Stable uric acid reagent with high anti-interference capacity and detection method
A detection method and technology of reagents, which are applied in material analysis by observing the influence of chemical indicators, and analysis by chemical reaction of materials, etc., can solve the problems of complex sample pretreatment, interference of reducing substances, and expensive equipment. , to achieve the effect of enhancing stability and anti-interference ability, improving stability, accuracy and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The detection reagent of uric acid comprises reagent R1 and reagent R2.
[0045] The composition of its R1 is:
[0046] PIPES (piperazine-1,4-diethanesulfonic acid) buffer (pH=7.6, 25°C) ···························· 100mmol / L
[0047] 4-Aminoantipyrine ···························································································· 1.3mmol / L
[0048] BSA ··············································································································· 1g / L
[0049] sucrose ··············································································································· 5g / L
[0050] Trehalose ··········································································································· 2g / L
[0051] peroxidase ··································································································· 12KU / L
[0052] ascorbate oxidase ···································································...
Embodiment 2
[0071] Interference test: take fresh mixed serum, divide it into 2 equal parts, then divide each equal part into 5 equal parts, add different interfering substances, so that the concentration in the serum reaches the requirements in Table 2. Then, the reagents obtained in Example 1 were used to compare with the common and recognized uric acid (UA) reagents in the market to measure the content of UA in serum at the same time. Relative deviation (%) = (measuring mean value of interference samples - measuring mean value of control samples) / measured mean value of control samples × 100%.
[0072] It can be seen from Table 2 that the reagent of Example 1 has no obvious interference on the test results when ascorbic acid ≤ 1704 μmol / L, bilirubin ≤ 684 μmol / L, hemoglobin ≤ 10 g / L, and triglyceride ≤ 22.6 mmol / L. However, the reagents of the control group were significantly interfered in the presence of the above-mentioned concentration of interfering substances, which shows that the a...
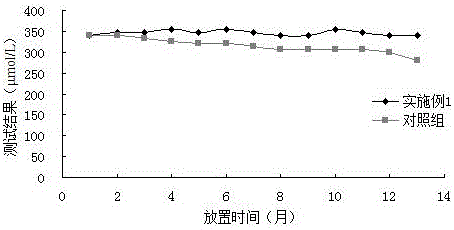
Embodiment 3
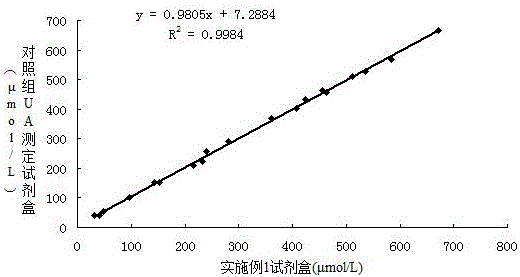
[0076] Correlation experiment: using the formula in Example 1 to prepare reagents, and conducting a control test with a uric acid kit from a company approved by the State Food and Drug Administration, which is common in the market, and testing 20 clinical serum samples at the same time, the test results are shown in Table 3 . And obtained the correlation curve of the two reagents (such as figure 1 Shown), the test results show that the correlation coefficient of the two kits is 0.9992, indicating that there is a great correlation between the two.
[0077] surface 3 Example 1 The test results are compared with the common and recognized uric acid assay kits in the market
[0078] 。
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com