Creatinine kit for eliminating calcium dobesilate and etamsylate and preparation method thereof
A technology of calcium dobesilate and ethylamine sulfonate, which is applied in the field of medical testing, can solve the problems of inaccurate creatinine measurement and misjudgment, achieve precise medication and treatment, simple and rapid operation, and avoid misjudgment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
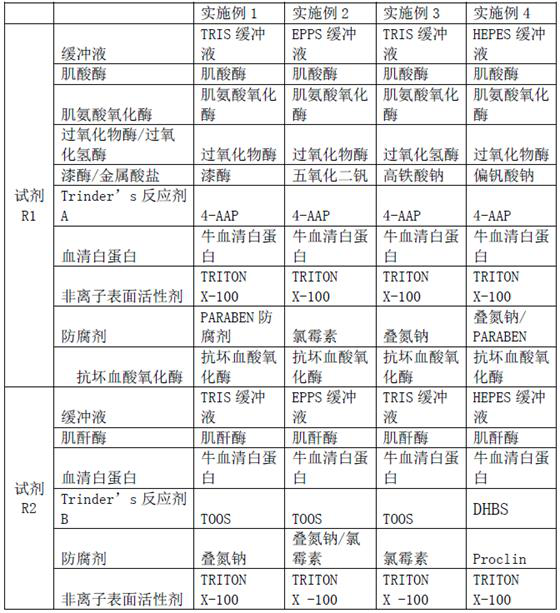
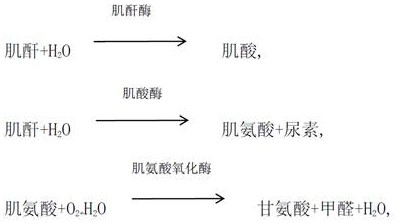
[0062] Reagent R1 components:
[0063] TRIS buffer 50mM PH8.0,
[0064] Creatinase 25ku / L,
[0065] Sarcosine oxidase 15 ku / L,
[0066] Peroxidase 10 ku / L,
[0067] Ascorbate Oxidase 10 ku / L,
[0068] Laccase 1 ku / L,
[0069] 4-AAP 3mM,
[0070] Bovine Serum Albumin 0.1%,
[0071] TRITON X-100 0.2%,
[0072] PARABEN preservative 0.1%;
[0073] Reagent R2 components:
[0074] TRIS buffer 50mM PH8.0,
[0075] Creatinase 300 ku / L,
[0076] Bovine Serum Albumin 0.1%,
[0077] TOOS 10mM,
[0079] TRITON X-100 0.1%;
Embodiment 2
[0081] Reagent R1 components:
[0082] EPPS buffer 50mM PH8.0,
[0083] Creatinase 25 ku / L,
[0084] Sarcosine oxidase 15 ku / L,
[0085] Peroxidase 5.5ku / L,
[0086] Ascorbate oxidase 6.4ku / L,
[0087] Vanadium Pentoxide 1 mM,
[0088] 4-AAP 3 mM,
[0089] Bovine Serum Albumin 0.1%,
[0090] TRITON X-100 0.2%,
[0091] Chloramphenicol 0.1%;
[0092] Reagent R2 components:
[0093] EPPS buffer 50 mM pH8.0,
[0094] Creatinase 500 ku / L,
[0095] Bovine Serum Albumin 0.1%,
[0096] TOOS 10 mM,
[0097] Sodium Azide / Chloramphenicol 0.1%,
[0098] TRITON X-100 0.1%.
Embodiment 3
[0100] Reagent R1 components:
[0101] TRIS buffer 50mM PH8.0,
[0102] Creatinase 25 ku / L,
[0103] Sarcosine oxidase 15 ku / L,
[0104] Catalase 100ku / L,
[0105] Ascorbate oxidase 7.5ku / L,
[0106] Sodium ferrate 2 mM,
[0107] 4-AAP 3 mM,
[0108] Bovine Serum Albumin 0.1%,
[0109] TRITON X-100 0.2%,
[0110] Sodium azide 0.1%;
[0111] Reagent R2 components:
[0112] TRIS buffer 50 mM pH8.0,
[0113] Creatinase 500 ku / L,
[0114] Bovine Serum Albumin 0.1%,
[0115] TOOS 10 mM,
[0116] Chloramphenicol 0.1%,
[0117] TRITON X-100 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com