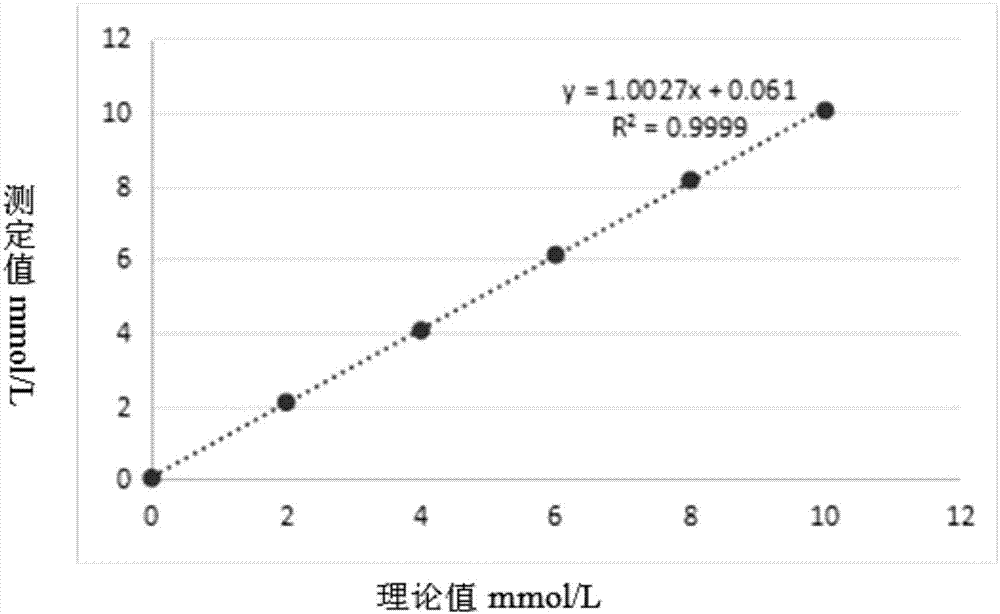
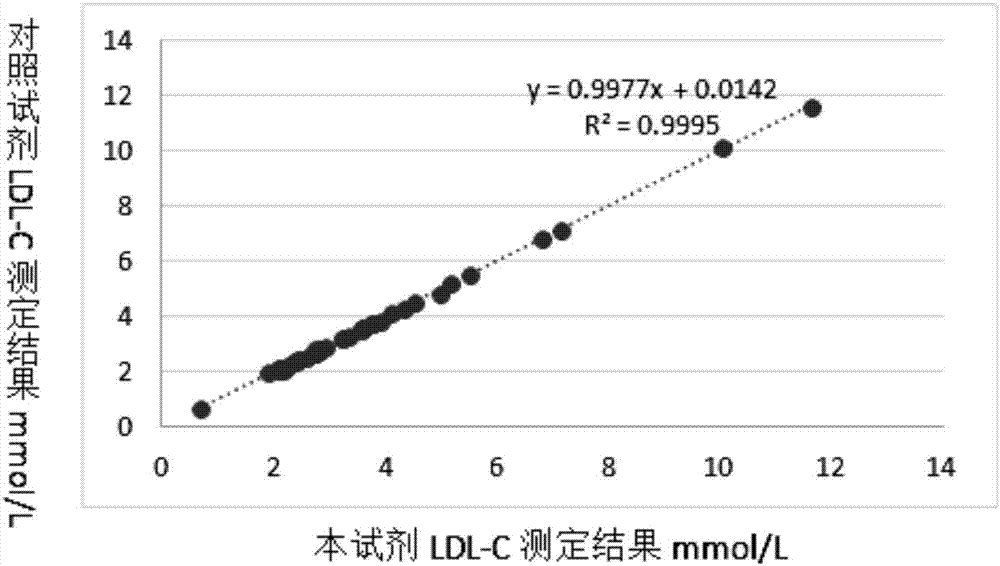
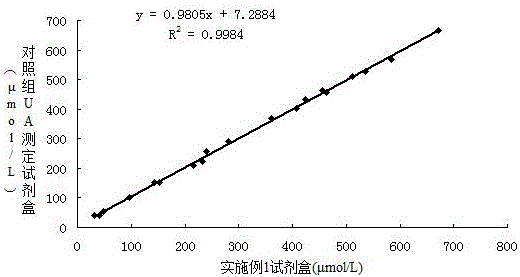
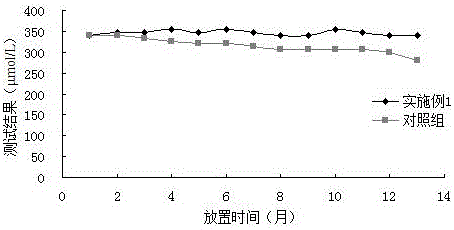
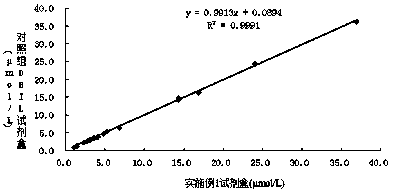
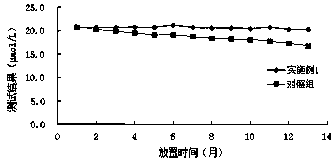
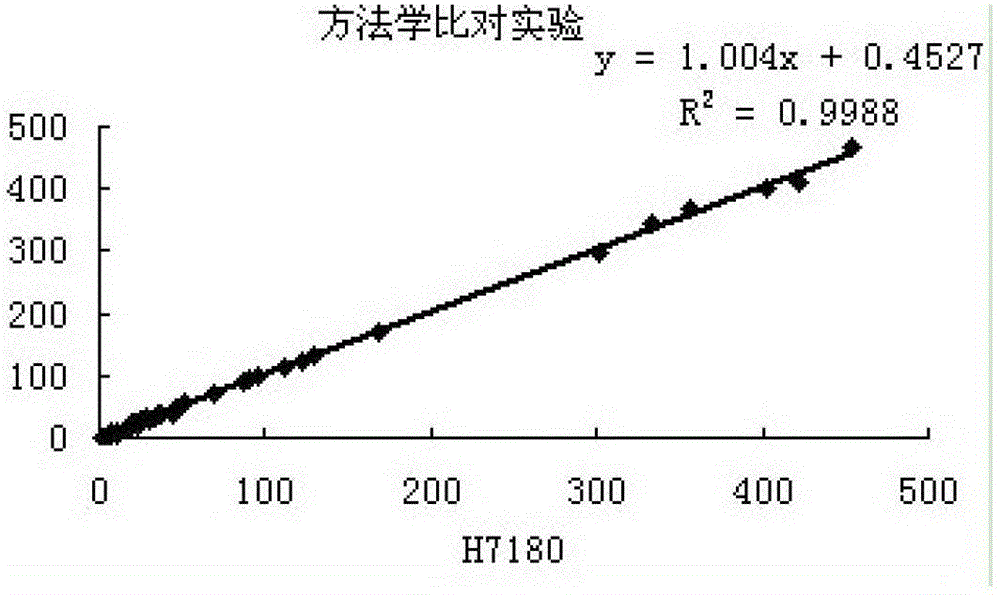
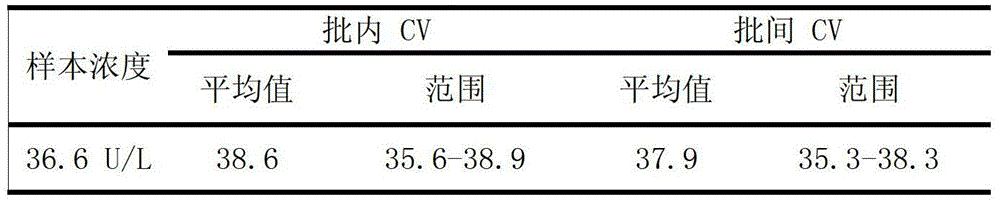
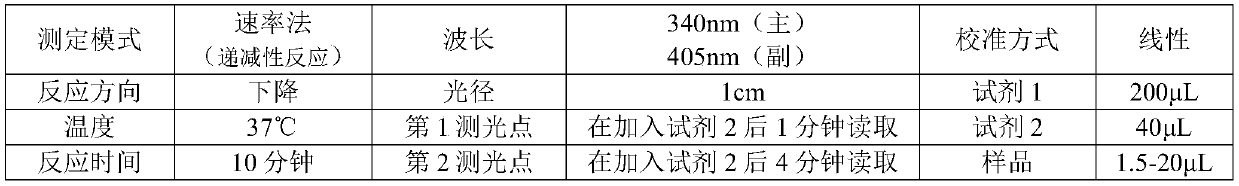
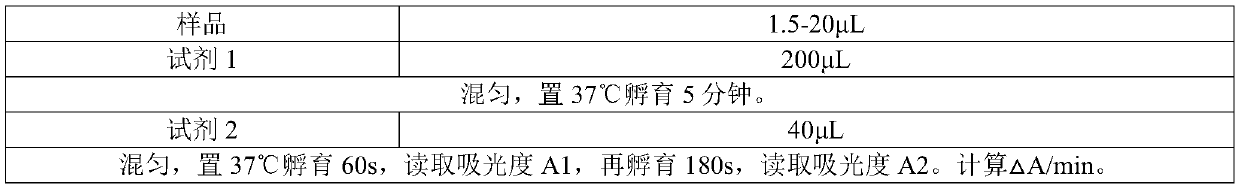
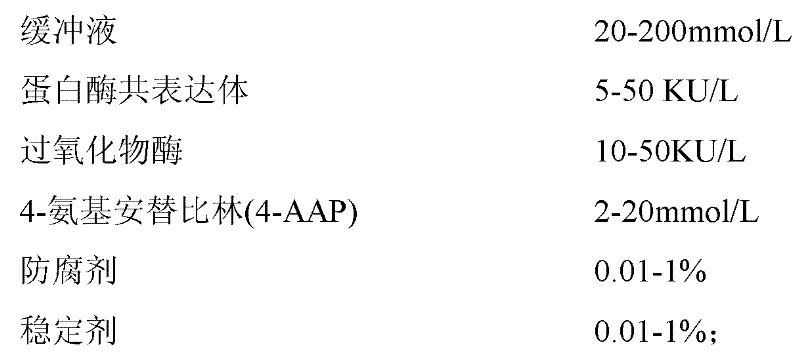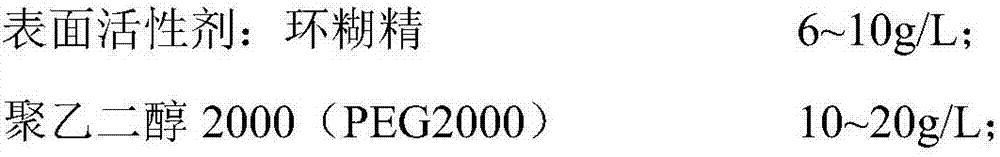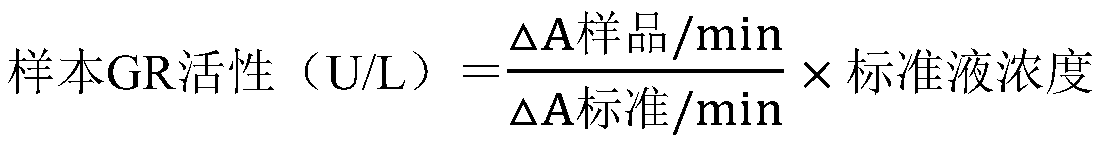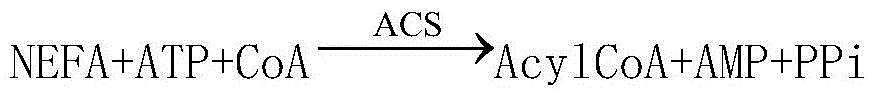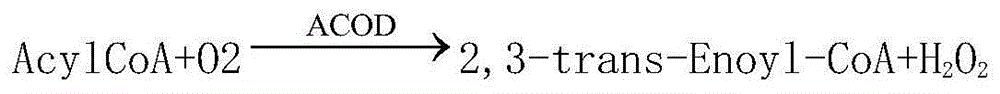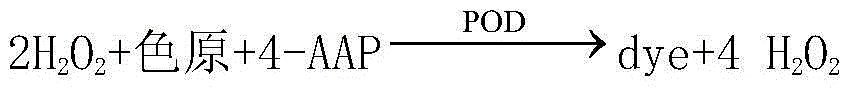Patents
Literature
90 results about "Ascorbate Oxidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In enzymology, a L-ascorbate oxidase (EC 1.10.3.3) is an enzyme that catalyzes the chemical reaction. 2 L-ascorbate + O 2 ⇌ 2 dehydroascorbate + 2 H 2 O. Thus, the two substrates of this enzyme are L-ascorbate and O 2, whereas its two products are dehydroascorbate and H 2 O
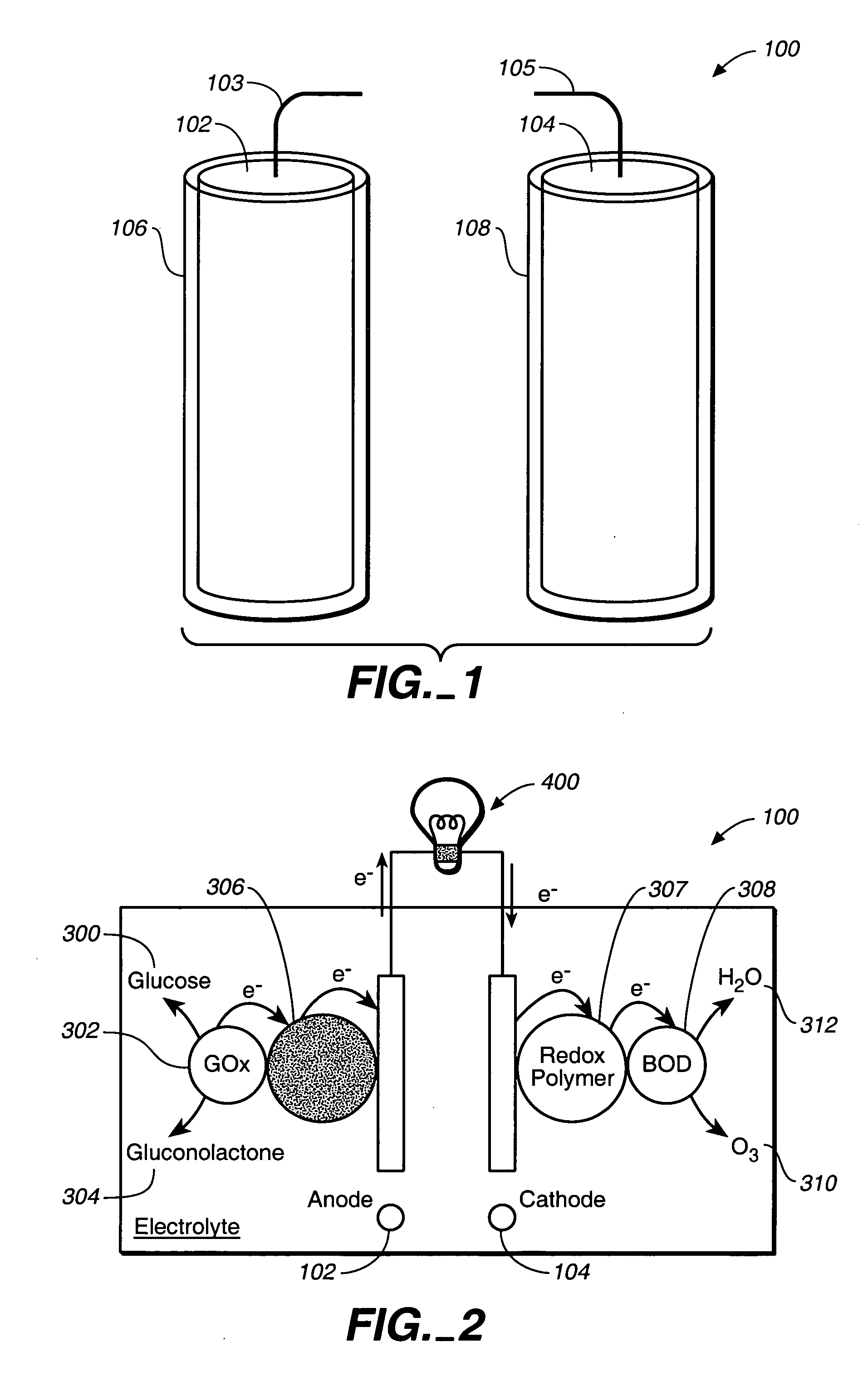
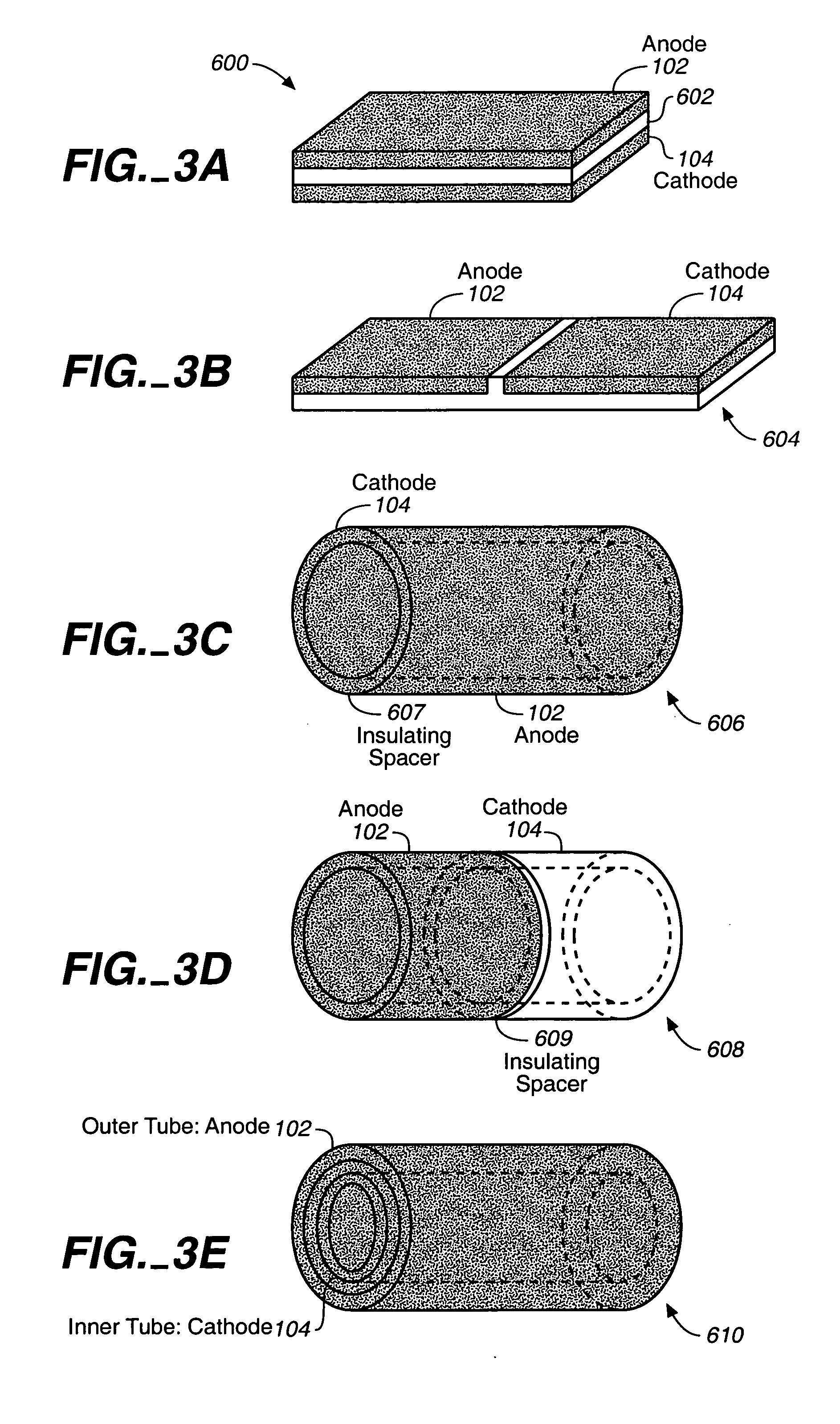
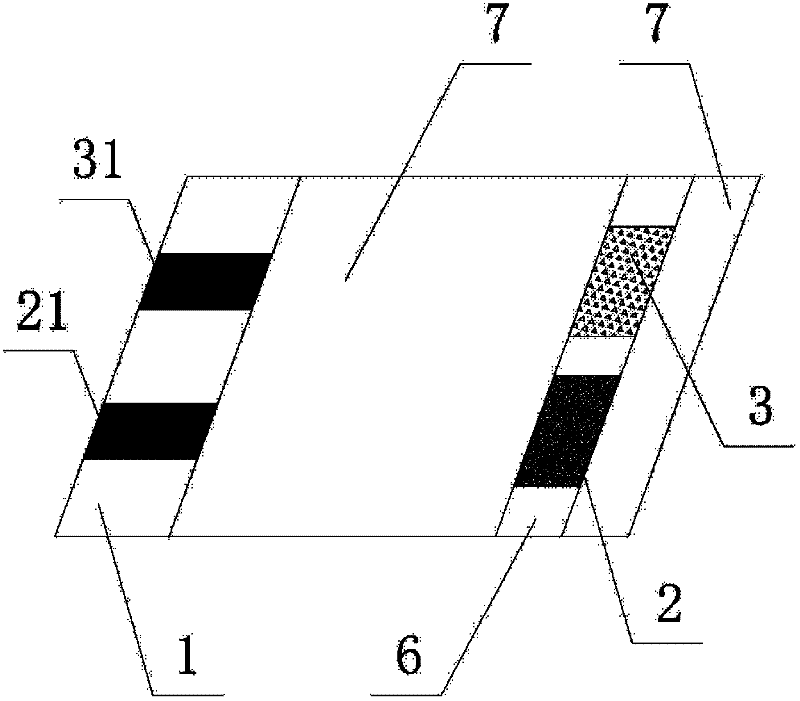
Miniature biological fuel cell that is operational under physiological conditions, and associated devices and methods
InactiveUS7368190B2Reduce physical sizeReduced dimensionMicrobiological testing/measurementVolume/mass flow measurementOperabilityRedox polymers
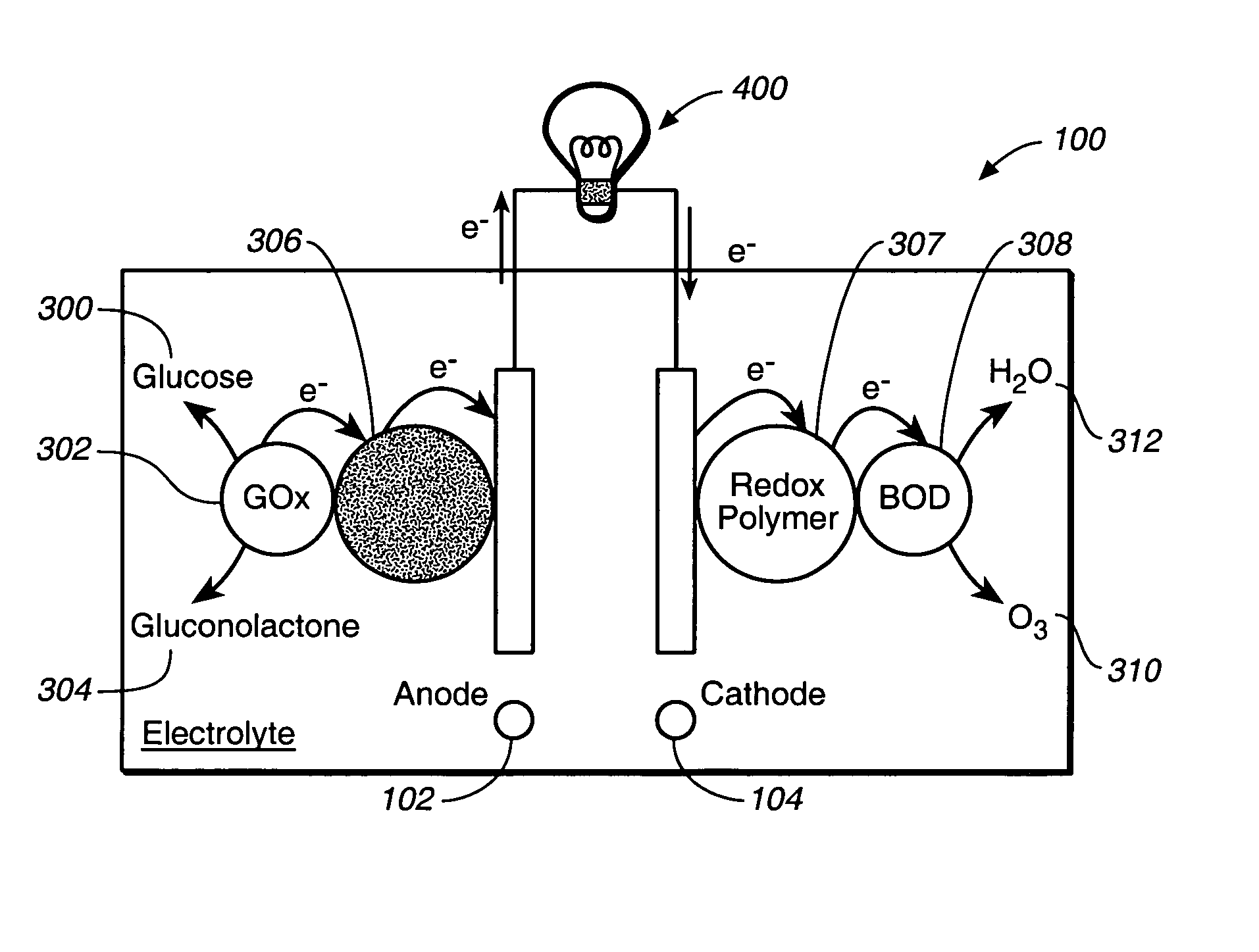
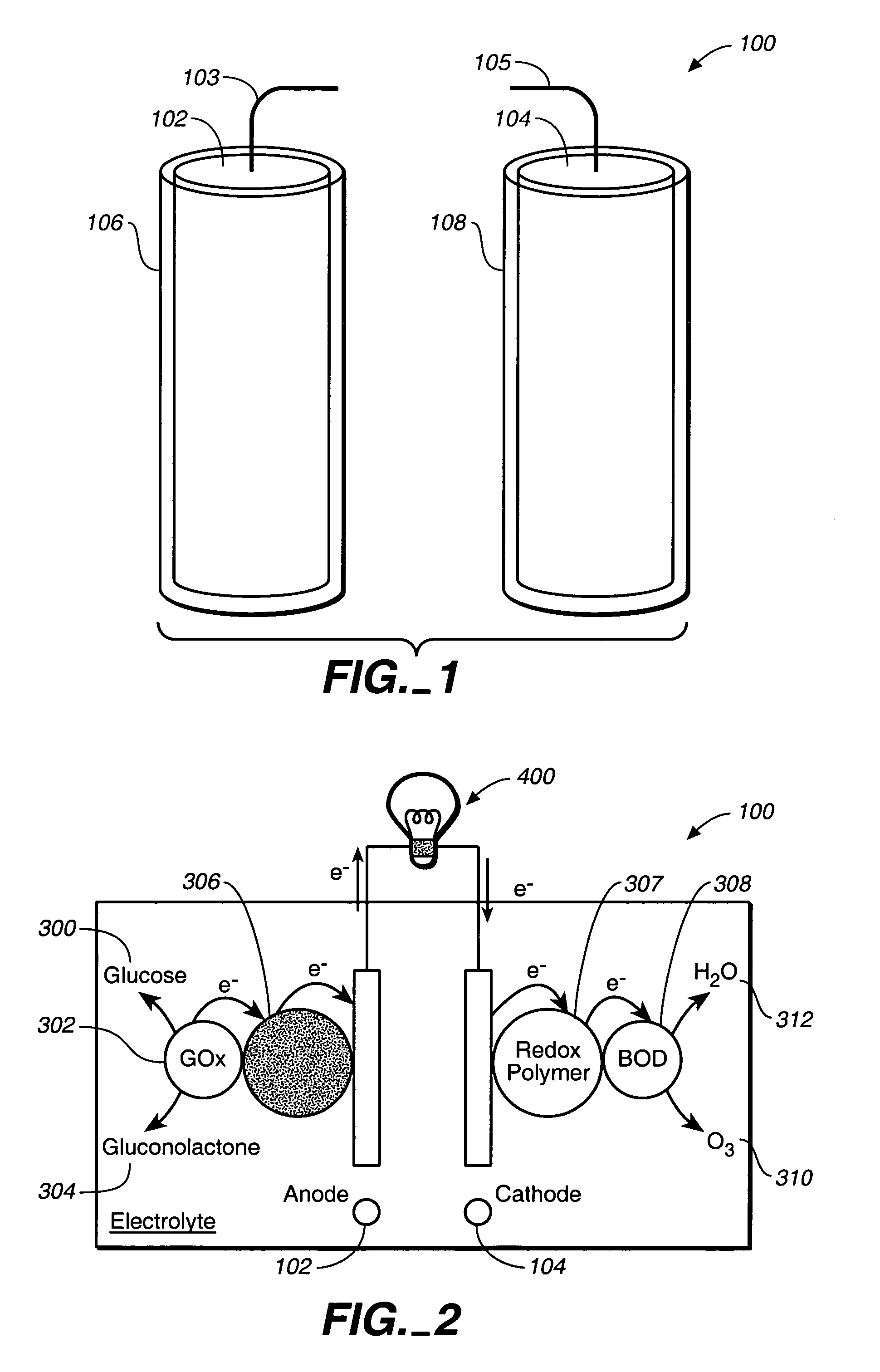
A fuel cell is provided with an anode and a cathode. The anode is in electrical communication with an anode enzyme and the cathode is in electrical communication with a cathode enzyme. The anode enzyme is preferably an oxidase or a dehydrogenase. The cathode enzyme is a copper-containing enzyme, such as a laccase, an ascorbate oxidase, a ceruloplasmine, or a bilirubin oxidase. Preferably, the cathode enzyme is operable under physiological conditions. Redox polymers serve to wire the anode enzyme to the anode and the cathode enzyme to the cathode. The fuel cell can be very small in size because it does not require a membrane, seal, or case. The fuel cell can be used in connection with a biological system, such as a human, as it may operate at physiological conditions. By virtue of its size and operability at physiological conditions, the fuel cell is of particular interest for applications calling for a power source implanted in a human body, such as a variety of medical applications.
Owner:ABBOTT DIABETES CARE INC
Miniature biological fuel cell that is operational under physiological conditions, and associated devices and methods
InactiveUS20080044721A1Reduce physical sizeReduced dimensionMicrobiological testing/measurementVolume/mass flow measurementOperabilityRedox polymers
A fuel cell is provided with an anode and a cathode. The anode is in electrical communication with an anode enzyme and the cathode is in electrical communication with a cathode enzyme. The anode enzyme is preferably an oxidase or a dehydrogenase. The cathode enzyme is a copper-containing enzyme, such as a laccase, an ascorbate oxidase, a ceruloplasmine, or a bilirubin oxidase. Preferably, the cathode enzyme is operable under physiological conditions. Redox polymers serve to wire the anode enzyme to the anode and the cathode enzyme to the cathode. The fuel cell can be very small in size because it does not require a membrane, seal, or case. The fuel cell can be used in connection with a biological system, such as a human, as it may operate at physiological conditions. By virtue of its size and operability at physiological conditions, the fuel cell is of particular interest for applications calling for a power source implanted in a human body, such as a variety of medical applications.
Owner:ABBOTT DIABETES CARE INC
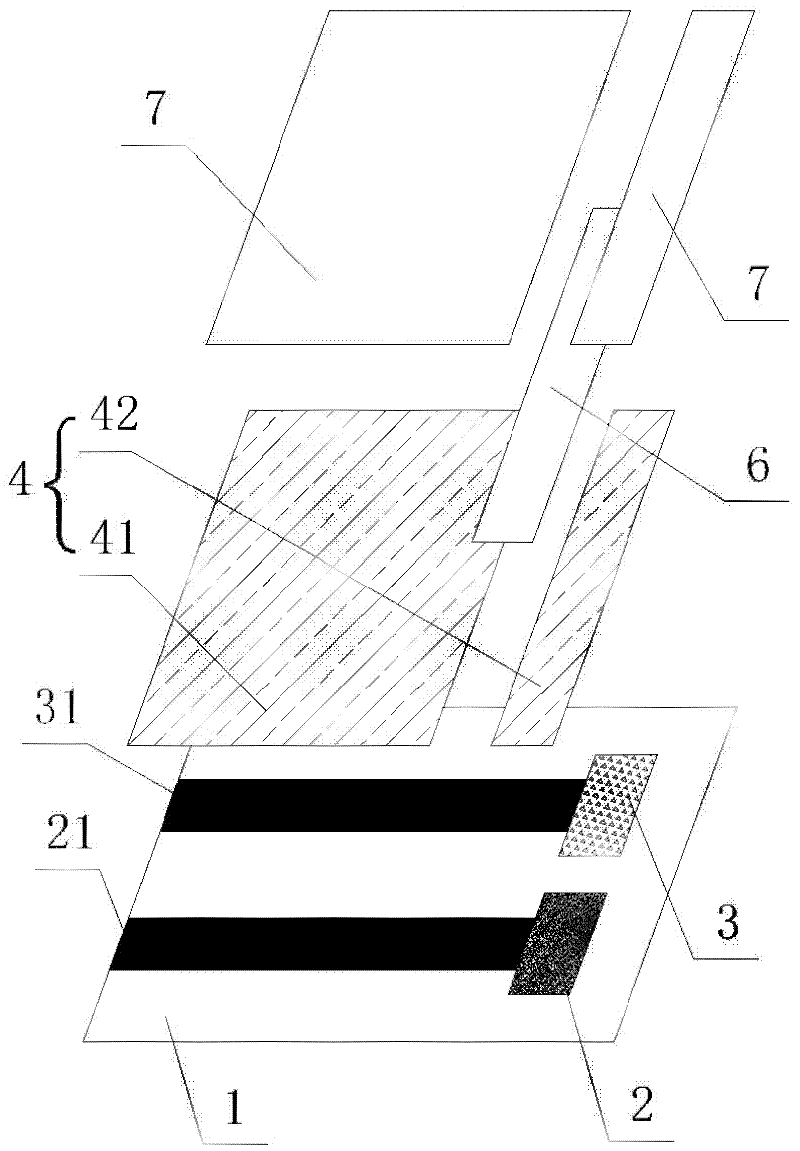
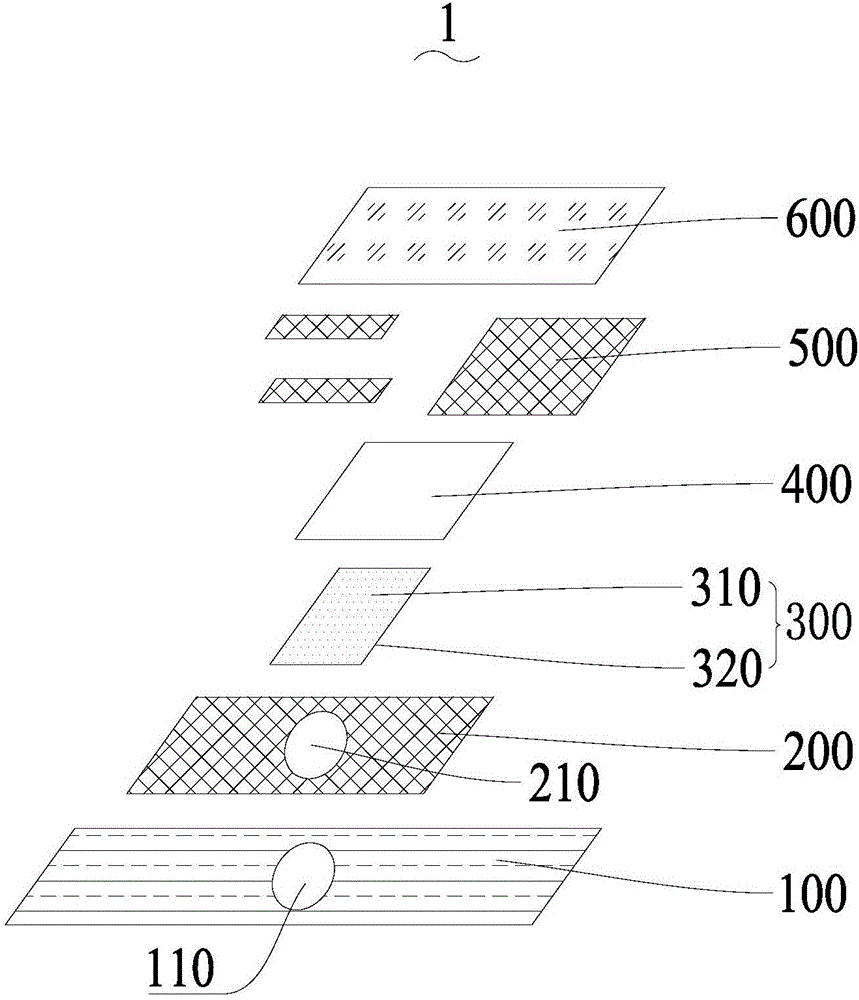
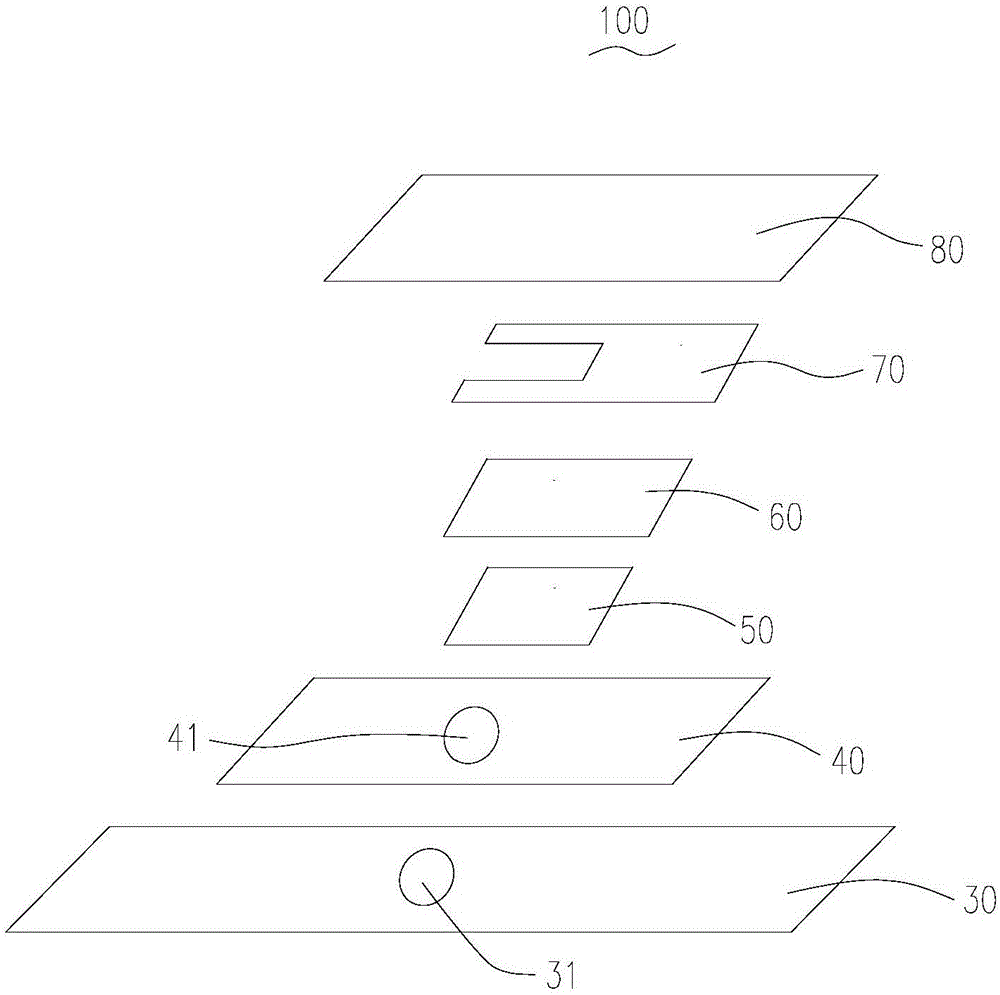
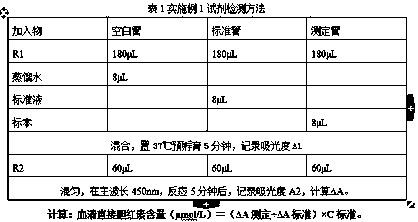
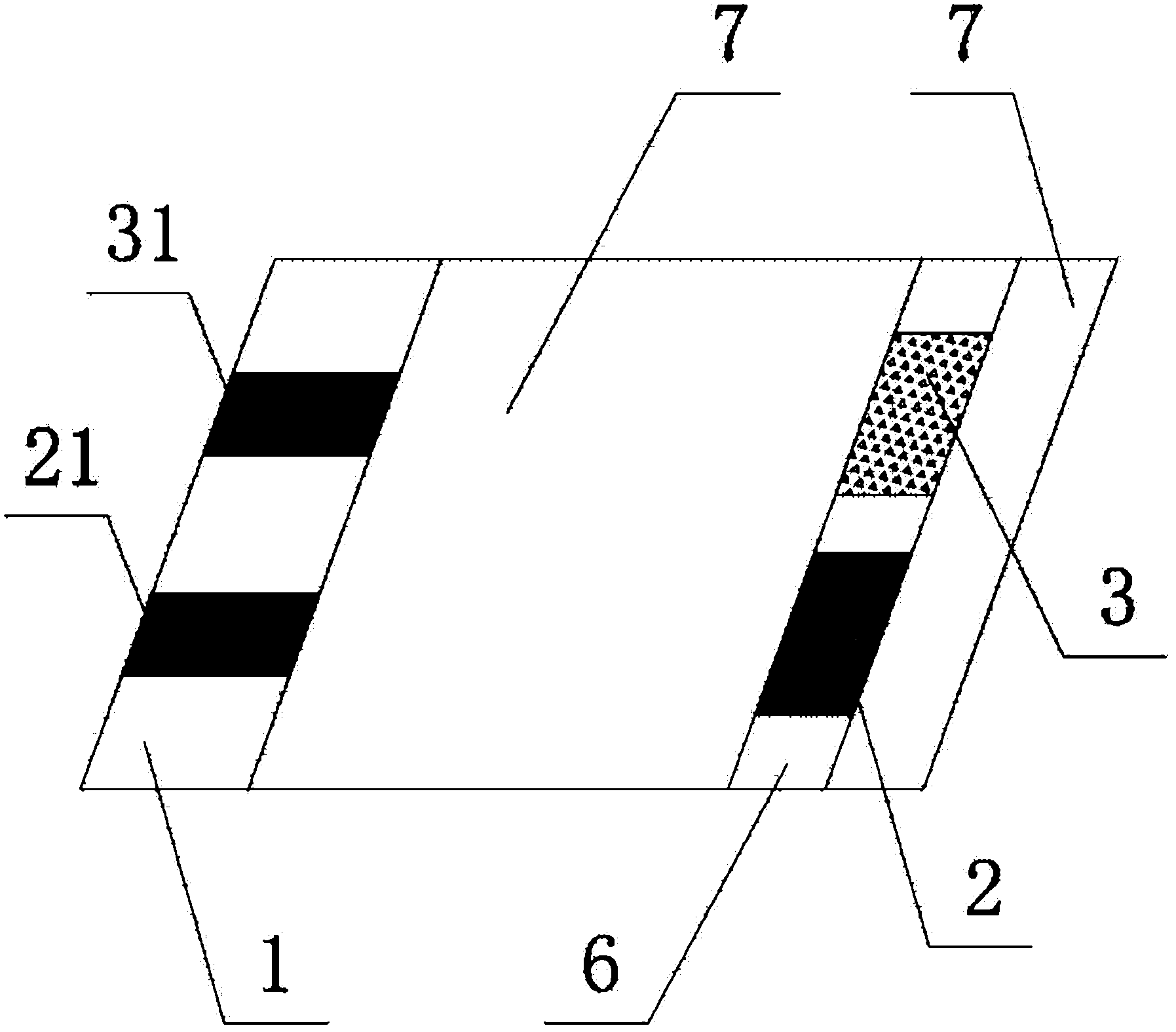
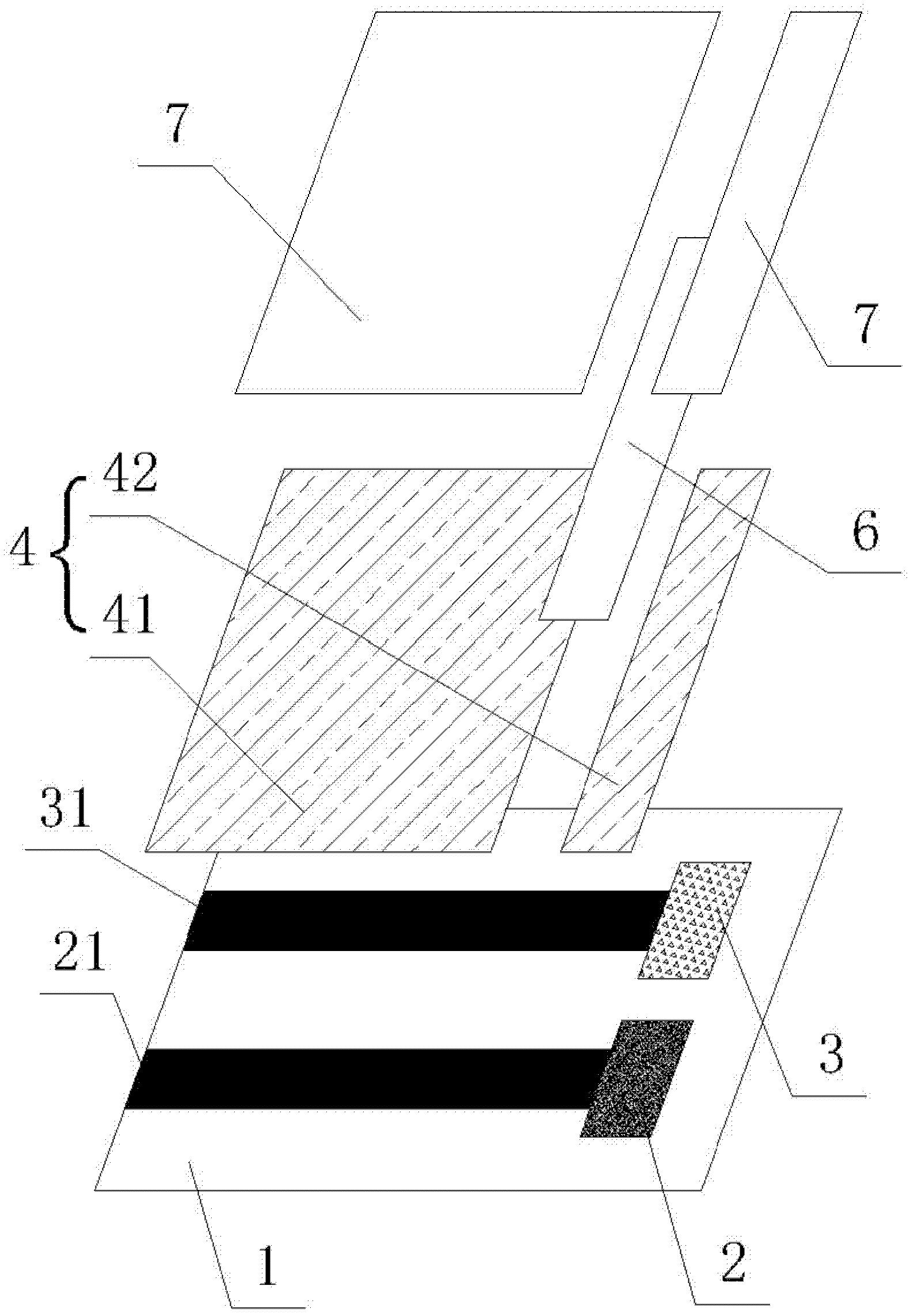
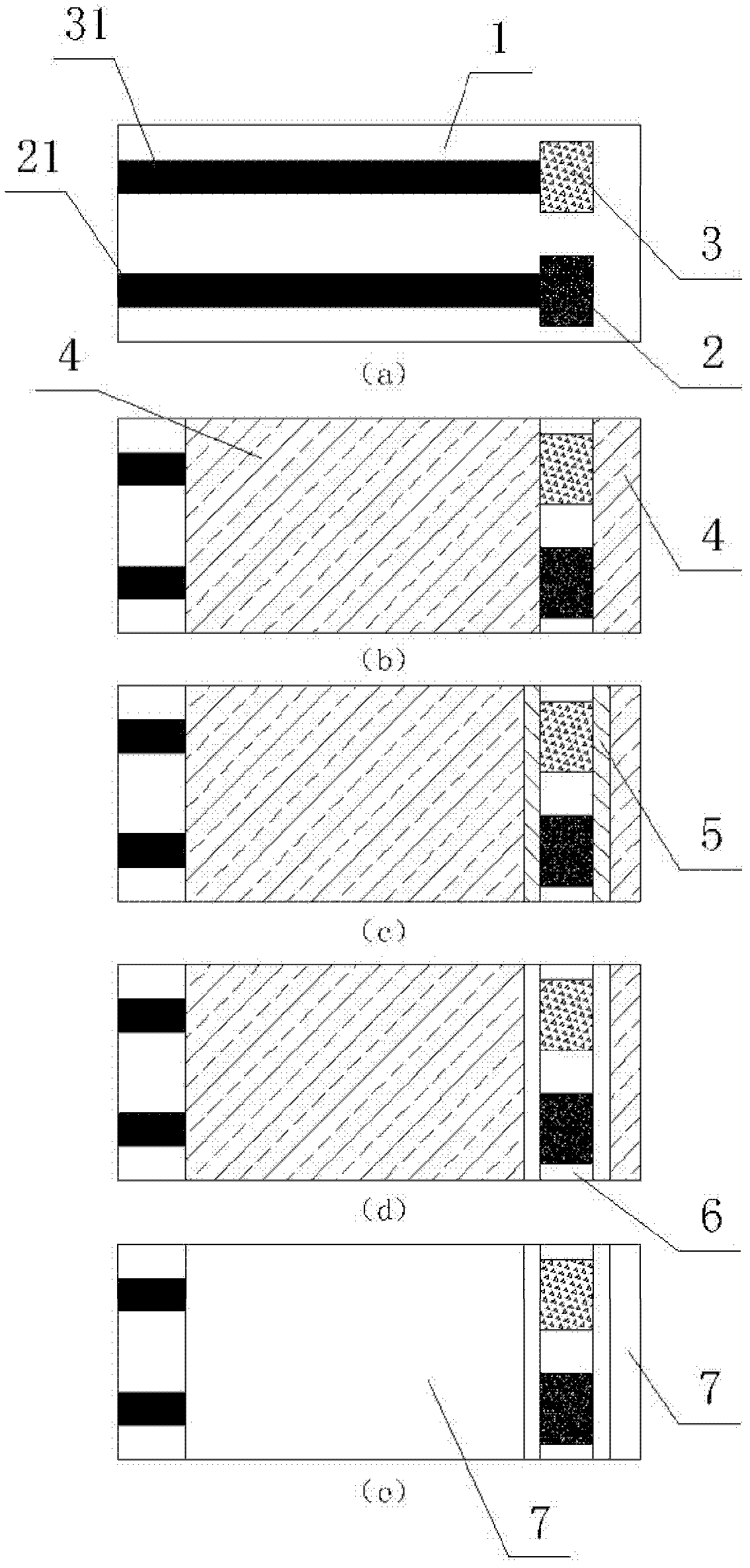
Electrochemical uric acid test strip and manufacturing method thereof
ActiveCN102507670AImprove storage conditionsImprove solubilityMaterial electrochemical variablesElectron transfer mediatorOxidation-Reduction Agent
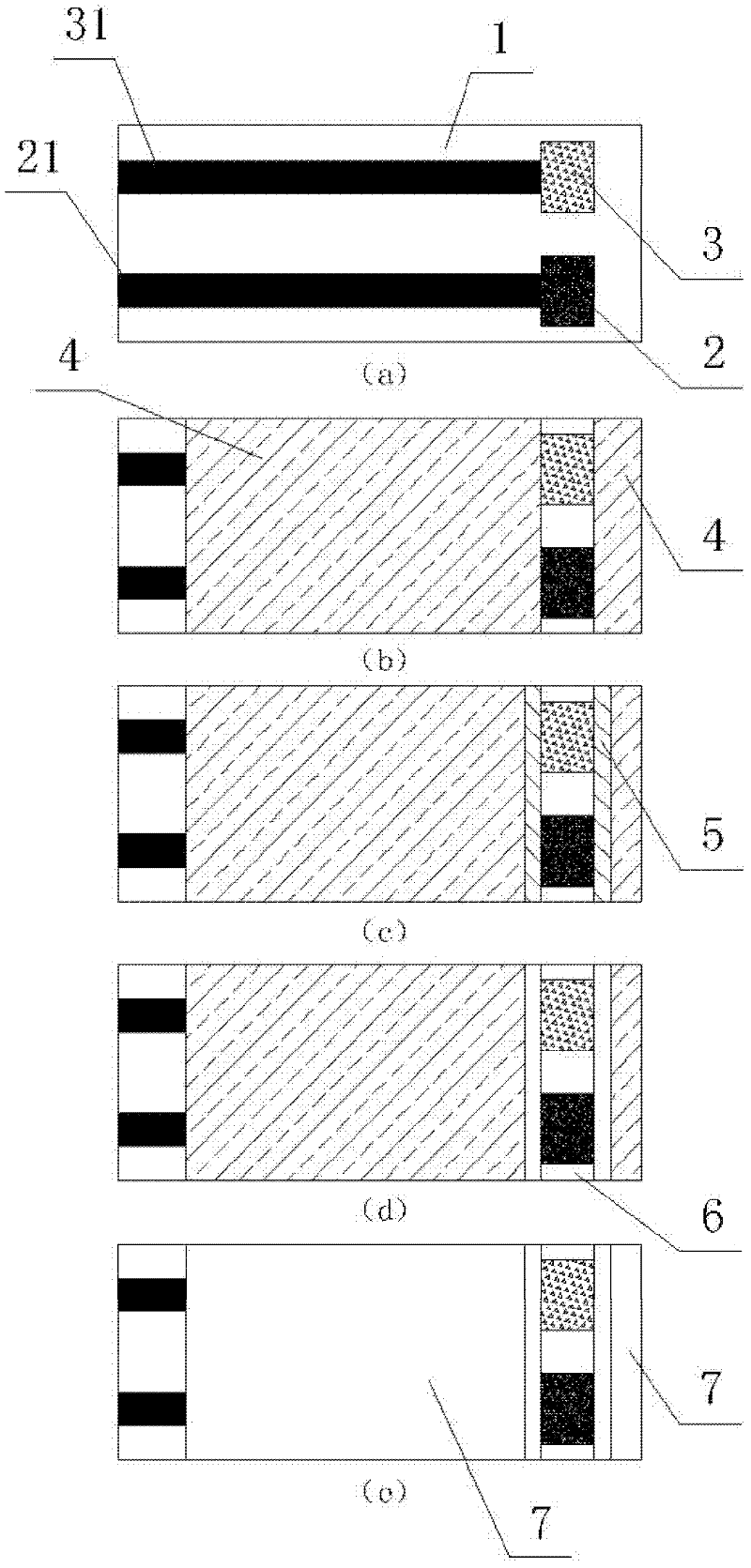
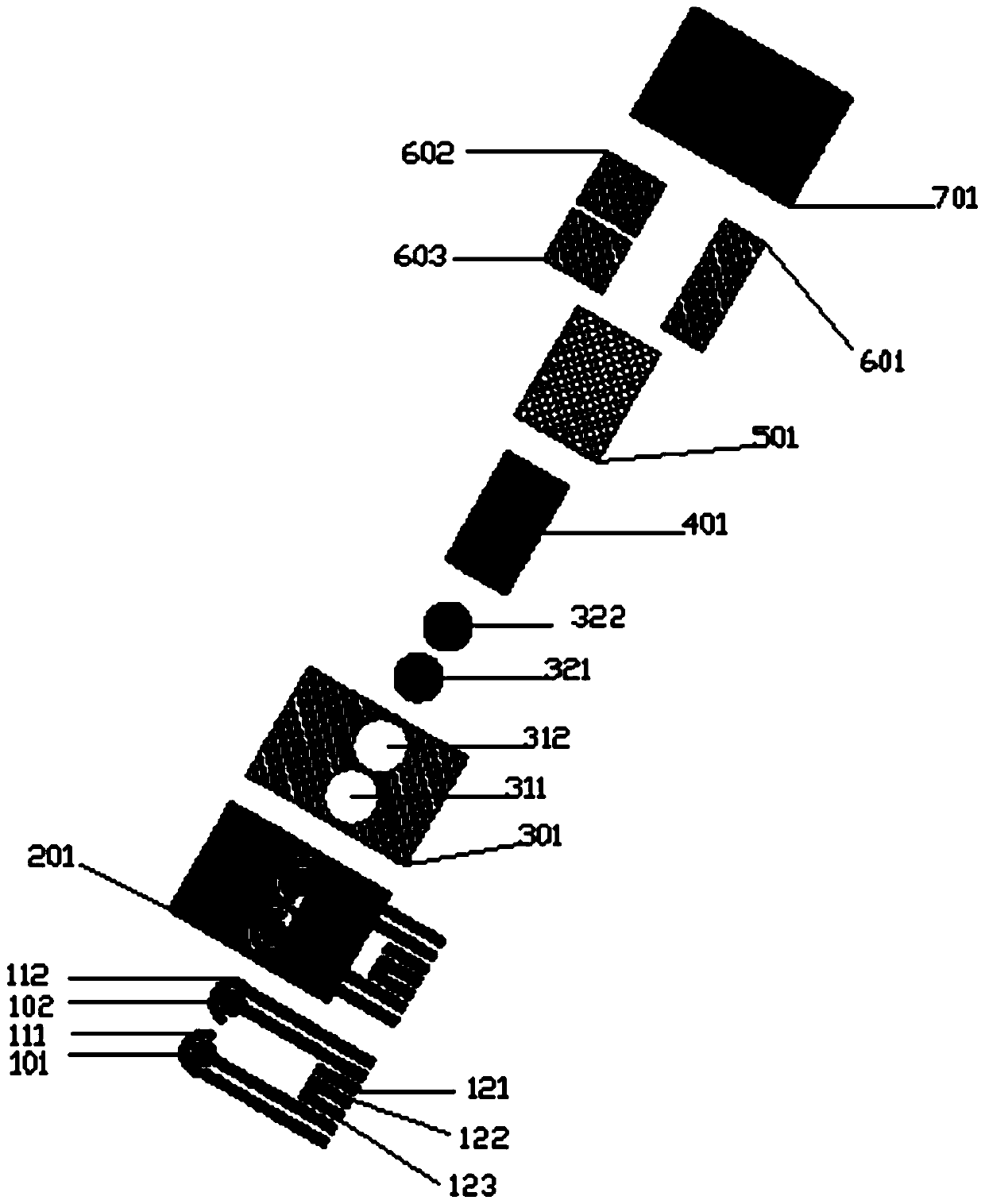
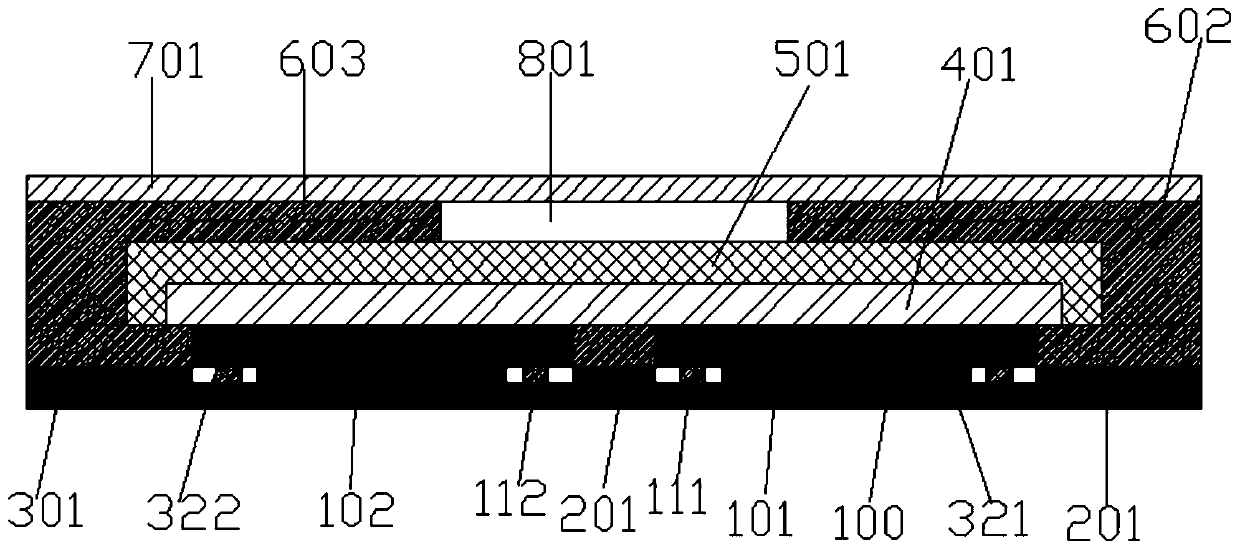
The invention provides an electrochemical uric acid test strip comprising an insulating substrate (1), a working electrode (2) and a counter electrode (3), wherein the working electrode (2) and the counter electrode (3) are arranged at a first end of the insulating substrate (1) side by side at an interval; the working electrode (2) is provided with an anti-interference reagent layer; the components of the reagent layer comprise an ascorbic acid oxidase, a macromolecular compound and a buffering solution; and the components of the working electrode (2) comprise a non-aqueous redox electron transfer mediator. The invention further provides a manufacturing method of the electrochemical uric acid test strip. The test strip provided by the invention utilizes the non-water-soluble electron transfer mediator and combines an interference-removing enzyme layer, so that the effect of removing ascorbic acid is obvious. In a test, the interference of the ascorbic acid on uric acid test is very small.
Owner:SINOCARE
Detection kit for determining content of creatinine in serum by enzymic method
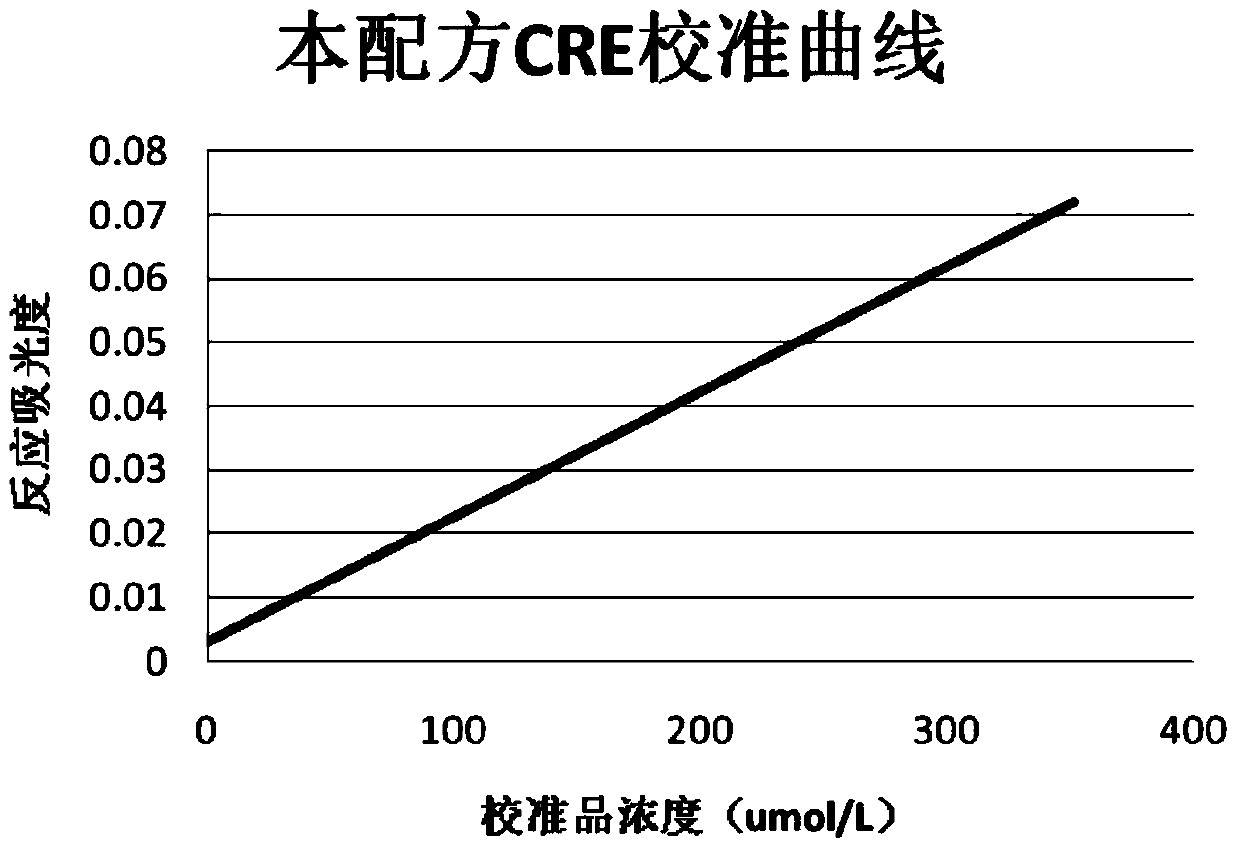
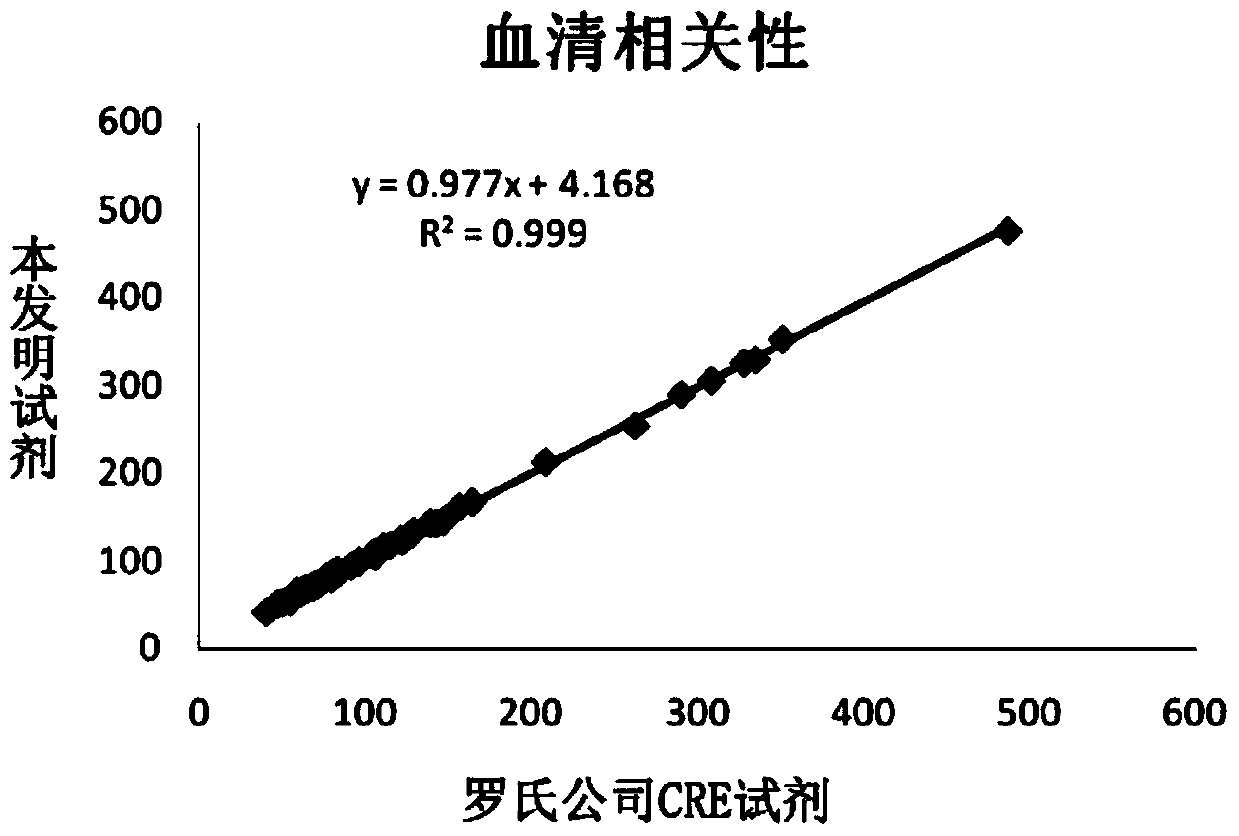
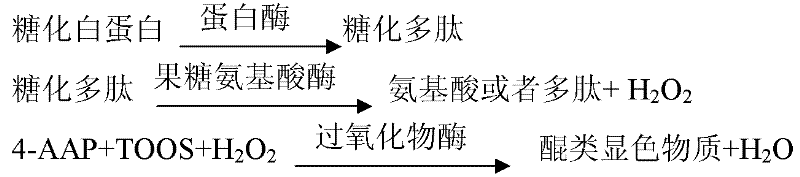
InactiveCN104198408AImprove stabilityEliminate distractionsMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsCreatinine riseCreatininase
The invention discloses a detection kit for determining the content of creatinine in serum by an enzymic method. The detection kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 contains 3-10g / L of buffering solution with the pH value being 7.5-8.0, 0.2-0.5g / L of EDTA, 0.5-2g / L of N-ethyl-N-(2-hydroxyl-3-sulfopropyl) m-toluidine sodium salt, 1 per mill-5 per mill of a surface active agent, 0.2-1g / L of sarcosine oxidase, 1-5KU / L of ascorbate oxidase, 2-5KU / L of creatinase and 0.5-1g / L of a stabilizing agent; the reagent R2 contains 3-10g / L of buffering solution with the pH value being 7.5-8.0, 0.1-0.5g / L of 4-aminoantipyrine, 0.1-0.4g / L of potassium ferricyanide, 2-8g / L of creatininase amidohydrolase, 1-6KU / L of peroxidase and 0.5-2g / L of a preservative. The detection kit disclosed by the invention is excellent in stability, strong in interference resistance and high in clinical application value.
Owner:上海睿康生物科技有限公司
Human serum glycated albumin array kit
ActiveCN102565420AUnchanged vitalityStrong specificityBiological testingMethylanilineFructose lysine
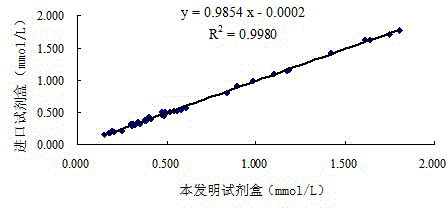
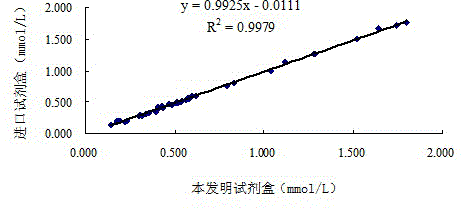
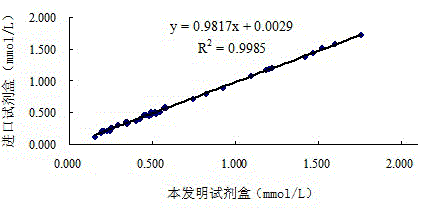
The invention provides a human serum glycated albumin assay kit which comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises the following components: 20-200mmol / L of buffer solution, 5-50KU / L of protease co-expression vector, 10-50KU / L of peroxisome, 2-20mmol / L of 4-amino antipyrine, 0.01-1% of preservative and 0.01-1% of stabilizer, and the protease co-expression vector is obtained by cloning a protease gene and an ascorbic acid oxidase gene onto a same vector for performing co-expression; and the reagent 2 comprises the following components: 20-200mmol / L of buffer solution, 5-50U / mL of fructose lysine enzyme, 1-10mmol / L of N, N-bis(4-sulfobutyl ether)-3-methylaniline, 0.01-1% of preservative and 0.01-1% of stabilizer. The kit can remove the interferences of globulin and ascorbic acid in human serum and has good stability and low cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Stable NEFA (non-esterified fatty acid) measuring kit
ActiveCN105203472AGood correlationExcellent linear fit correlation coefficientColor/spectral properties measurementsBiological testingBlood lipidsFatty acid
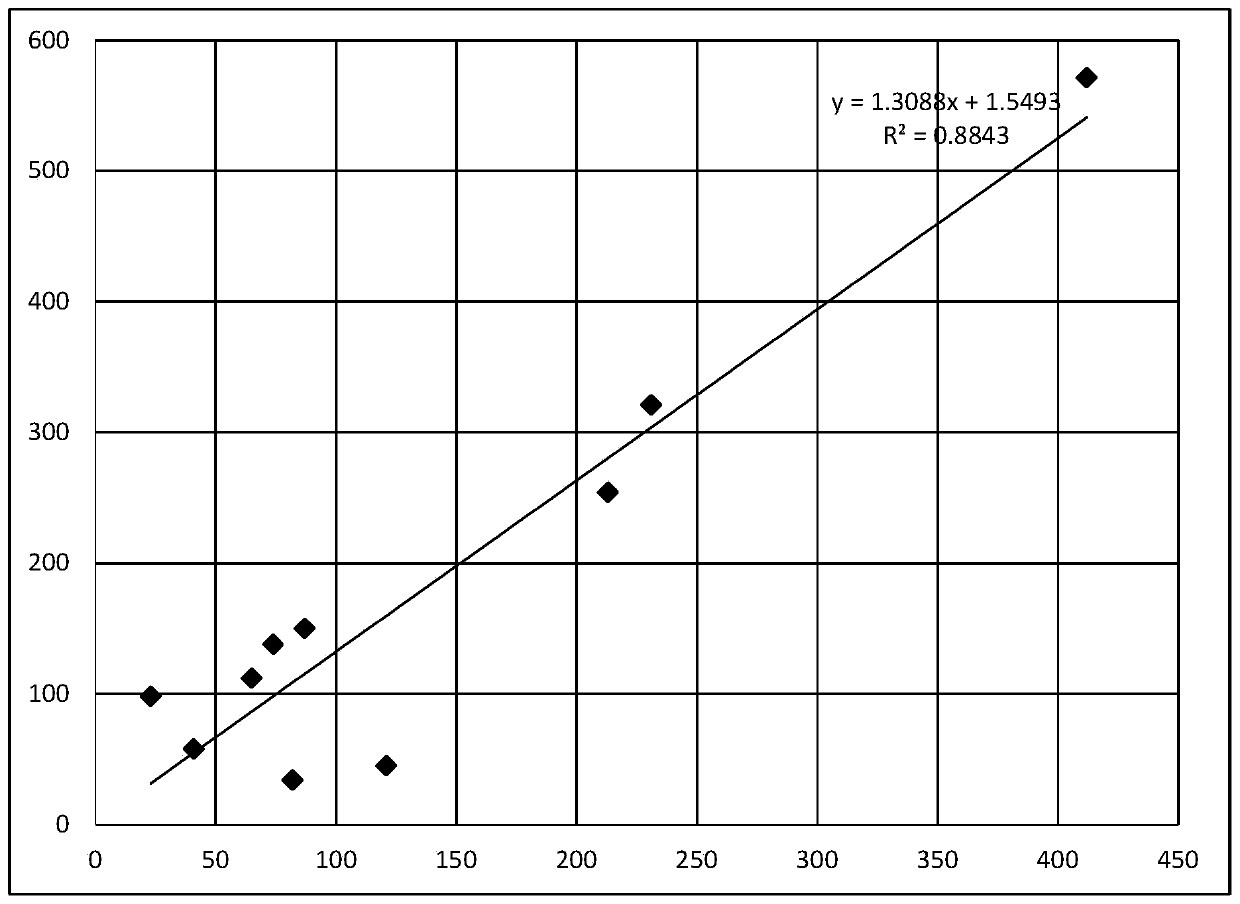
The invention relates to a stable kit for measuring NEFA (non-esterified fatty acid) through an enzymic method. The kit comprises two separate liquid reagents, namely, a reagent 1 and a reagent 2 which are respectively placed, and is good in stability and anti-interference performance. The enzymic method is adopted to measure NEFA, and the method is easy, convenient and easy to operate. According to the kit, novel chromogen is selected, and anti-interference agents such as ascorbic acid oxidase and potassium ferrocyanide are added into the formula at the same time, so that the reagent has the characteristics of being high in sensitivity and anti-interference performance; a liquid stabilization technology is adopted, and a series of special stabilizers, chelating agents and enzymatic protective agents are added, so that the reagent is good in stability, and can be widely applied to most semi / full automatic biochemical analyzers, and used for monitoring human body fat metabolic status, lipid level, and the like, and clinical popularization is facilitated.
Owner:北京万泰德瑞诊断技术有限公司
Detection kit for LDL (low-density lipoprotein) cholesterol and use method of detection kit
ActiveCN107505272AImprove accuracyNo precipitationColor/spectral properties measurementsPeroxidasePolyethylene glycol
The invention discloses a detection kit for LDL (low-density lipoprotein) cholesterol and a use method of the detection kit, relates to the field of biochemical detection and aims to provide a detection kit, having high stability, accuracy and precision as well as low toxicity, for LDL cholesterol and a use method of the detection kit. The kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 is prepared from a surfactant, polyethylene glycol, SDS (sodium dodecyl sulfate), Emulgen A9 series, CHOD (cholesterol oxidase), CHER (cholesterol esterase), ascorbic acid oxidase, CAT (catalase), 4-AAP (4-aminoantipyrine) and the like; the reagent 2 is prepared from octylphenyl polyethylene glycol, cholate, a compound stabilizer, bovine serum albumin, POD (peroxidase), TOPS (sodium 3-(N-ethyl-3-methylanilino)propanesulfonate) and the like. The proclin series added to the kit is a novel biological preservative, has good compatibility with various enzymes, better stability and low toxicity, and the stability of the reagents is maintained.
Owner:WHITMAN BIOTECH NANJING
Detection reagent for triglyceride and test paper for triglyceride
InactiveCN107064123AQuick checkAccurate detectionMaterial analysis by observing effect on chemical indicatorReaction layerSiphon
Owner:复星诊断科技(长沙)有限公司
Working electrode biological reactant and electrode type test strip
InactiveCN105572199AReduce distractionsNo distractionMaterial analysis by electric/magnetic meansResponse sensitivityTarget analysis
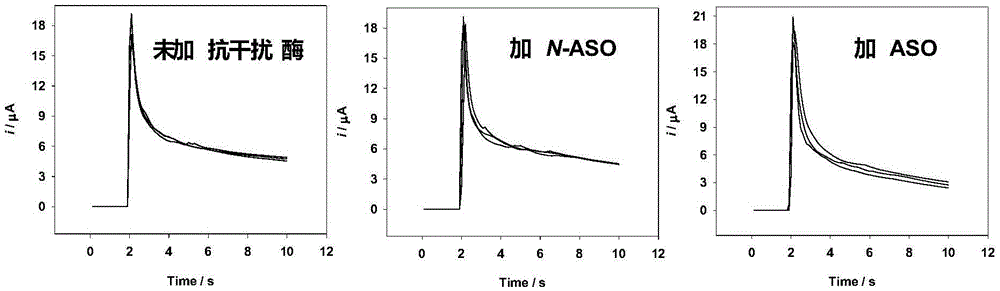
The invention relates to the technical field of electromechanical detection, in particular to a working electrode biological reactant and an electrode type test strip. The working electrode biological reactant comprises ascorbic acid oxidase of microorganism origin, a biological enzyme corresponding to a substance to be detected, and an electronic mediator. In the invention, interference of ascorbic acid is reduced by means of directly mixing a target analyte special enzyme and novel ascorbic acid oxidase (N-ASO), with no interference (for example, no influence on precision of the test strip and no reduction in the current response sensitivity) on enzyme reaction current response signals of the target analyte special enzyme; an electrochemical detection method of the invention is applicable to an electrochemical test strip using single or multiple working electrodes, enabling a reduction in the interference of the ascorbic acid upon the electromechanical test strip using oxidized current as a detection signal and a reduction in the interference of the ascorbic acid upon the electrochemical test strip using reduction electric current as a detection signal.
Owner:SINOCARE
Detection reagent and test paper for uric acid
InactiveCN106645128AHigh-precision detectionEliminate distractionsMaterial analysis by observing effect on chemical indicatorReaction layerSiphon
The invention discloses a detection reagent and test paper for uric acid. The detection reagent comprises 12 to 18 KU / L of urate oxidase, 5 to 10 KU / L of horseradish peroxidase, 15 to 20 KU / L of ascorbic acid oxidase, 0.20 to 0.35 g / L of 4-aminoantipyrine and 0.20 to 0.30 g / L of a chromogenic substance. The detection reagent provided by the invention contains a plurality of specific enzymes in a specific ratio and can achieve the purpose of rapid and accurate detection of the content of uric acid. The test paper for uric acid comprises a reaction substrate layer, a reaction layer, a hemofiltration layer and a sample suction layer. The above-mentioned layers are superposed to form a siphon system, so the test paper has better anti-interference capability, effectively eliminates interference by endogenous substances and further guarantees rapid and accurate detection of the content of uric acid.
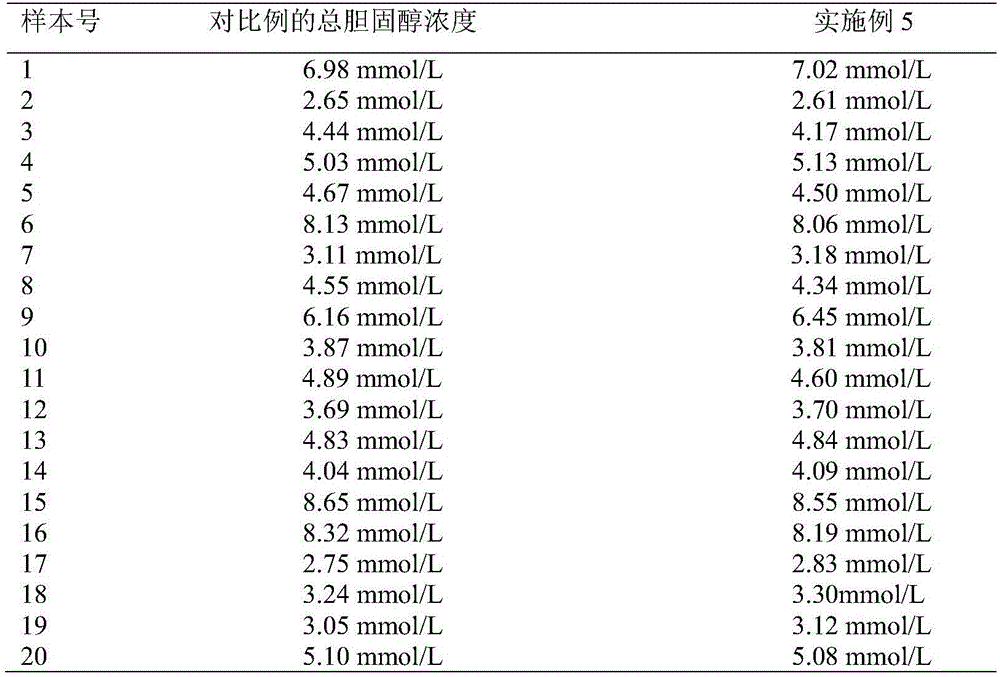
Total cholesterol detection reagent and total cholesterol detection paper
The invention discloses a total cholesterol detection reagent and total cholesterol detection paper. The total cholesterol detection reagent comprises 6-9 KU / L cholesterol esterase, 3-5 KU / L cholesterol oxidase, 15-20 KU / L horseradish peroxidase, 3-5 KU / L ascorbic acid oxidase, 0.20-0.35 g / L 4-ampyrone, and 0.20-0.30 g / L color-showing matter. The detection reagent contains a plurality of specific enzymes in a specific ratio, and can achieve the purposes of rapid detection and relatively accurate detection. The detection paper comprises a reaction base layer, a reaction layer, a blood filtering layer and a hydrophilic layer, which are overlapped to form a siphon system, thereby further ensuring rapid detection and relatively accurate detection.
Owner:复星诊断科技(长沙)有限公司
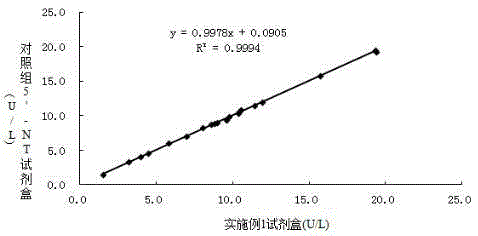
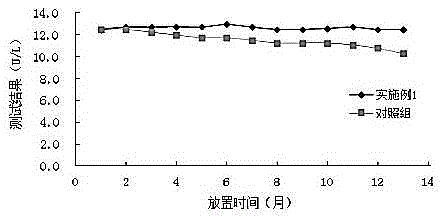
Stable serum 5'-ribotide hydrolase detection reagent with strong anti-interference capability and detection method
ActiveCN105420345AImprove stabilityAvoid turbidityMicrobiological testing/measurementBetaineSodium phosphates
The invention relates to the technical field of serum 5'-ribotide hydrolase detection, in particular to a serum 5'-ribotide hydrolase detection reagent. A PIPES buffer solution is adopted, and various stabilizers are added to remarkably improve the stability of the reagent; beta-sodium glycerophosphate, bilirubin oxidase and ascorbic acid oxidase are added to effectively prevent interference caused by alkaline phosphatase, cholerythrin and ascorbic acid and greatly enhance the anti-interference capability of the reagent. Besides, a preferred novel amphoteric surfactant, dodecyl dimethyl betaine (BS-12), is added to prevent a reaction system from turbidity, enhance the stability of a substrate and improve the anti-interference capability of the reagent.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Enzymic uric acid detection agent with high interference preventing capacity
ActiveCN108287233AGuaranteed cushioning effectGuaranteed not to interfere with the responseBiological testingAmpyroneBetaine
The invention relates to an enzymic uric acid detection agent with high interference preventing capacity. The agent is characterized in that an agent R1 includes a buffering solution, 4-ampyrone, sodium nitrite, an ion balancing agent, ascorbic acid oxidase, a heavy metal ion chelator, bovine serum albumin, dodecyl trimethyl betaine, triton-305 and a preservative; an agent R2 includes a bufferingsolution, F-DAOS (N-ethyl-N-(2-hydroxyl-3-sulfopropyl)-3, 5-dimethoxy-4-fluoroaniline), uricase, bovine serum albumin, peroxidase, a preservative, and other components. The agent is high in interference preventing capacity and particularly capable of accurately detecting a sample of a newborn.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Stable uric acid reagent with high anti-interference capacity and detection method
InactiveCN106290323AImprove stabilityImprove anti-interference abilityMaterial analysis by observing effect on chemical indicatorSucroseAlkylphenol
The invention relates to the technical field of uric acid detection, in particular to a uric acid detection reagent. A reagent R1 is prepared from a buffer solution, 4-aminoantipyrine, BSA, sucrose, trehalose, peroxidase, ascorbic acid oxidase, bilirubin oxidase, alkylphenol ethoxylates (APEO) and a preservative; a reagent R2 is prepared from a buffer solution, TOOS uricase.BSA sucrose.trehalose alkylphenol ethoxylates (APEO) and a preservative. The PIPES (piperazine-1,4-bisethanesulfonic acid) solution is adopted, the stabilizer BSA, sucrose and trehalose are added, and the stability of the reagent is greatly improved; the novel surfactant alkylphenol ethoxylates (APEO) is adopted, and therefore not only is the determination property of the product significantly improved, but also the stability and anti-interference capacity of the reagent are improved.
Owner:章丘美高义医疗器械有限公司
Stable high-interference-resistance direct bilirubin (oxidase method) detection reagent and detection method
InactiveCN109991177AImprove stabilityAvoid interferenceColor/spectral properties measurementsSucroseTurbidity
The invention relates to the field of serum direct bilirubin detection technologies, in particular to a serum direct bilirubin (oxidase method) detection reagent. A reagent R1 contains a buffer solution, sodium fluoride, NAC(N-acetylcysteine), sodium 4-methylbenzenesulfonate, lipase, ascorbic acid oxidase, alpha-cyclodextrin, sucrose, trehalose, PEG-6000, fatty alcohol polyoxyethylene ether (AEO-9) and a preservative; and a reagent R2 contains a buffer solution, bilirubin oxidase, sucrose, trehalose, PEG-6000, fatty alcohol polyoxyethylene ether (AEO-9) and a preservative. Through the reagent,the specificity and accuracy of direct bilirubin testing are obviously improved, turbidity of a reaction system can be prevented, a reaction is promoted, and the stability and interference resistanceof the reagent are further improved.
Owner:济南宇鑫生物科技有限公司
Electrochemical uric acid test strip and manufacturing method thereof
ActiveCN102507670BGuaranteed not to interfere with each otherEase of mass productionMaterial electrochemical variablesElectron transfer mediatorOxidation-Reduction Agent
The invention provides an electrochemical uric acid test strip comprising an insulating substrate (1), a working electrode (2) and a counter electrode (3), wherein the working electrode (2) and the counter electrode (3) are arranged at a first end of the insulating substrate (1) side by side at an interval; the working electrode (2) is provided with an anti-interference reagent layer; the components of the reagent layer comprise an ascorbic acid oxidase, a macromolecular compound and a buffering solution; and the components of the working electrode (2) comprise a non-aqueous redox electron transfer mediator. The invention further provides a manufacturing method of the electrochemical uric acid test strip. The test strip provided by the invention utilizes the non-water-soluble electron transfer mediator and combines an interference-removing enzyme layer, so that the effect of removing ascorbic acid is obvious. In a test, the interference of the ascorbic acid on uric acid test is very small.
Owner:SINOCARE
A stable serum phospholipid detecting reagent high in interference-resisting capability and a detecting method
ActiveCN105543336AAvoid interferenceImprove anti-interference abilityMicrobiological testing/measurementGlycerolTurbidity
The invention relates to the technical field of serum phospholipid detection and particularly relates to a serum phospholipid detecting reagent. A reagent R1 comprises a buffer solution, phospholipase D, DAOS, ascorbic acid oxidase, bilirubin oxidase, polyethylene glycol 6000, cane sugar, xanthan gum, mannitol, trehalose, BSA, glycerol propoxylate-block-ethoxylate (Pluranic L64) and an aseptic. A reagent R2 comprises the buffer solution, 4-aminoantipyrine, peroxidase, choline oxidase, the polyethylene glycol 6000, the cane sugar, the xanthan gum, the mannitol, the trehalose, the BSA, the glycerol propoxylate-block-ethoxylate (Pluranic L64) and the aseptic. The HEPES buffer solutions and a novel Trinder reaction chromogen DAOS are adopted. A plurality of stabilizers are added to obviously improve stability of the detecting reagent. The bilirubin oxidase and the ascorbic acid oxidase are added, thus effectively avoiding interference caused by bilirubin and ascorbic acid and greatly improving interference-resisting capability of the detecting reagent. In addition, addition of the glycerol propoxylate-block-ethoxylate (Pluranic L64) which is an optional nonionic surfactant prevents reaction system turbidity, enhances substrate stability and improves the interference-resisting capability of the detecting reagent.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
N-acetyl-beta-D glucosidase reagent and detection method
InactiveCN105424934AGood water solubilityImprove stabilityMaterial analysisBovine serum albuminOxidative enzyme
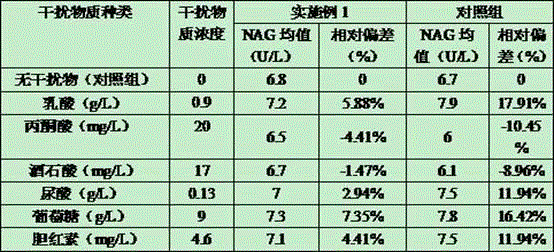
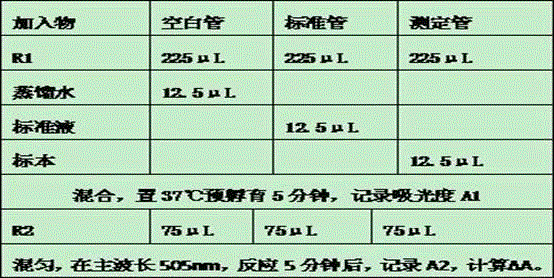
The invention relates to the field of reagent NAG detection technologies, in particular to an N-acetyl-beta-D glucosidase reagent. The reagent R1 contains a buffer solution, VRA-NAG, bovine serum albumin, TritonX-100, ethylene glycol, ascorbic acid oxidase and a preservative. The reagent R2 contains a buffer solution and a preservative. The reagent adopts a novel substrate VRA-NAG, the substrate has better solubility in water compared with other substrates, is good in stability and is a reliable NAG substrate. The reagent uses citric acid-disodium hydrogen phosphate as the buffer solution, the buffering capacity of the reagent is greatly improved, and meanwhile a reaction system is not destroyed. The added TritonX-100 and the ethylene glycol serve as cosolvents to promote dissolution of the substrate and have an obvious solubilization assisting effect. BSA is added to serve as a stabilizer, and the stability and anti-interference capability of the reagent are greatly enhanced. The N-acetyl-beta-D glucosidase reagent is simple in configuration and low in price and is very suitable for large-area clinic popularization.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Detection reagent and test strip for creatinine
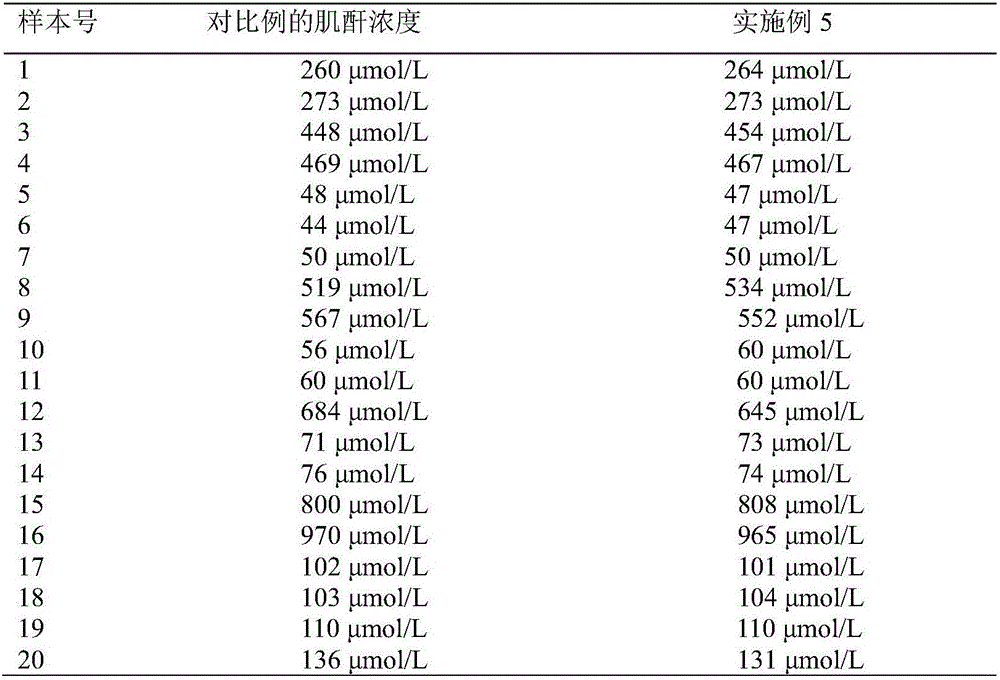
The invention discloses a detection reagent and a test strip for creatinine. Each liter of the detection reagent contains 12-18 KU of creatininase, 12-20 KU of creatinase, 9-12 KU of sarcosine oxidase, 15-20 KU of horseradish peroxidase, 5-8 KU of ascorbic acid oxidase, 0.20-0.35 g of 4-aminoantipyrine and 0.20-0.30 g of color developing matter. The detection reagent contains various specific enzymes for specific proportioning, and can achieve the purposes of rapid detection and more accurate detection. The test strip comprises a reaction base layer, a reaction layer, a blood filter layer and a hydrophilic layer which are stacked to form a siphon system, thereby further guaranteeing rapid detection and more accurate detection.
Owner:复星诊断科技(长沙)有限公司
Test strip for detecting creatinine by electrochemical method and preparation method thereof
PendingCN111189899ARapid quantitationQuantitatively accurateMaterial analysis by electric/magnetic meansDisease diagnosisCreatininasePeroxidase
The invention discloses a test strip for detecting creatinine by an electrochemical method. The test strip comprises: a substrate, a working electrode, a reference electrode, a counter electrode, a startup electrode, an insulating layer, a first enzyme carrier layer, a second enzyme carrier layer, a blood filtering membrane, a diffusion layer, a double faced adhesive tape layer, siphon holes and ahydrophilic layer. A formula of a first reaction solution on the first enzyme carrier layer comprises: creatininase, sarcosine oxidase, creatine hydrolase, ascorbic acid oxidase, peroxidase, a dispersing agent, a surfactant and a buffer solution; a formula of a second reaction solution on the second enzyme carrier layer comprises: sarcosine oxidase, creatine hydrolase, ascorbic acid oxidase, peroxidase, a stabilizer, a surfactant and a buffer solution; the detection result of the invention is well matched with a hospital inspection result, and the use is simple.
Owner:杭州联晟生物科技有限公司
Urine glucose test paper capable of resisting ascorbic acid interference and preparation method thereof
InactiveCN109856128AImprove anti-VC interference abilityHigh sensitivityMaterial analysis by observing effect on chemical indicatorPeroxidasePotassium iodine
The invention belongs to the field of urine analysis and detection, relates to urine glucose test paper capable of resisting ascorbic acid interference and a preparation method thereof and solves thetechnical problem of existence of ascorbic acid interference in the detection of urine glucose in an existing dry chemistry test paper. The urine glucose test paper capable of resisting ascorbic acidinterference is formed by a substrate and filter paper fixedly arranged on the substrate. The test paper is obtained by being immersed into immersion liquid consisting of a buffer solution, glucose oxidase, peroxidase, potassium iodide, ascorbic acid oxidase, anti-interference substance xanthan gum, a surfactant, an enzyme stabilizer and a blue dye substance, so that anti-VC interference capability of the glucose test paper with potassium iodide as the substrate can be significantly improved, and sensitivity and accuracy of the detection result are enhanced; and the glucose detection result isnot influenced when the content of the VC in the sample does not exceed 3.5mmol / L. The preparation method of the urine glucose test paper capable of resisting ascorbic acid interference is simple andeasy to operate, stable in performance and accurate in test result.
Owner:DIRUI MEDICAL TECH CO LTD
Boron-doping diamond film based ascorbic acid oxidase sensor electrode
InactiveCN102590306AExtended service lifeExcellent physical and chemical propertiesMaterial analysis by electric/magnetic meansOvervoltageElectrochemical biosensor
The invention discloses a boron-doping diamond film based ascorbic acid oxidase sensor electrode which is composed of a boron-doping diamond film, an amino monomolecular layer and an enzyme membrane layer. The preparation method of the ascorbic acid oxidase sensor electrode comprises the steps of: firstly, depositing a boron-doping diamond film on a tantalum sheet which is subjected to a surface pre-treatment by utilizing a hot filament CVD (Chemical Vapor Deposition) device; then performing amination modification on the boron-doping diamond film; and finally fixing the ascorbic acid oxidase on the surface of the ammoniated diamond film by adopting a chemical crosslinking way, wherein the electrode can be used on an electrochemical biosensor. The boron-doping diamond film based ascorbic acid oxidase sensor electrode, disclosed by the invention, has the advantages of having the limit of detection of 1*10-11 mol / L, and having high sensitivity, good reproducibility and long service life as the electrode inherits the advantages of corrosion resistance, extremely high oxygen evolution overvoltage, wide electric potential window, high electrochemical stability and the like of the diamond.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Homocysteine detecting kit and using method thereof
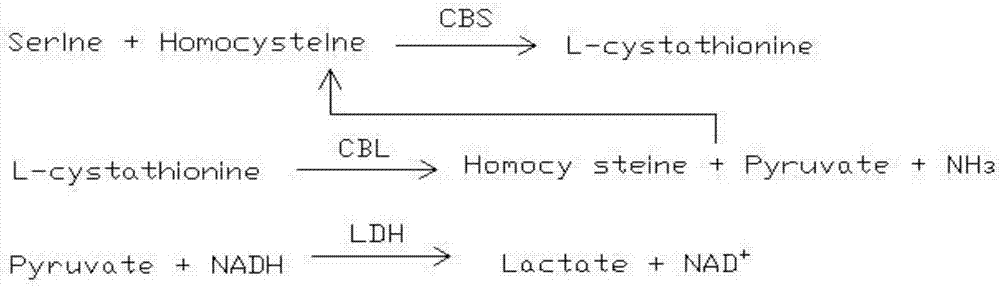
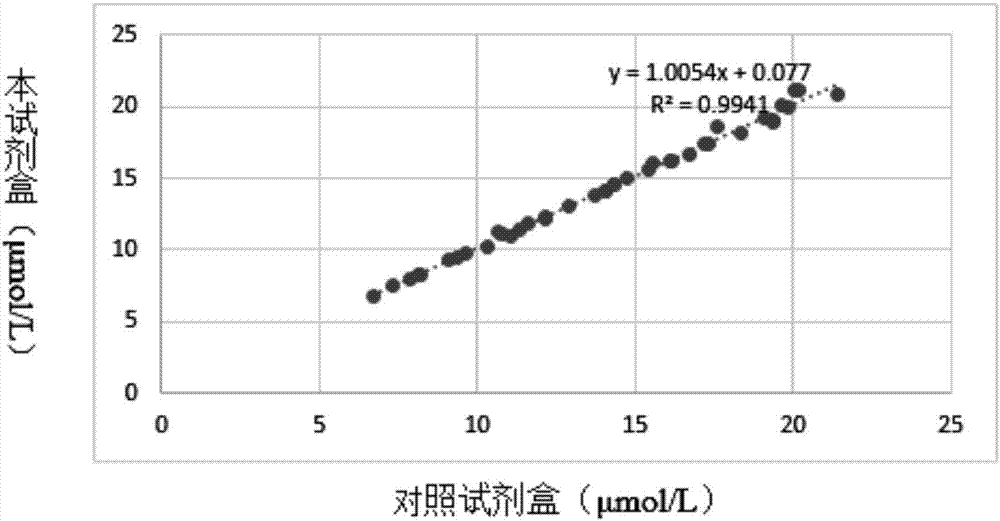
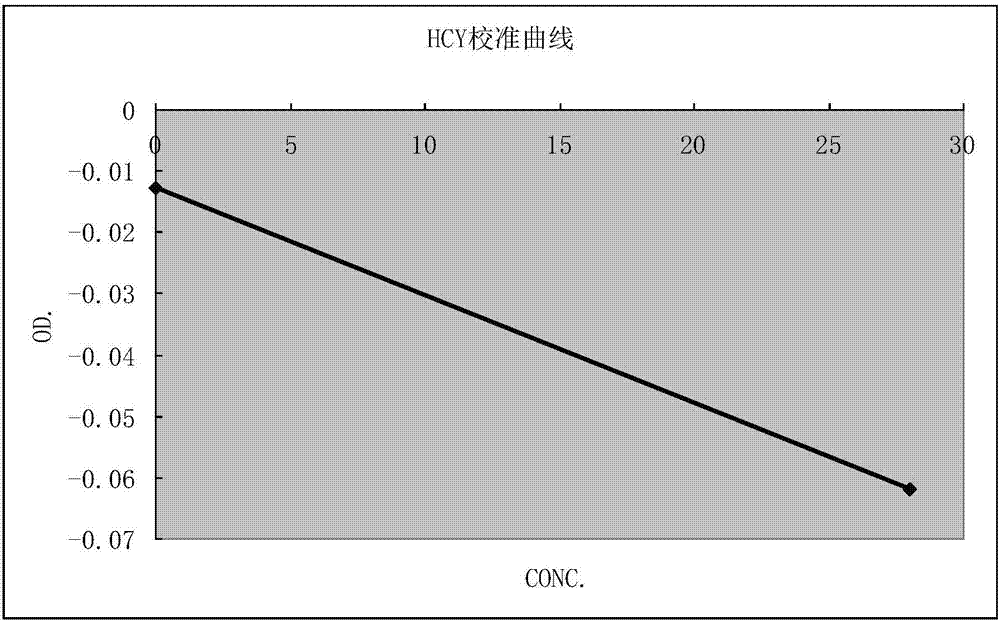
The invention provides a homocysteine detecting kit and a using method thereof, relates to the field of biochemical detection, and aims to provide a homocysteine detecting kit which is high in stability, high in accuracy and high in precision and a using method of the homocysteine detecting kit. The homocysteine detecting kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises a capso buffer solution, lactic dehydrogenase (LDH), L-serine, reduced coenzyme Thio-NADH, tris((2-carboxyethyl)phosphine, mercaptoethanol, an alkylphenol polyoxyethylene series, EDTA, polyethylene glycol 300, ascorbic acid oxidase, a composite stabilizer, and a proclin series preservative; and the reagent 2 comprises a capso buffer solution, cystathionine-beta-synthase, methionine synthase, cystathionine-beta-lyase, methionine-gamma lyase, a surfactant and sodium dodecylbenzene sulfonate. By the various added mixed enzymes and methionine-gamma lyases, the accuracy of the kit on detection of homocysteine can be improved.
Owner:WHITMAN BIOTECH NANJING
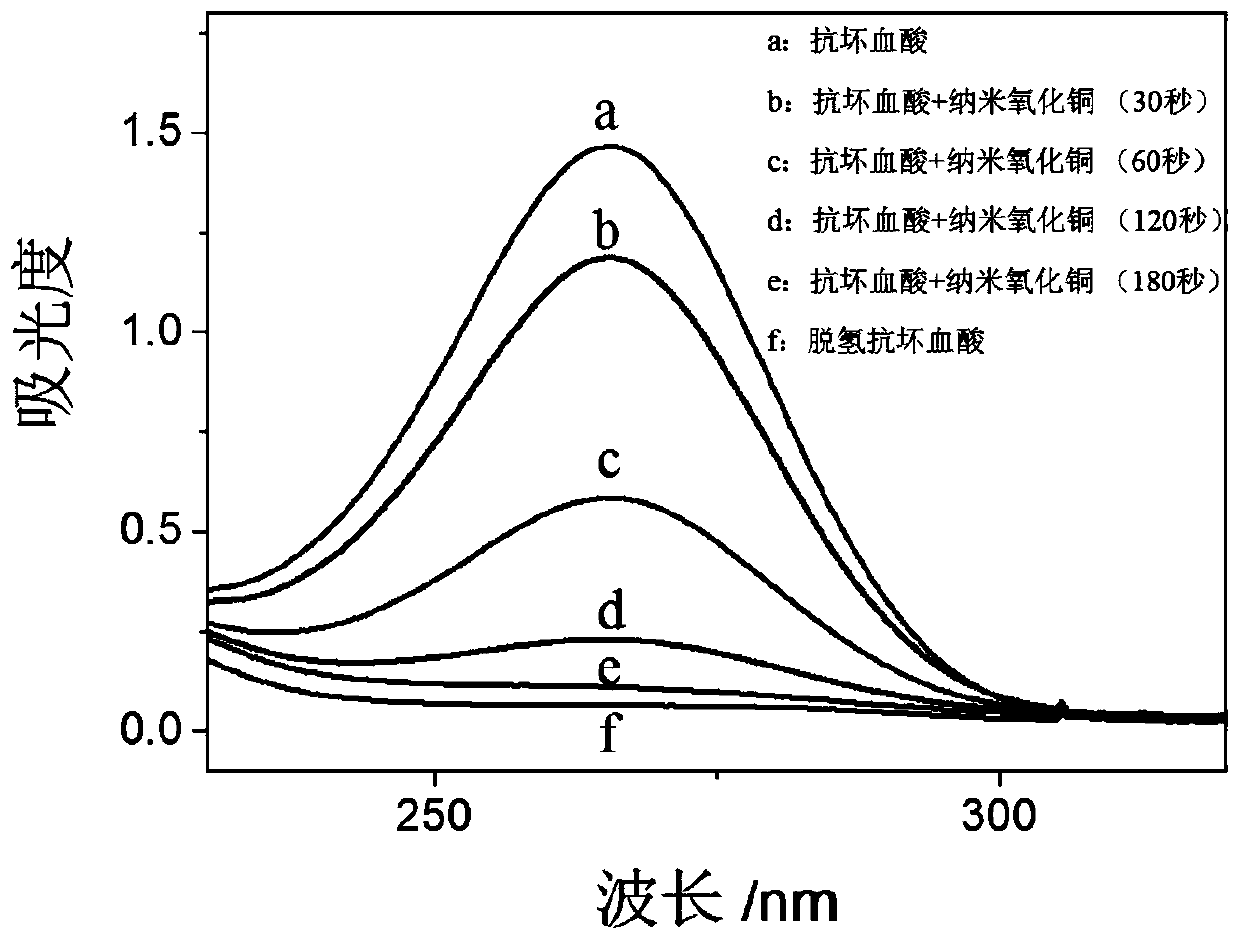
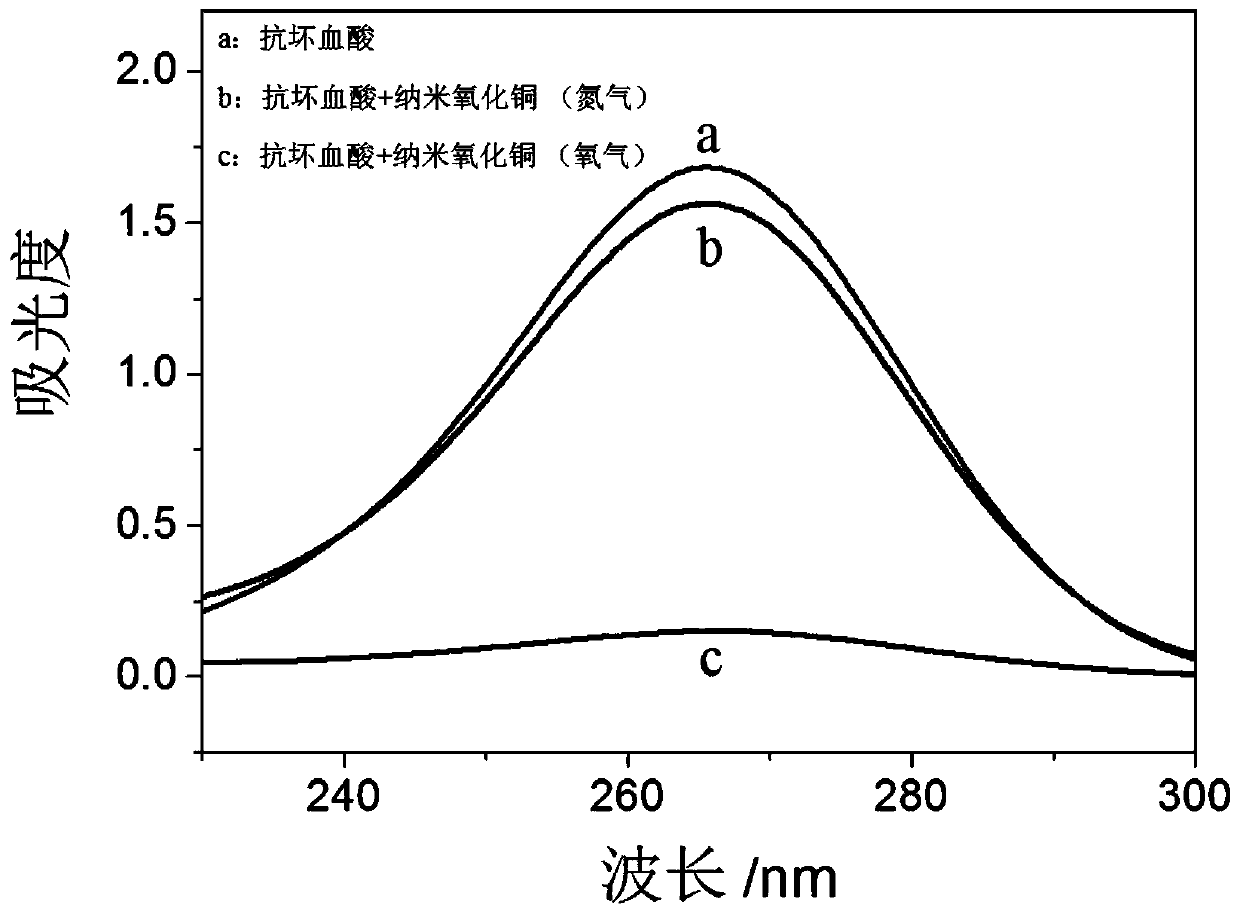
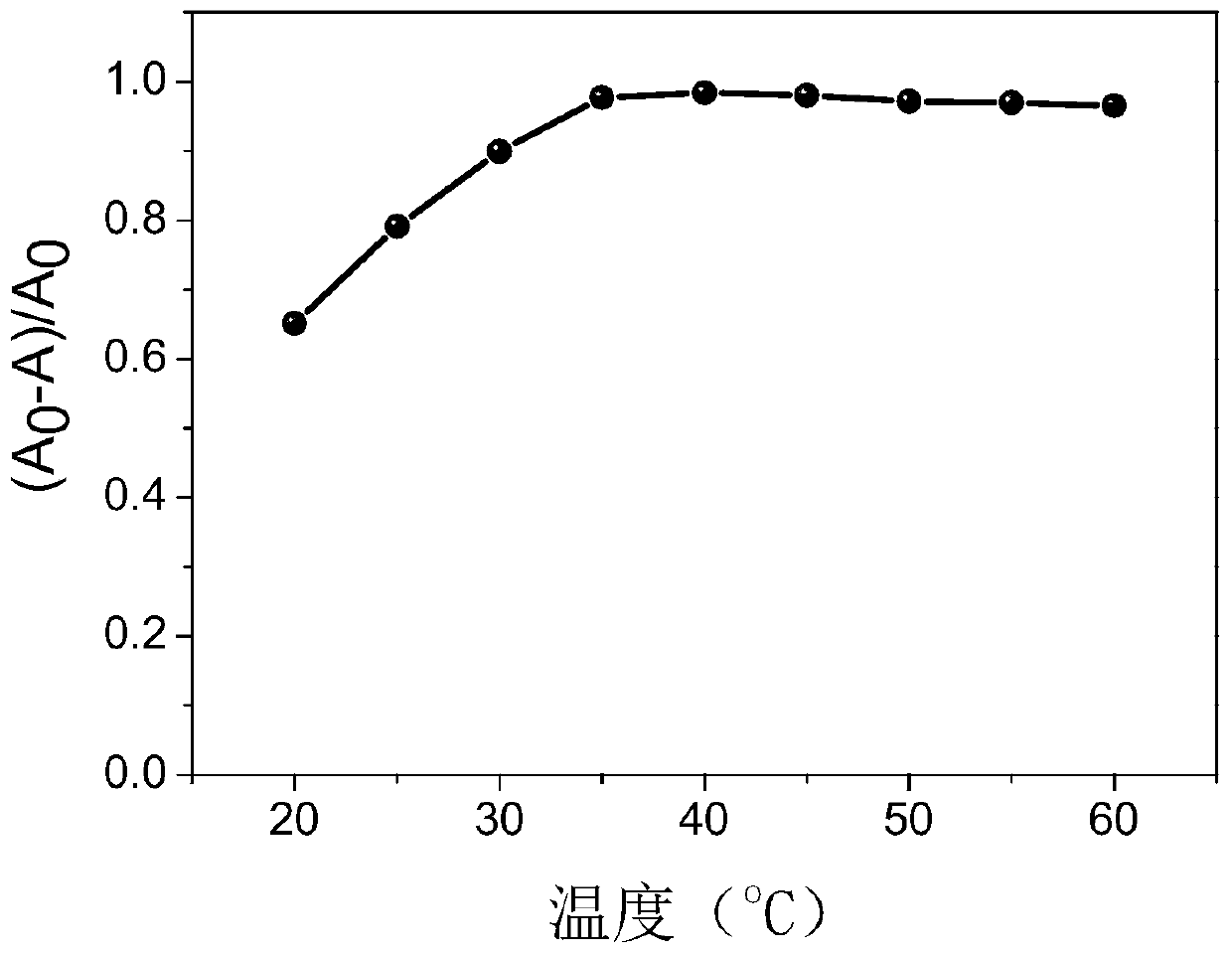
Nano copper oxide with activity of ascorbic acid oxidization mimic enzyme
InactiveCN110196234AImprove stabilityMaterial nanotechnologyCopper oxides/halidesFluorescenceStrong acids
The invention discloses a nano copper oxide with the activity of an ascorbic acid oxidization mimic enzyme, and aims at effectively catalyzing ascorbic acid oxidization to generate dehydroascorbic acid under aerobic environment and keeping the activity of the ascorbic acid oxidization mimic enzyme under strong acid, strong base or high temperature. By utilizing an ultraviolet spectrophotometry, the influences, on the nano copper oxide as ascorbic acid oxidase, of the temperature, pH and reaction time are respectively researched. The steady-state kinetic parameters of the nano copper oxide as the ascorbic acid oxidase are equivalent to the natural ascorbic acid oxidase, and the stability of the nano copper oxide as the ascorbic acid oxidase is obviously stronger than the natural ascorbic acid oxidase. By utilizing a deoxidization experiment and a fluorescence analysis method, a mechanism of the nano copper oxide as the ascorbic acid oxidase to catalyze ascorbic acid oxidization is verified. The activity of the ascorbic acid oxidization mimic enzyme shows a relatively tempting application prospect in the technical fields of nano enzymes, nanometers and bionic.
Owner:FUJIAN MEDICAL UNIV
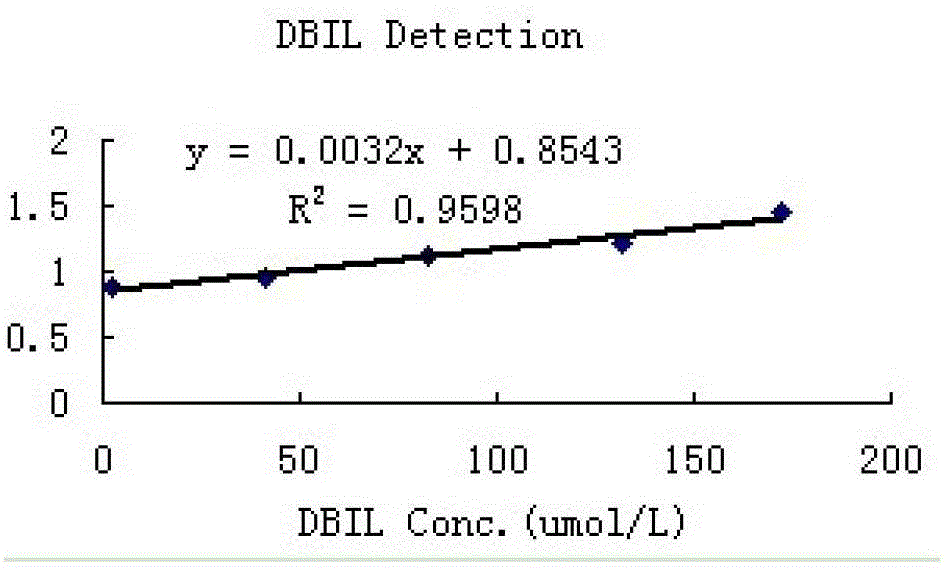
Direct bilirubin detection reagent
ActiveCN103333945AHigh sensitivityImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsVitamin CFreeze-drying
The invention discloses a direct bilirubin detection reagent. The direct bilirubin detection reagent comprises diluents and reaction reagents. The diluents comprise a buffer 1, a surfactant, an antiseptic and ascorbate oxidase. The reaction reagents comprise a buffer 2, common salt, disodium ethylene diamine tetraacetate, hydroxylamine sulphate, etidronic acid, sodium persulfate, sulfuric acid, an antiseptic and a freeze-drying protective agent. The direct bilirubin detection reagent has good sensitivity, accuracy, precision and linearity and can satisfy clinical examination requirements.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
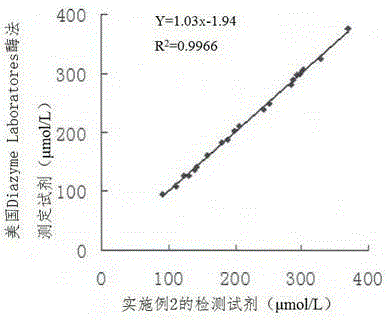
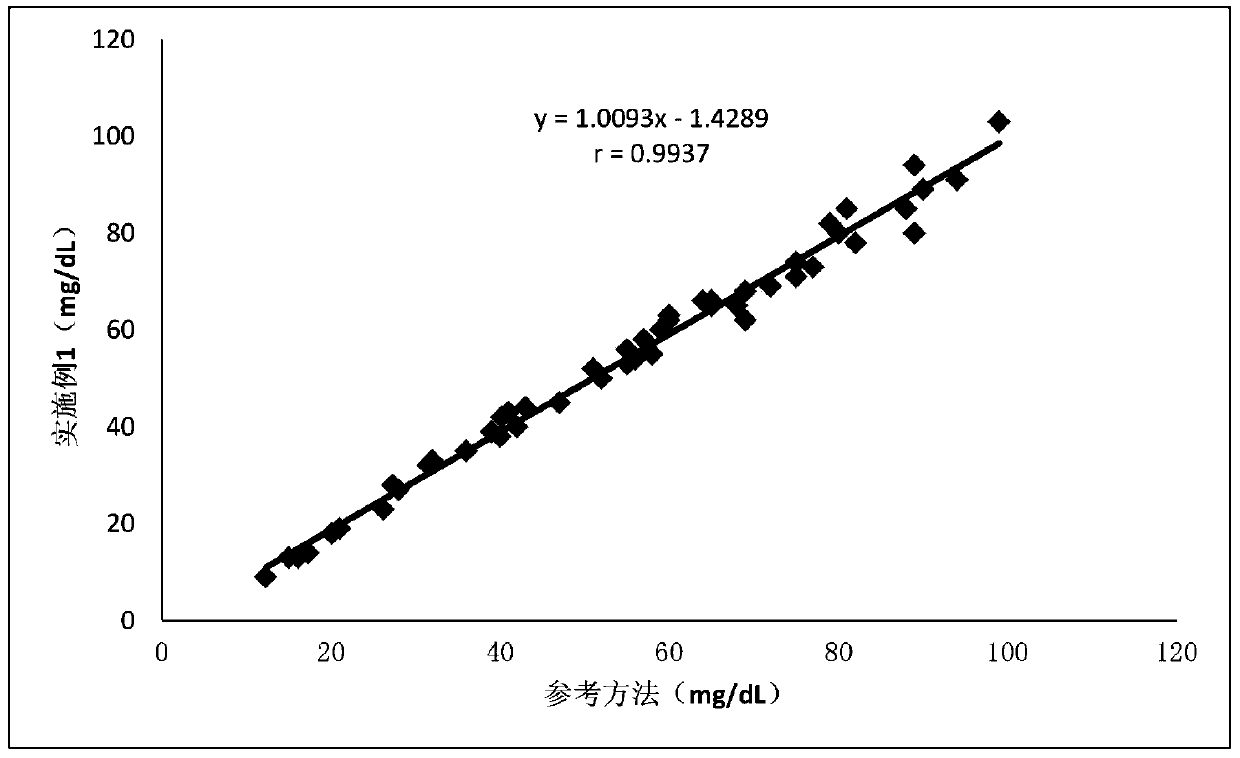
Glutathione reductase determination kit, and preparation method and applications thereof
ActiveCN111321198AImprove accuracyImprove linear rangeMicrobiological testing/measurementBiological material analysisEGTAActive agent
Owner:浙江达美生物技术有限公司
Glycosylated serum protein detection reagent and application thereof
ActiveCN105223192AImprove anti-interference abilityHigh measurement sensitivityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsTetrazoleDioxyethylene Ether
Belonging to the technical field of medical examination, the invention in particular relates to a glycosylated serum protein detection reagent and application thereof. The reagent comprises a reagent R1 and a reagent R2 in a volume ratio of 3:1, the reagent R1 is mainly composed of a potassium phosphate buffer solution, uricase, ascorbic acid oxidase, alkanolamide polyoxyethylene ether, polyol, calcium chloride and a preservative, and the reagent R2 is mainly composed of an MES buffer solution, alkyl polyglucoside, 2-(4-iodobenzene)-3-(2, 4-dinitrobenzene1)-5-(2, 4-di-sulfobenzene1)-2H-tetrazole and a preservative. The glycosylated serum protein detection reagent provided by the invention has the advantages of stronger anti-interference ability, high determination sensitivity and precision, and good repeatability of determination result, also the detection reagent has high stability and good water solubility, and is conducive to clinical application of the glycosylated serum protein detection reagent.
Owner:郑州金域临床检验中心有限公司
Test paper for measuring high density lipoprotein cholesterol and preparation method and application thereof
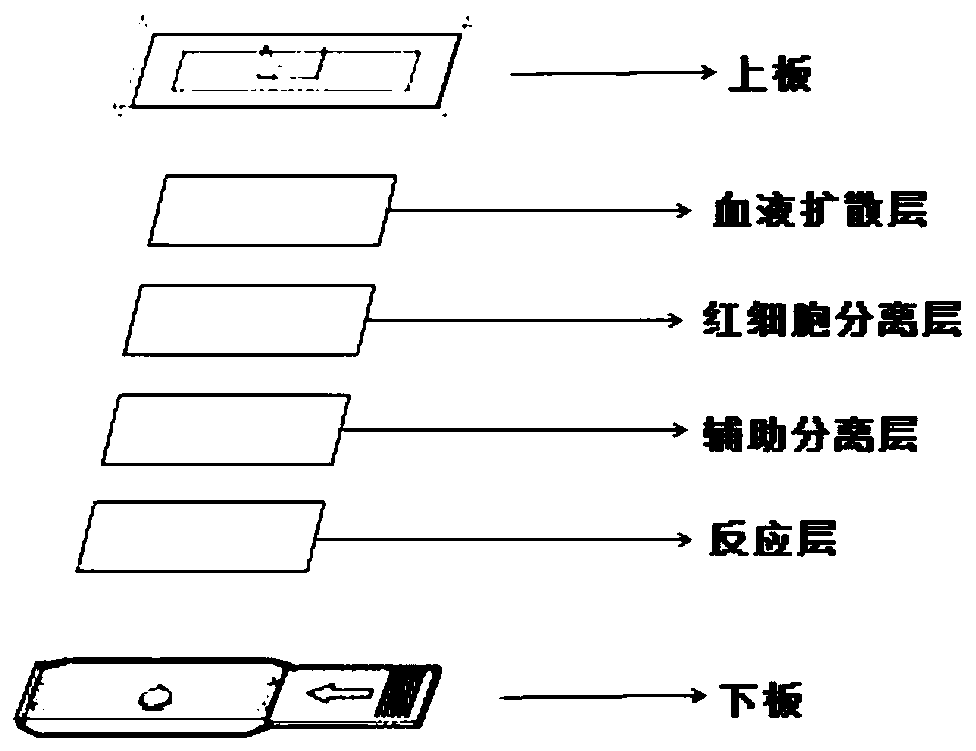
InactiveCN109884042AContent is simple and fastImprove stabilityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsHemagglutininReaction layer
The invention discloses test paper for measuring high density lipoprotein cholesterol and a preparation method and application thereof. The test paper comprises a red blood cell separation layer, an auxiliary separation layer and a reaction layer, wherein the red blood cell separation layer, the auxiliary separation layer and the reaction layer are prepared from a separation material, a separationmembrane and a reaction membrane through carrying out steeping by red blood cell separation solution, auxiliary separation solution and reaction solution and then carrying out drying; the red blood cell separation solution comprises the following components: buffer salt, a divalent metal ion, dextran sulfate sodium salt with a molecular weight of 30000-500000, hemagglutinin or an Anti-RBC antibody; the auxiliary separation solution comprises the following components: buffer salt, a surfactant I and polyhydric alcohol; and the reaction solution comprises the following components: buffer salt,a surfactant II, polysaccharide, cholesterol esterase, cholesterol oxidase, peroxidase, ascorbic acid oxidase, an oxidizing substance and a color developing agent. The test paper is capable of simplyand rapidly detecting the contents of high density lipoprotein cholesterol. The invention furthermore provides a preparation method and application of the test paper.
Owner:WUHAN J H BIO TECH
Method, reagent and kit for quantitatively detecting free fatty acid
InactiveCN104678107AAccurate detectionSimple and fast operationBiological testingAcyl Coenzyme A SynthetasesPeroxidase
The invention relates to a reagent for quantitatively detecting the content of free fatty acid in human serum. The reagent comprises a reagent I and a reagent II which are respectively placed, wherein the reagent I comprises sodium dihydrogen phosphate, disodium hydrogen phosphate, magnesium chloride, fatty acyl-coenzyme A synthetase, ascorbic acid oxidase, coenzyme A, ATP and 4-aminoantipyrine, and the reagent II contains sodium dihydrogen phosphate, disodium hydrogen phosphate, phenoxy ethanol, fatty acyl-coenzyme A oxidase, peroxidase, chromogen and a surfactant. The kit and the detection method only need dozens of microlitres of serum without centrifugal or electrophoresis and other separation treatments, are simple and convenient to operate, can be used for meeting the requirement of full-automatic analysis, and are suitable for timely accurate detection of large-scale samples.
Owner:ZHEJIANG KAICHENG BIOTECH
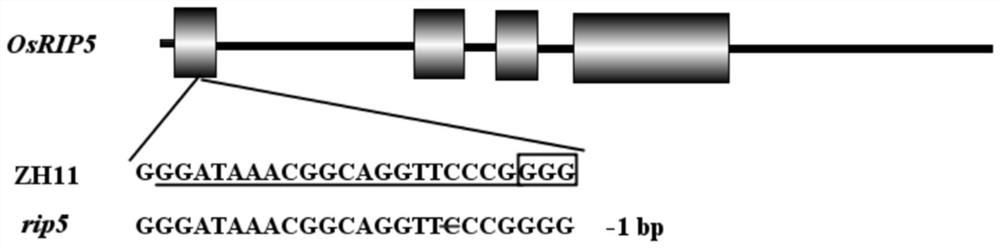
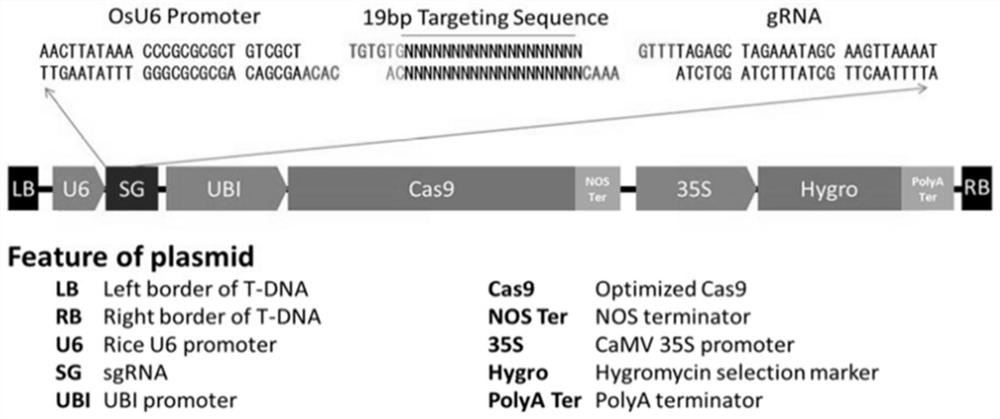
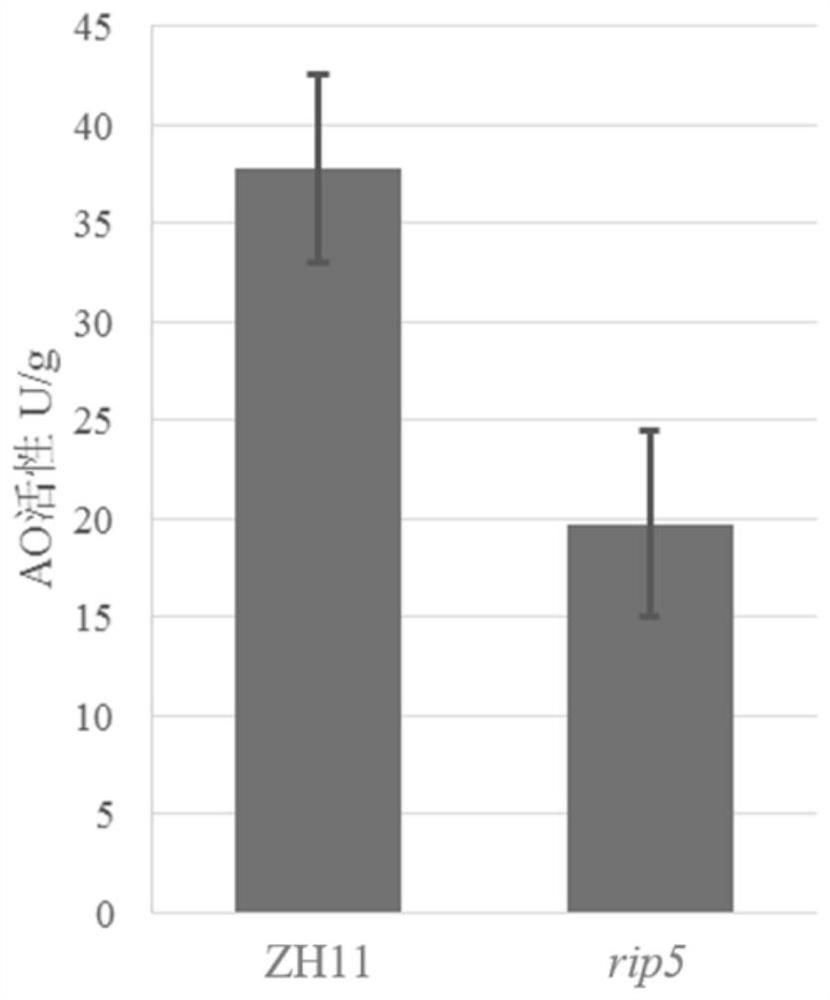
Application of ascorbic acid oxidase RIP5 to regulating and controlling of drought resistance of rice
InactiveCN111876394AImprove drought resistanceReduced activityOxidoreductasesFermentationBiotechnologyOxidative enzyme
The invention discloses an application of ascorbic acid oxidase RIP5 to regulating and controlling of drought resistance of rice. An RIP5 gene knockout mutant is constructed by targeted mutation of anRIP5 gene through a GRISPR / Cas9 system, and the inventor finds that the drought resistance of plants can be effectively improved by inhibiting the expression quantity and / or activity of the RIP5 protein, that is, the ascorbic acid oxidase gene RIP5 of rice can negatively regulate and control the drought resistance of the plants. The invention also provides a gRNA target sequence for editing the ascorbic acid oxidase gene RIP5 of the rice, and the gRNA target sequence cooperates with a CRISPR / Cas9 gene editing system to realize targeted mutation of the coding gene of the RIP5 protein, so thatthe drought resistance of the rice can be regulated and controlled. The research of the invention provides new gene targets and resources for drought resistance genetic breeding of the plants, and knockout mutant plants obtained by editing the gene have important application value.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com