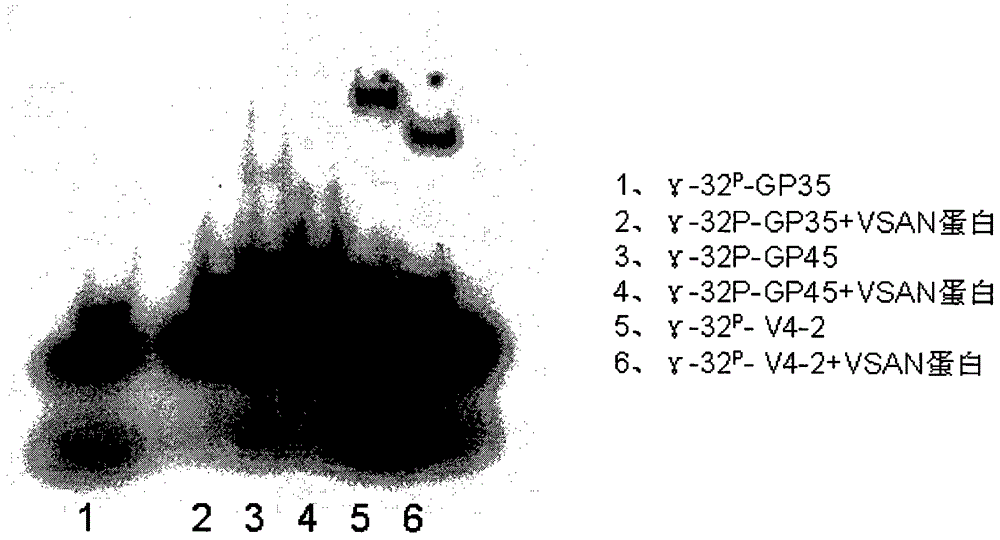
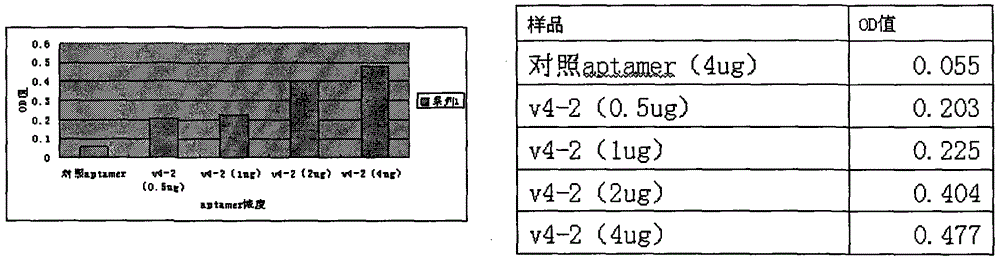
Sequence and application of oligonucleotide ligand V4-2 for specifically recognizing Vasorin (VASN) protein
ActiveCN104450712AEasy to operateLower synthesis costBiological testingDNA/RNA fragmentationDiseaseBasic research
The invention discloses a sequence and application of an oligonucleotide ligand V4-2 for specifically recognizing Vasorin (VASN) protein, belonging to the technical field of molecular biomedicine. The V4-2 capable of specifically recognizing the Vasorin (VASN) protein is detected by designing the specific sequence of V4-2 and adopting a 5'-end or 3'-end modification and marking (e.g. FITC (fluorescein isothiocyanate), biotin or digoxin) method. The V4-2 serving as a composition part of a kit or a detection index is used for auxiliary diagnosis and targeted therapy of liver cancer or basic research related with occurrence, development and progress of liver cancer diseases and the like. The method is simple, fast and sensitive, is suitable for auxiliary diagnosis of clinical tumor serum specimens and targeted biotherapy of tumors, and has a wide clinical application and basic application prospect.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
PCR (Polymerase Chain Reaction) primer, PCR primer group, PCR detection probe and PCR detection kit for detecting hepatitis B virus as well as detection method
ActiveCN104004856BAvoid false negativesAvoid pollutionMicrobiological testing/measurementMicroorganism based processesNew medicationsUracil
The invention discloses a PCR (Polymerase Chain Reaction) primer, a PCR primer group, a PCR detection probe and a PCR detection kit for detecting hepatitis B virus as well as a detection method. The kit contains the high-sensitivity detection primer group and the high-sensitivity detection probe and can be used for detecting single copy HBVDNA (Hepatitis B virus-Deoxyribonucleic acid), the limit of detection of the kit in a serum specimen can reach 2IU / mL, and compared with an imported reagent Roche COBAS, the kit is relatively sensitive. The detection method disclosed by the invention can be used for detecting different subtypes of hepatitis B pathogens around the world; due to addition of an internal control system in the detection method, false negative can be effectively prevented; due to addition of a UNG (uracil-N-glycosylase) system in the detection method, pollution can be effectively avoided; the kit and the detection method have the beneficial effects that the kit and the detection method can be applied to diagnosis of hepatitis B, evaluation of research, development and screening of anti-hepatitis-B new drugs and evaluation of an anti-hepatitis-B treatment effect and relapsing and have wide clinical application effects, and the popularization and application of the kit and the detection method are facilitated.
Owner:GUANGZHOU SUPBIO BIO TECH & SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com