Patents
Literature
47 results about "Dry chemistry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
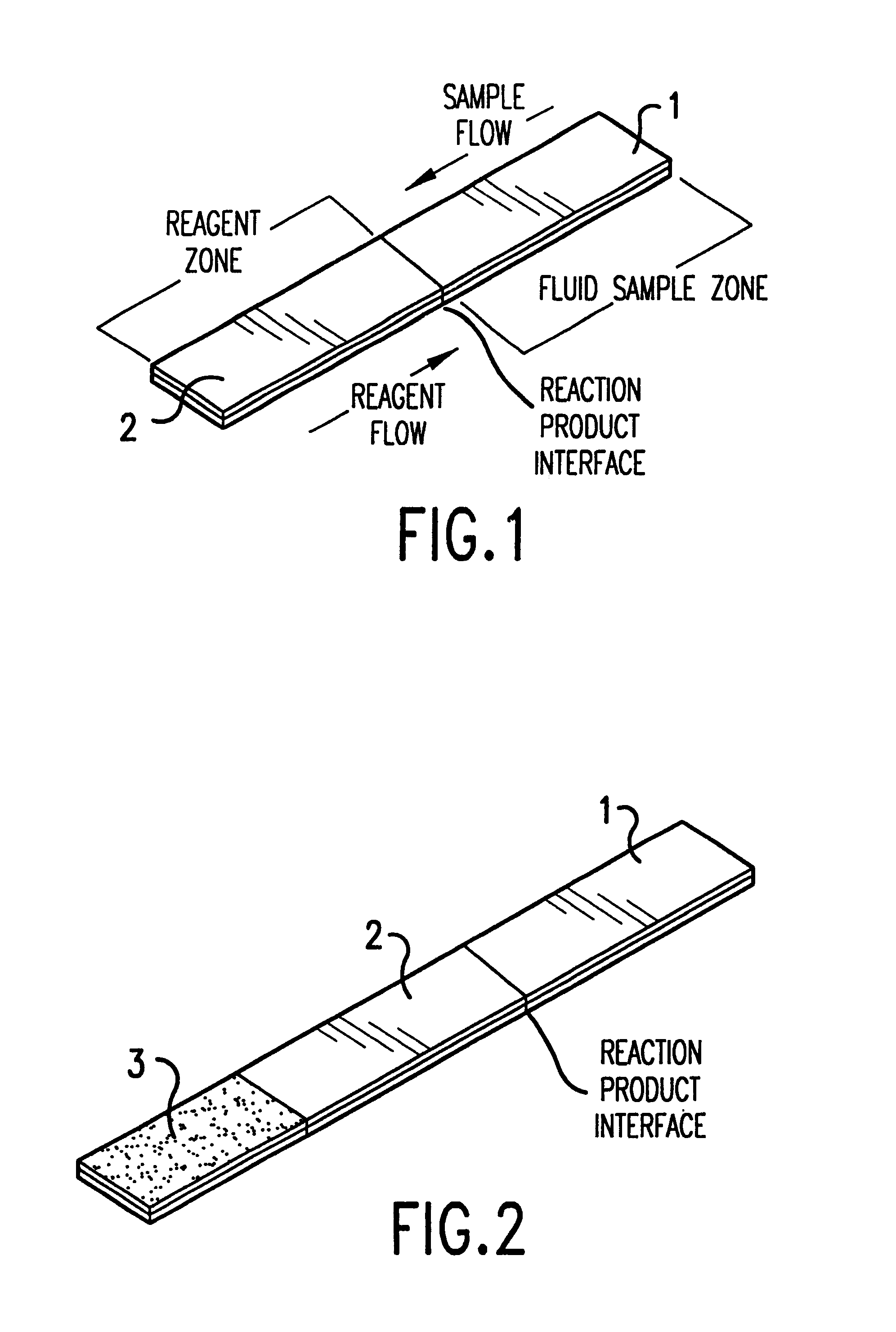
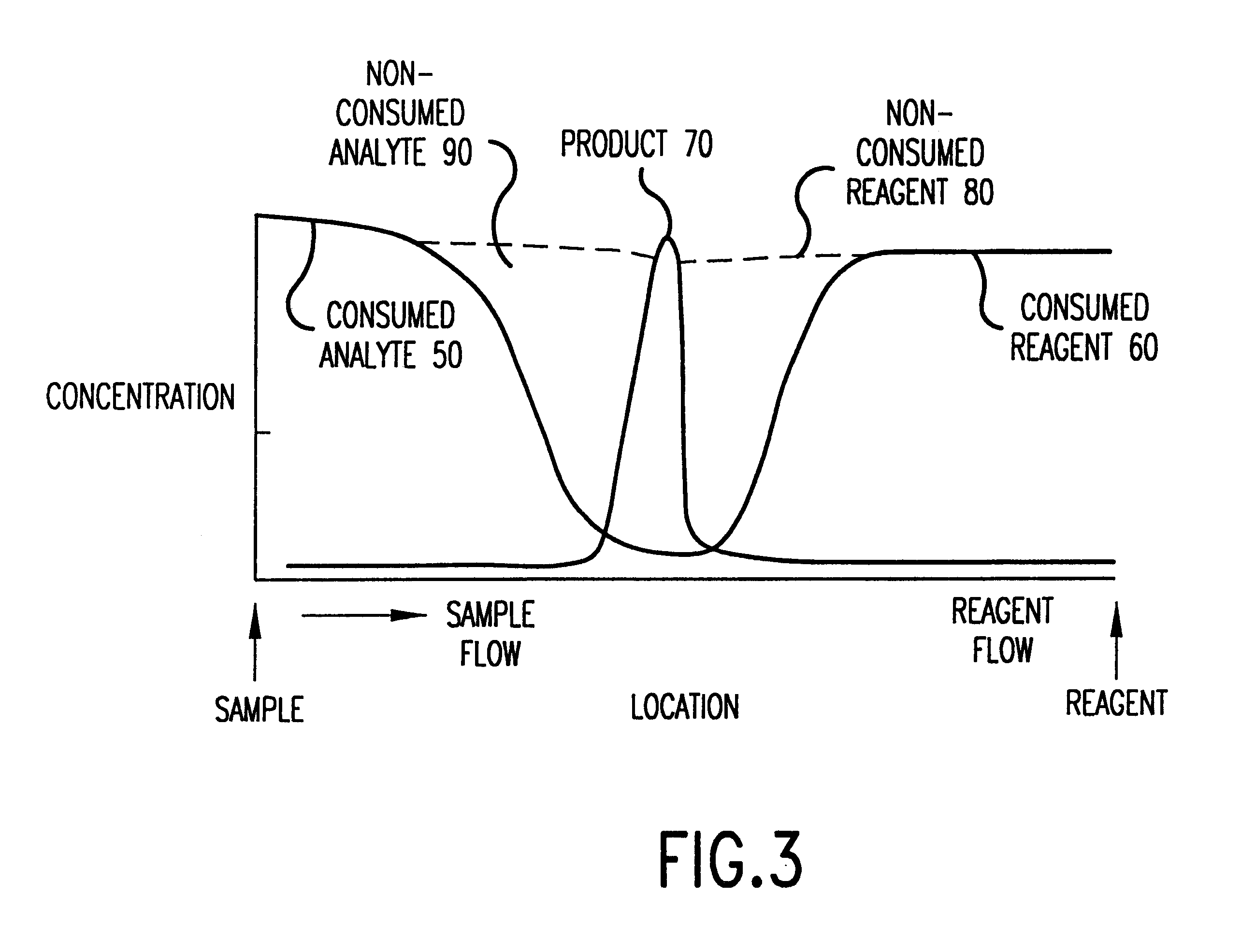
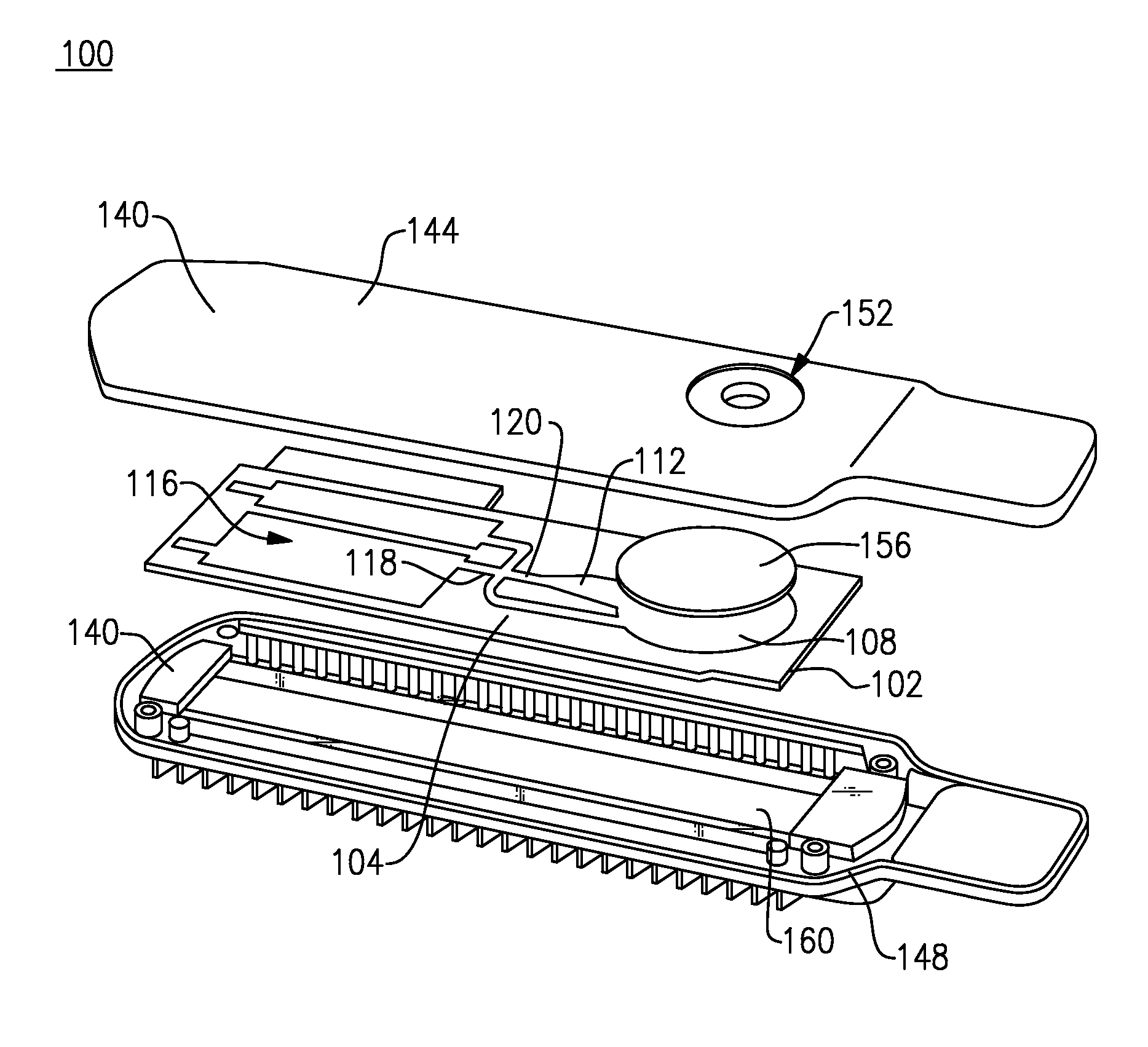
Interstitial fluid methods and devices for determination of an analyte in the body
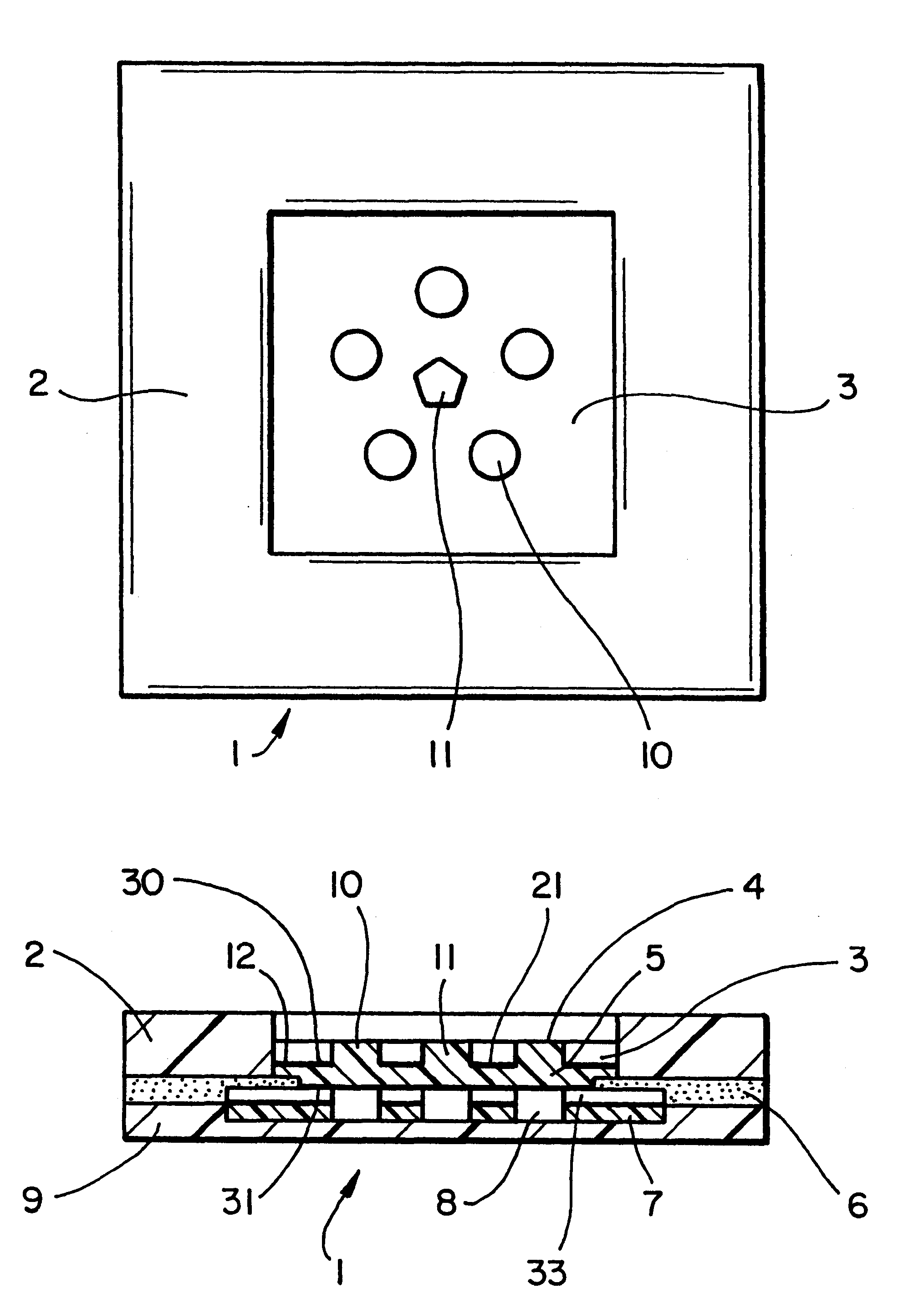
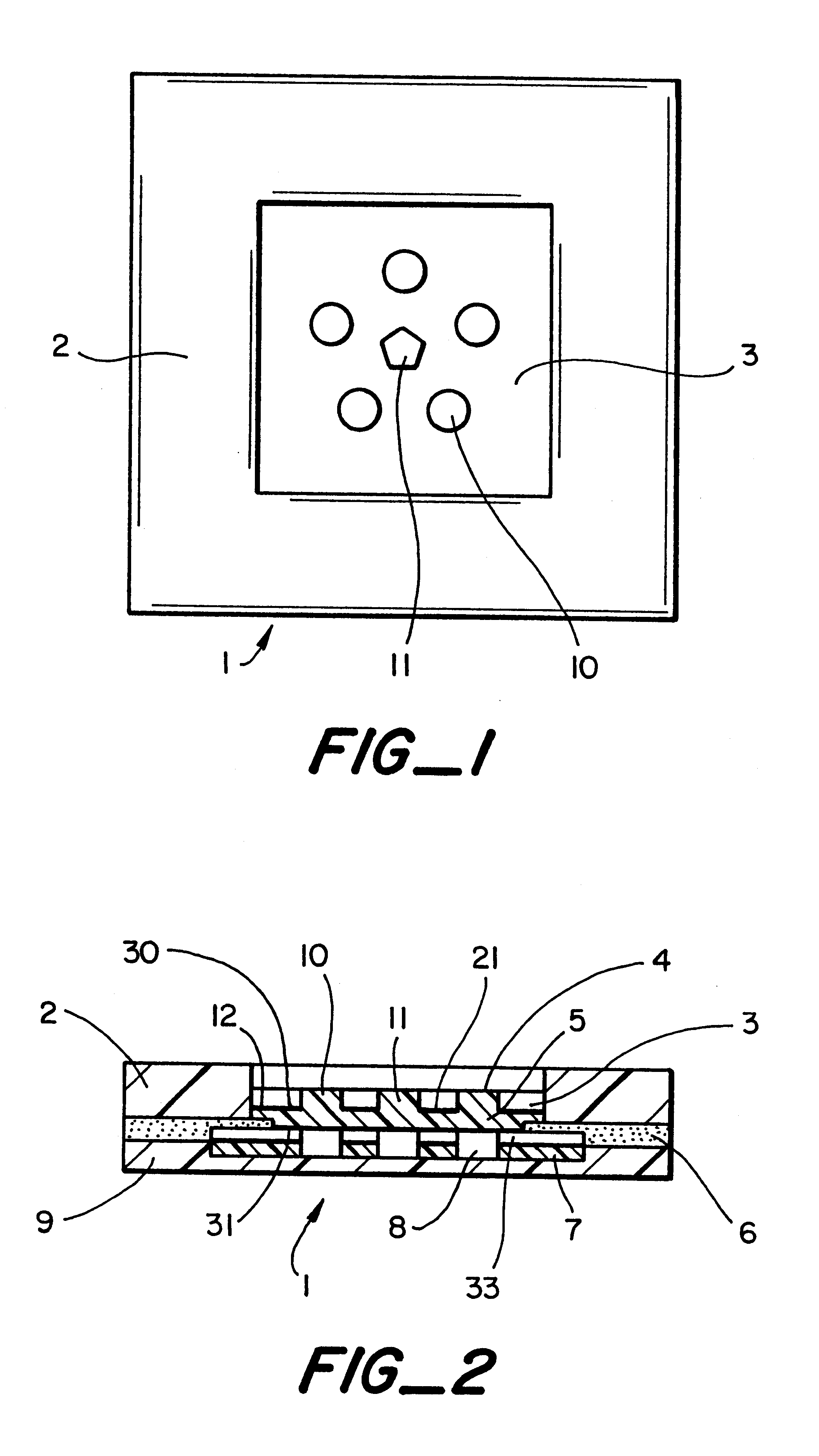
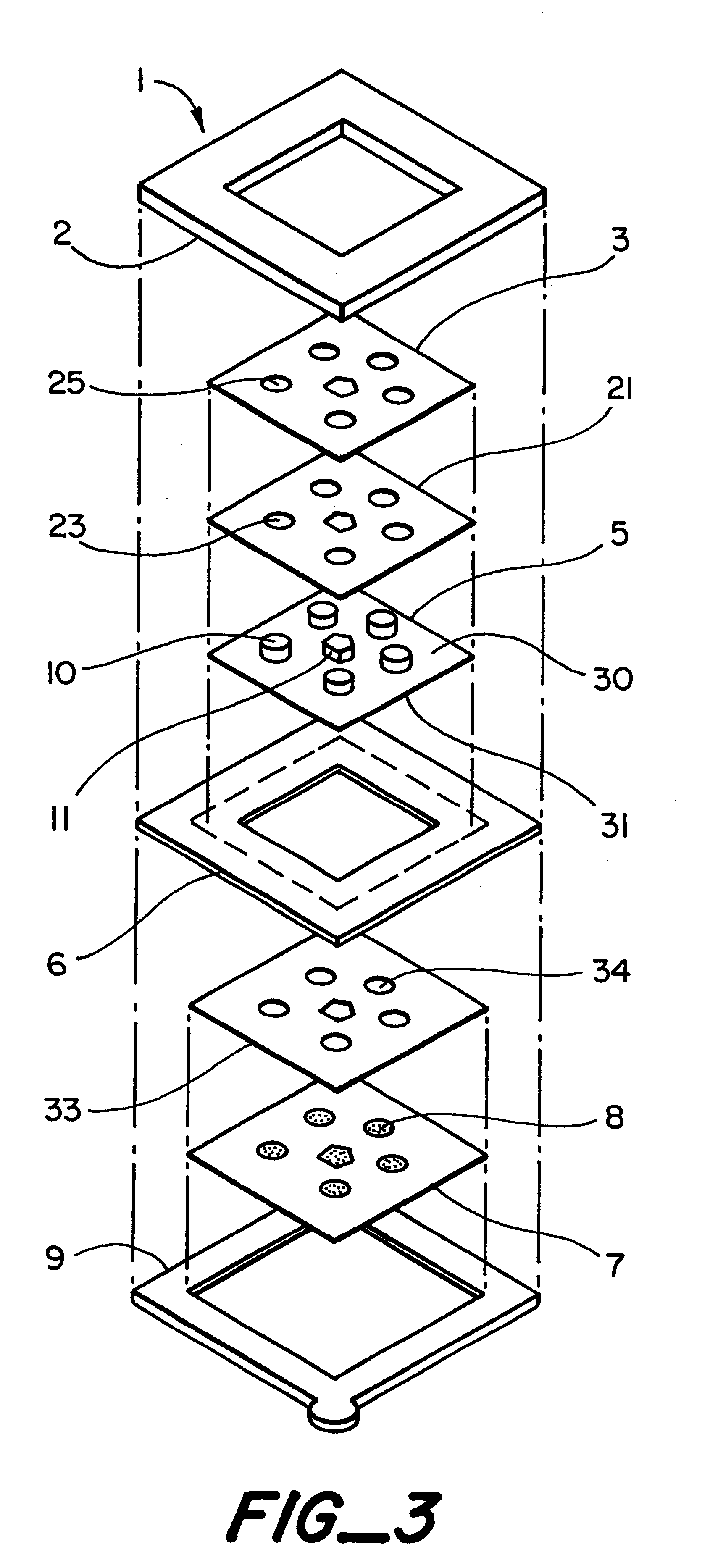
InactiveUS6251083B1Simpler to useFacilitate increased patient complianceDiagnostic recording/measuringSensorsAnalytePorous membrane
Devices and methods for utilizing dry chemistry dye indicator systems for body fluid analysis, such as glucose level provided by incorporating a porous membrane in a disposable patch. The devices also provide for microtitration of fluid samples in fixed volumetric openings containing indicator reagent. The devices provided are low cost due to efficient manufacturing methods provided.
Owner:ROCHE DIABETES CARE INC
Method and device for detecting analytes in fluids
InactiveUS6602719B1Bioreactor/fermenter combinationsBiological substance pretreatmentsHigh concentrationBlood plasma
Owner:IDEXX LABORATORIES
Noninvasive transdermal systems for detecting an analyte in a biological fluid and methods
InactiveUS7577469B1Easy to useImprove complianceInvestigating moving sheetsDiagnostic recording/measuringTransdermal patchColor changes
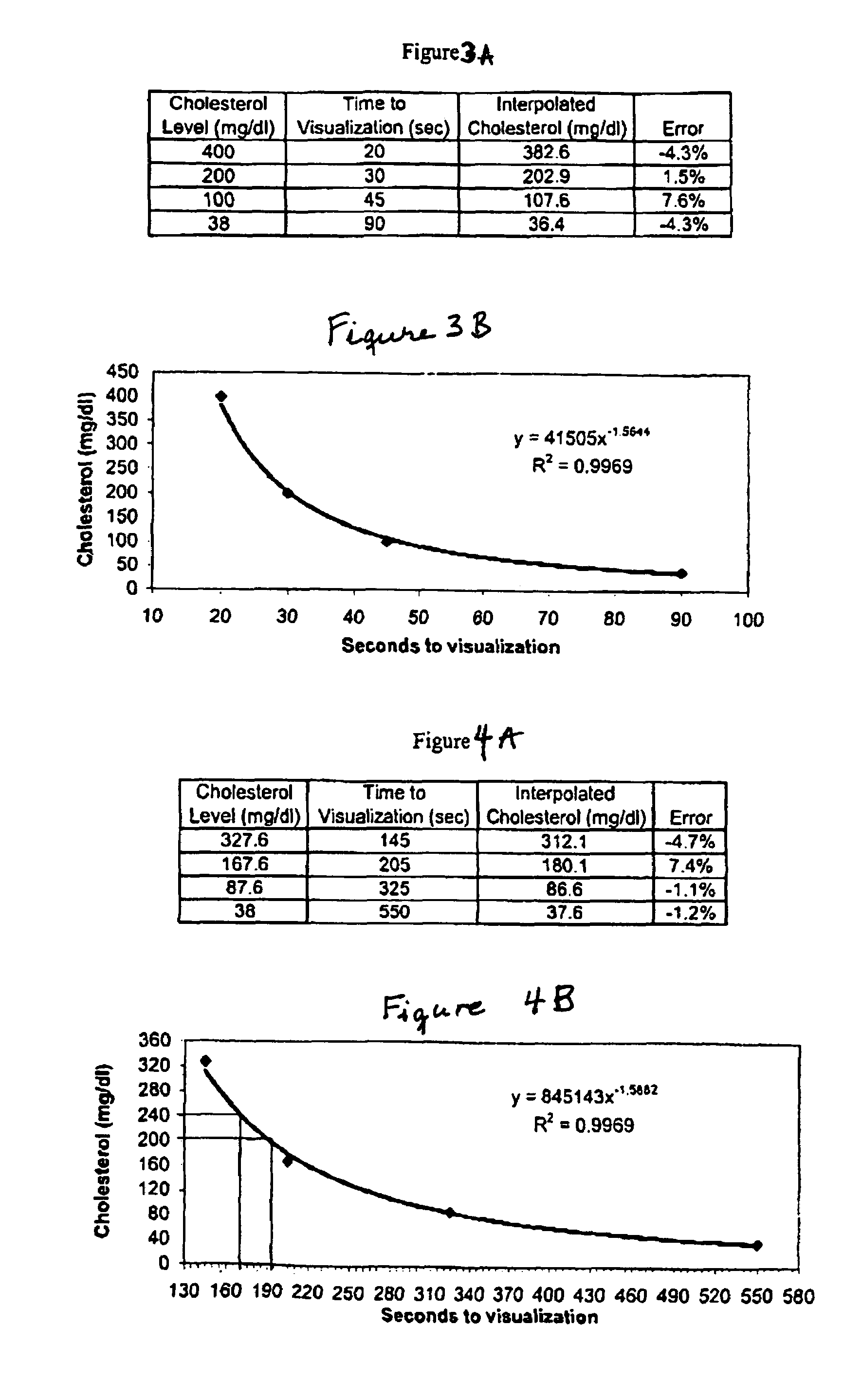
The present invention relates to noninvasive transdermal systems comprised of a noninvasive transdermal patch and a reflectometer. The noninvasive transdermal patches are comprised of a wet chemistry component and a dry chemistry component. The wet chemistry component is a liquid transfer medium in the form of a gel layer for the extraction and liquid bridge transfer of the analyte of interest from the biological fluid within or beneath the skin to the dry chemistry component. The dry chemistry component is a reagent system for interacting with the analyte of interest (glucose) to generate a color change. The reflectometers include a modulated light source for emitting light to illuminate a target surface which possesses a certain color and shade of color for detection by an optical detector. The output signal is processed for determining a corresponding quantity or quality measurement.
Owner:PLDHC ACQUISITIONS LLC
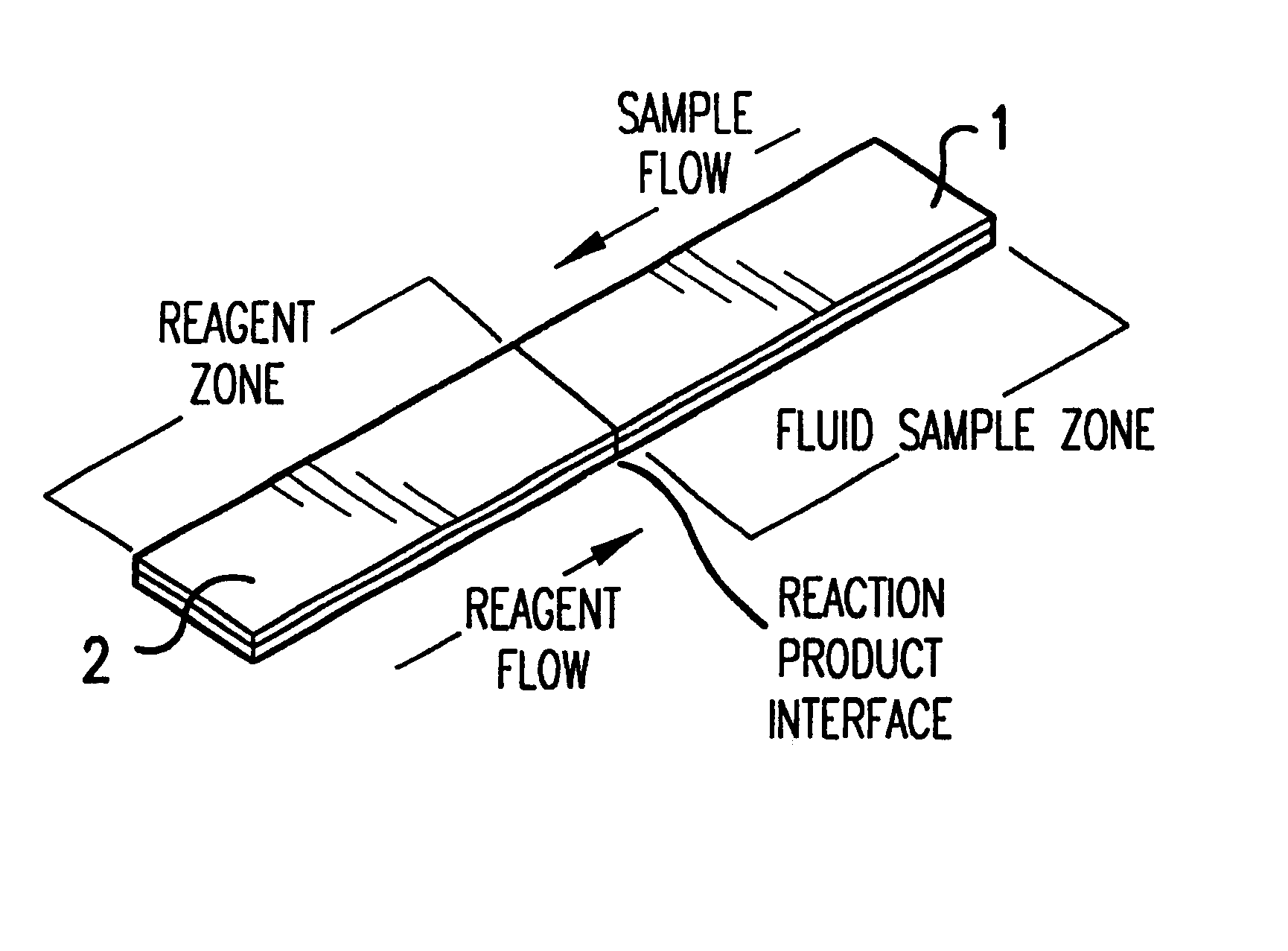
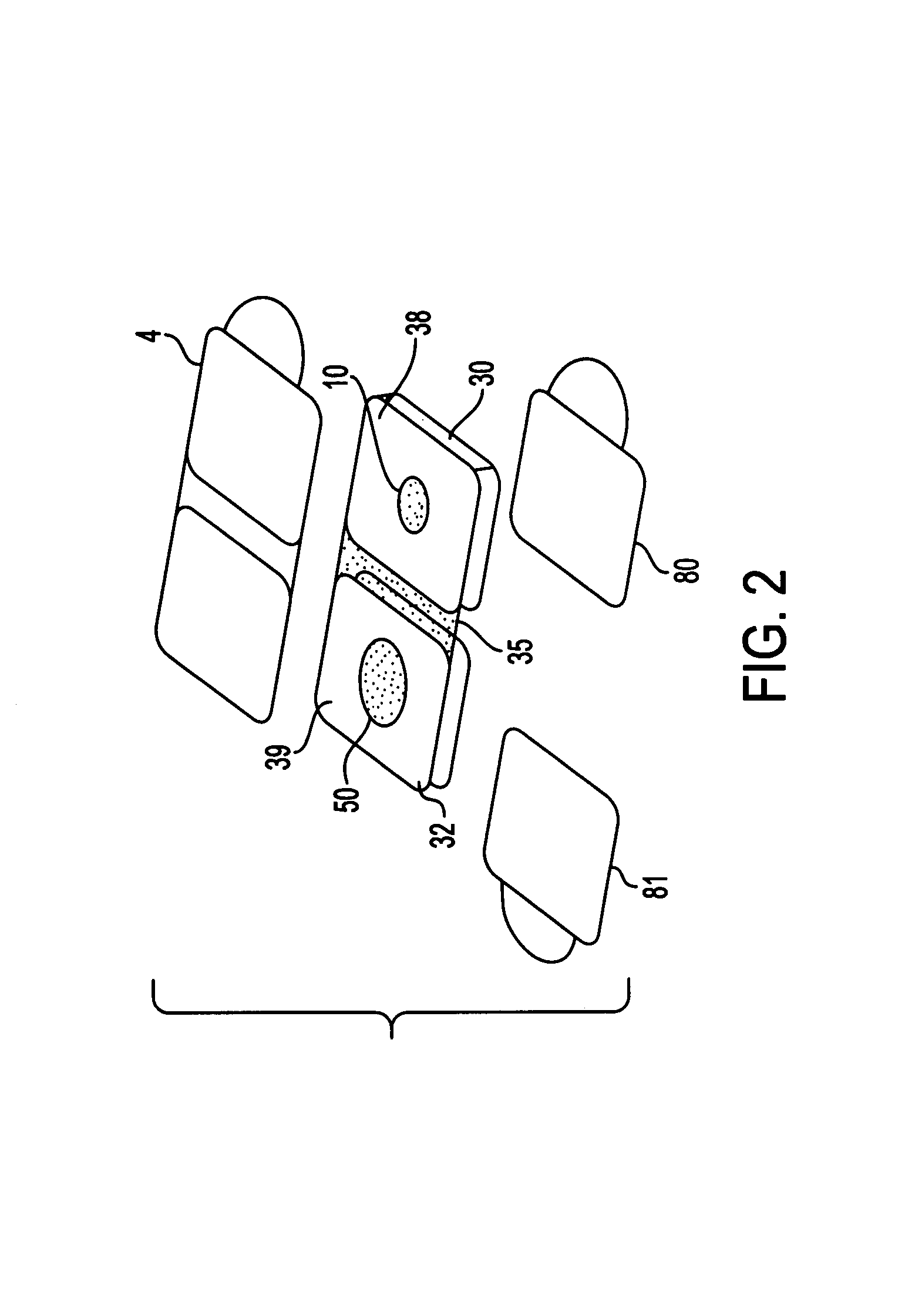
Dry chemistry, lateral flow-reconstituted chromatographic enzyme-driven assays
InactiveUS20040241779A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSpecific enzymeAssay
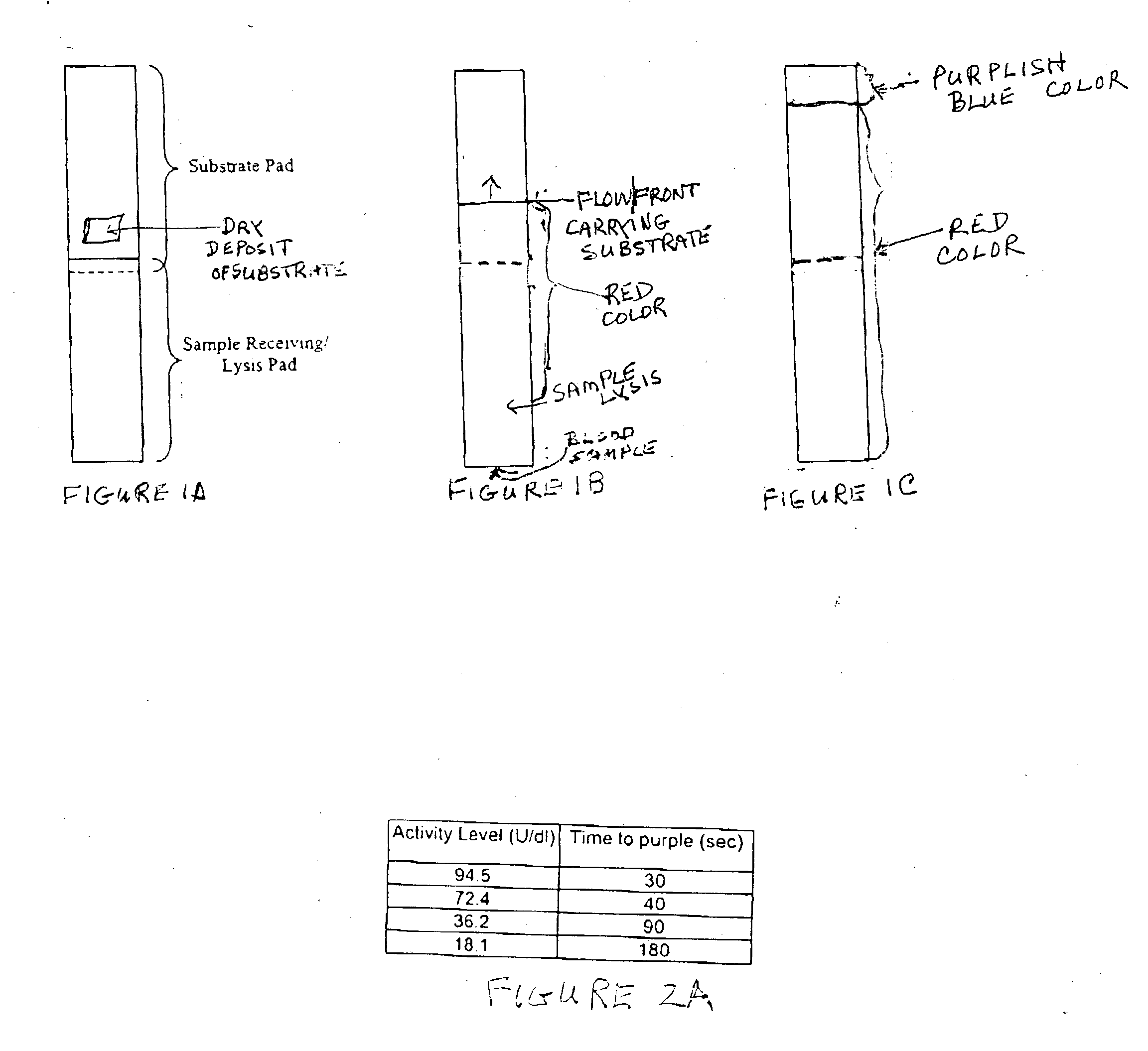
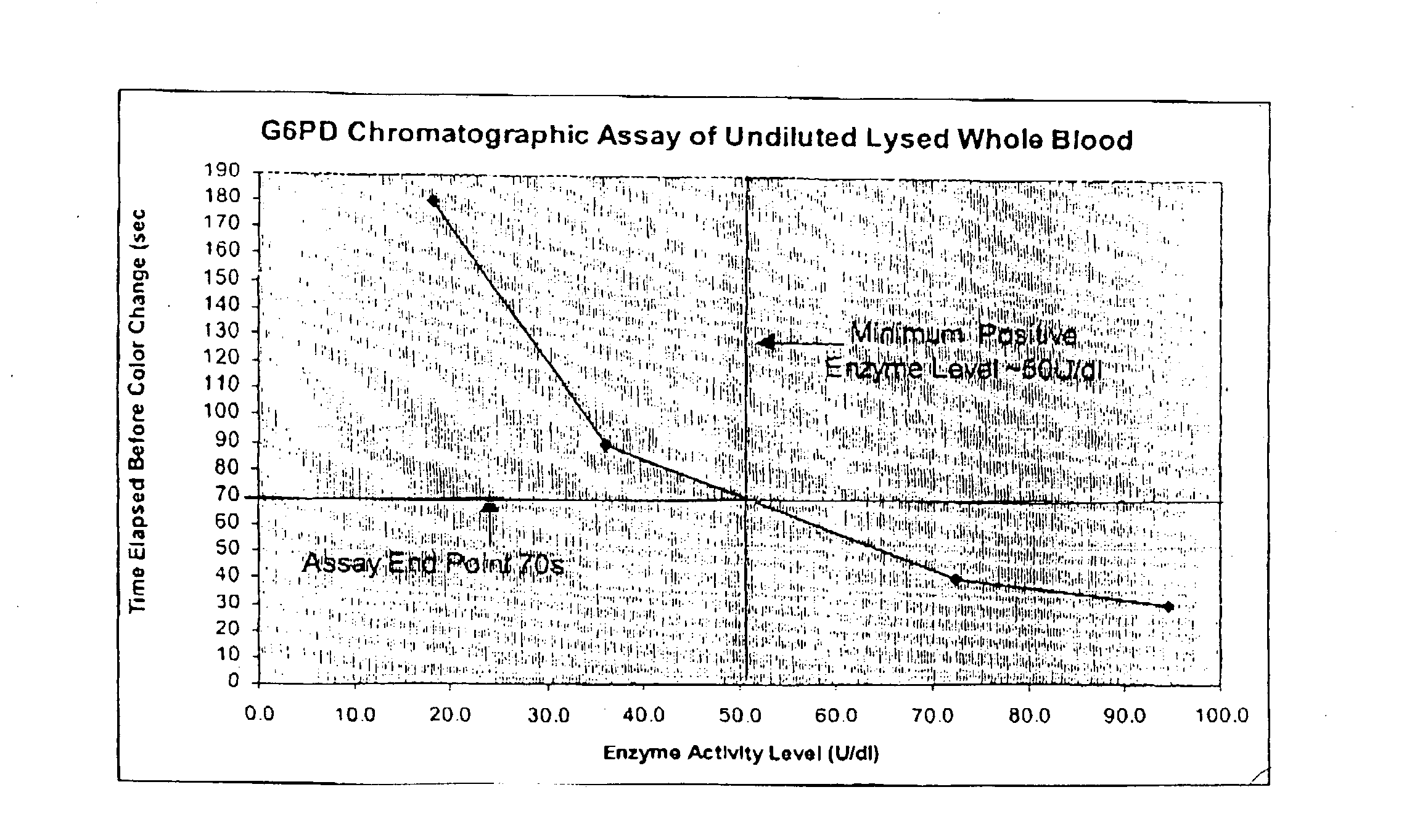
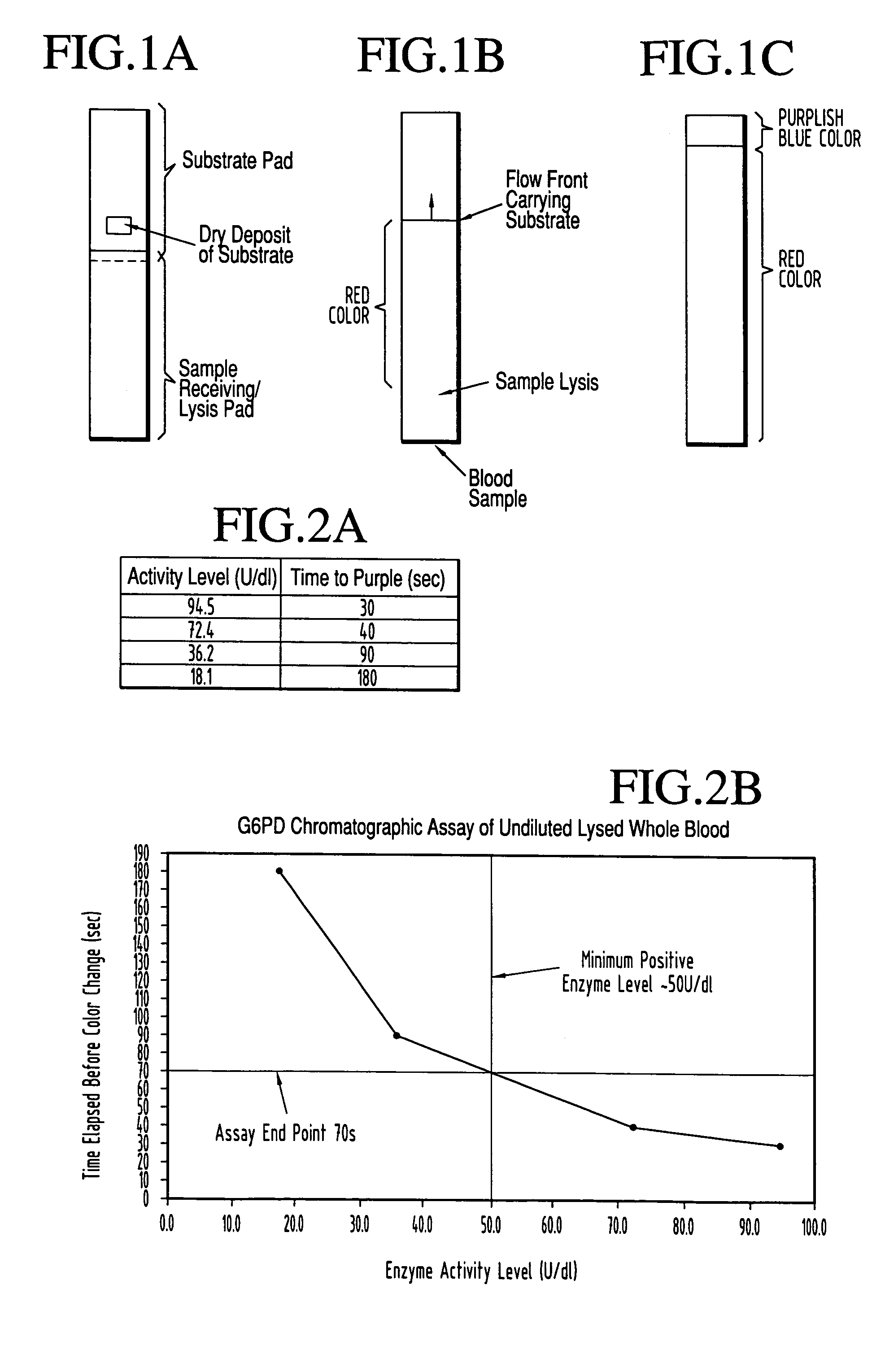
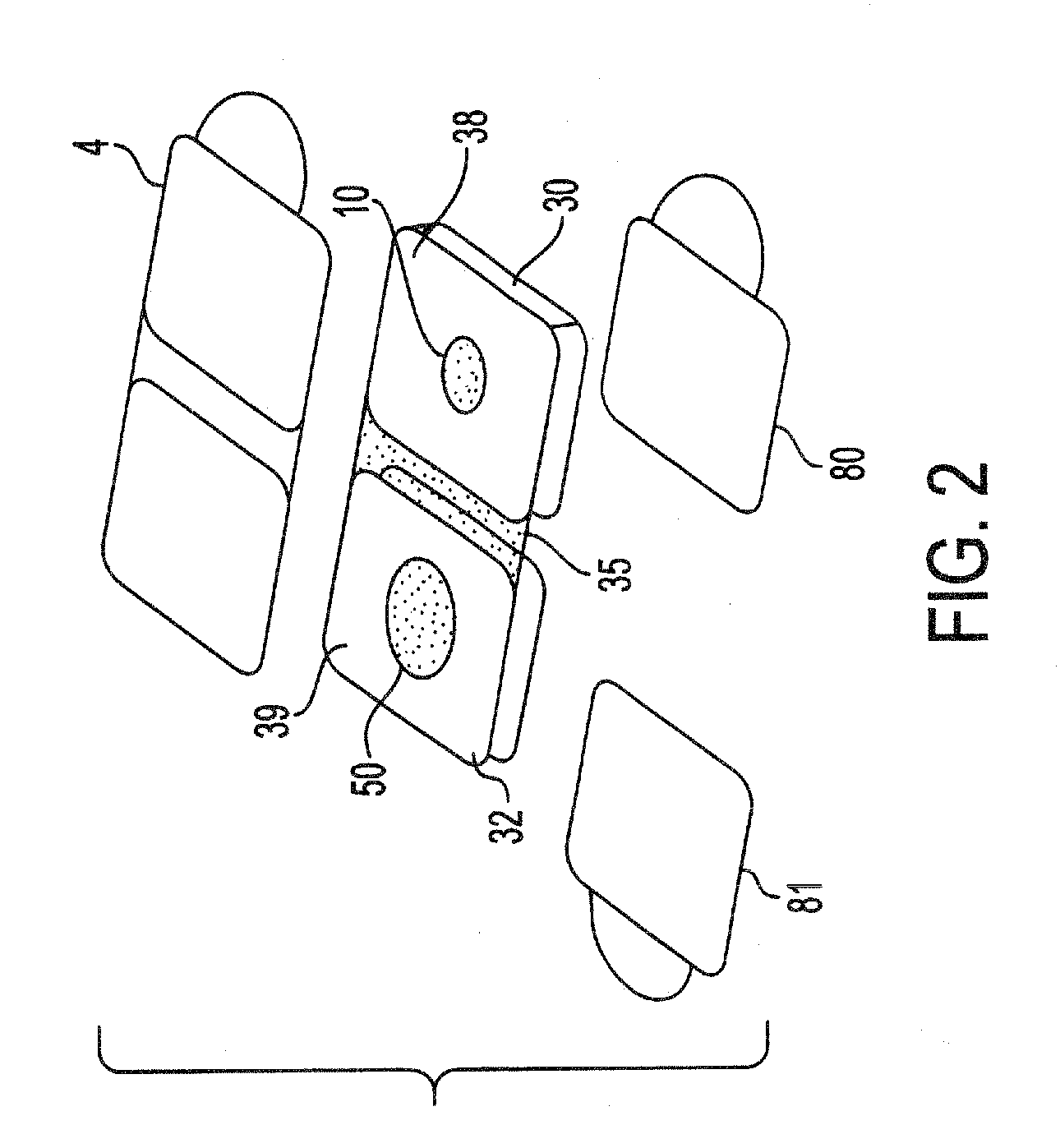
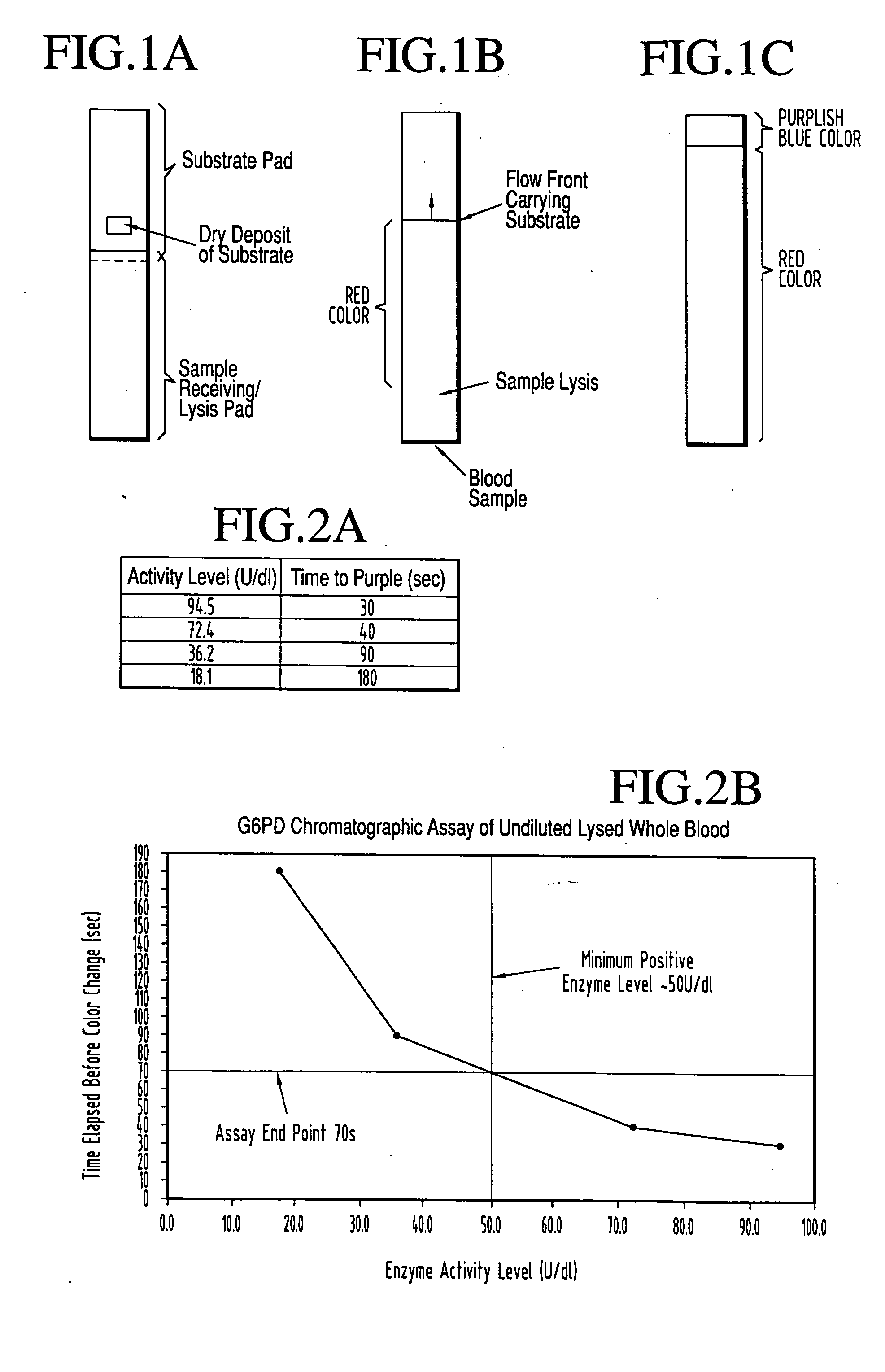
A lateral flow chromatographic assay format for the performance of rapid enzyme-driven assays is described. A combination of components necessary to elicit a specific enzyme reaction, which are either absent from the intended sample or insufficiently present therein to permit completion of the desired reaction, are predeposited as substrate in dry form together with ingredients necessary to produce a desired color upon occurrence of the desired reaction. The strip is equipped with a sample pad placed ahead of the substrate deposit in the flowstream, to which liquid sample is applied. The sample flows from the sample pad into the substrate zone where it immediately reconstitutes the dried ingredients while also intimately mixing with them and reacting with them at the fluid front. The fluid front moves rapidly into the final "read zone" wherein the color developed is read against predetermined color standards for the desired reaction. Pretreatment pads for the sample, as needed, (e.g. a lysing pad for lysing red blood cells in whole blood) are placed in front of the sample pad in the flow path as appropriate. The assay in the format of the invention is faster and easier to perform than analogous wet chemistry assays. A specific assay for glucose-phosphate dehydrogenase ("G-6PD") in this format is disclosed.
Owner:BINAX INC
Dry chemistry, lateral flow-reconstituted chromatographic enzyme-driven assays
InactiveUS7425302B2Easy to observeHigh detection sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsAdditive ingredientPeroxidase
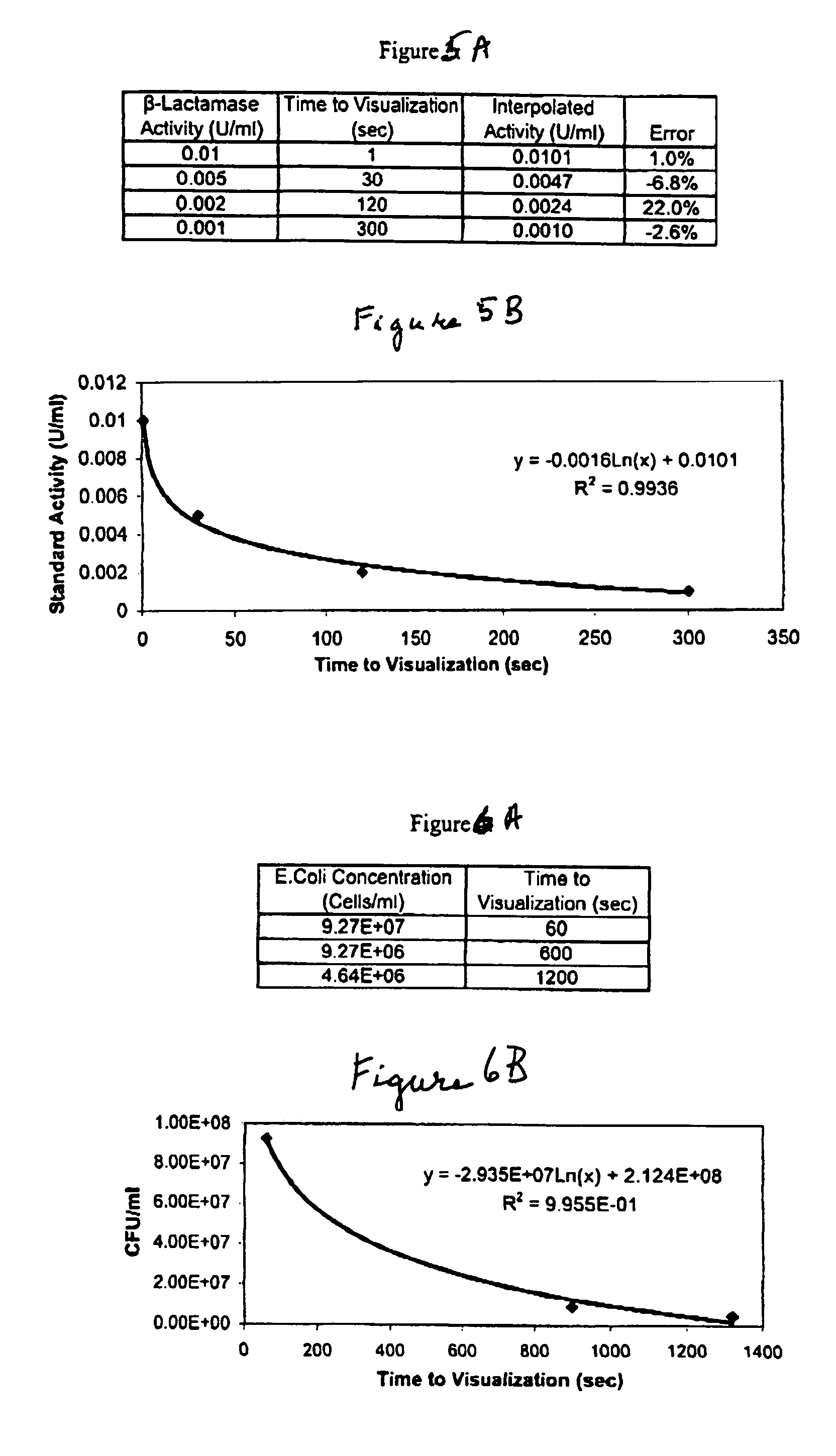
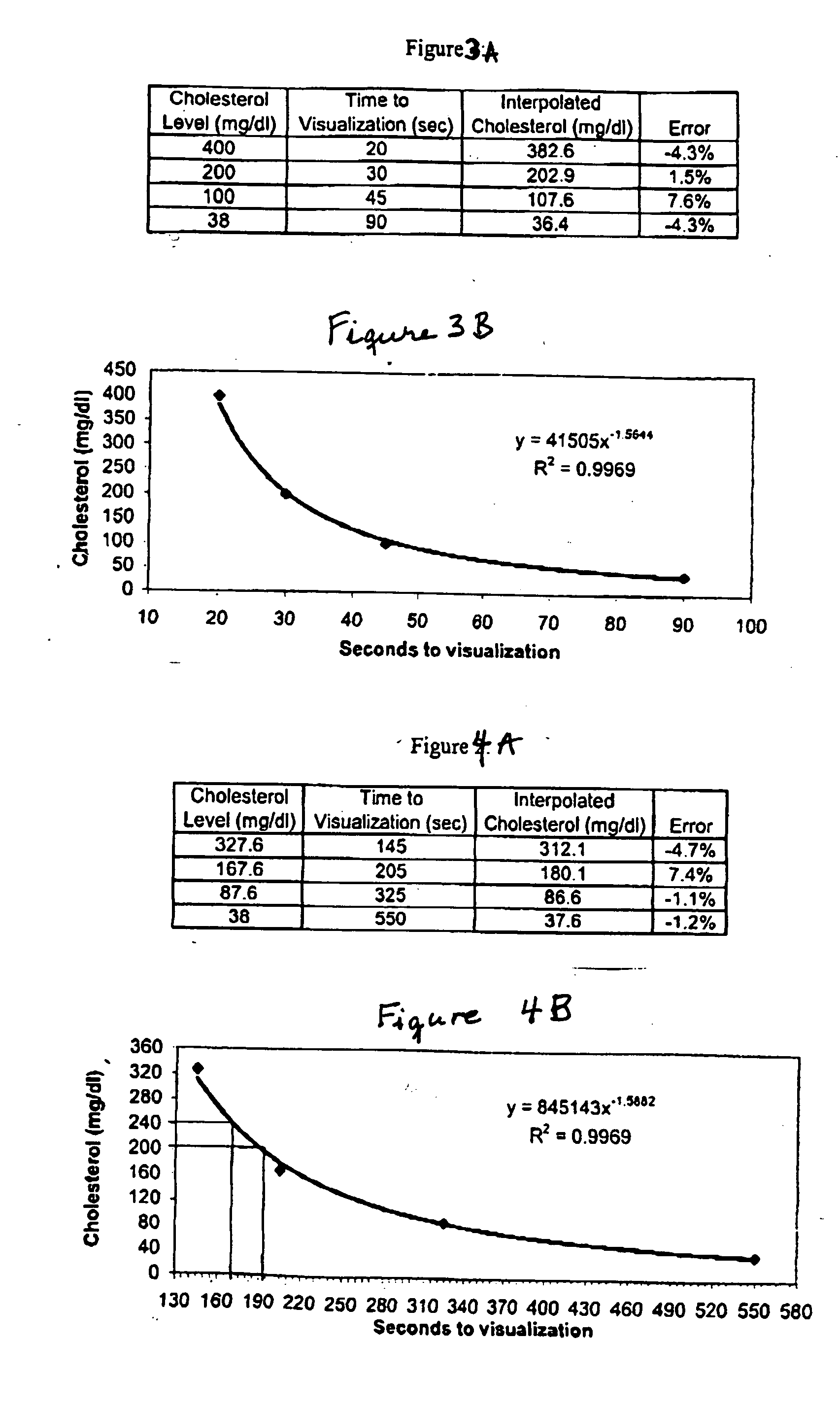
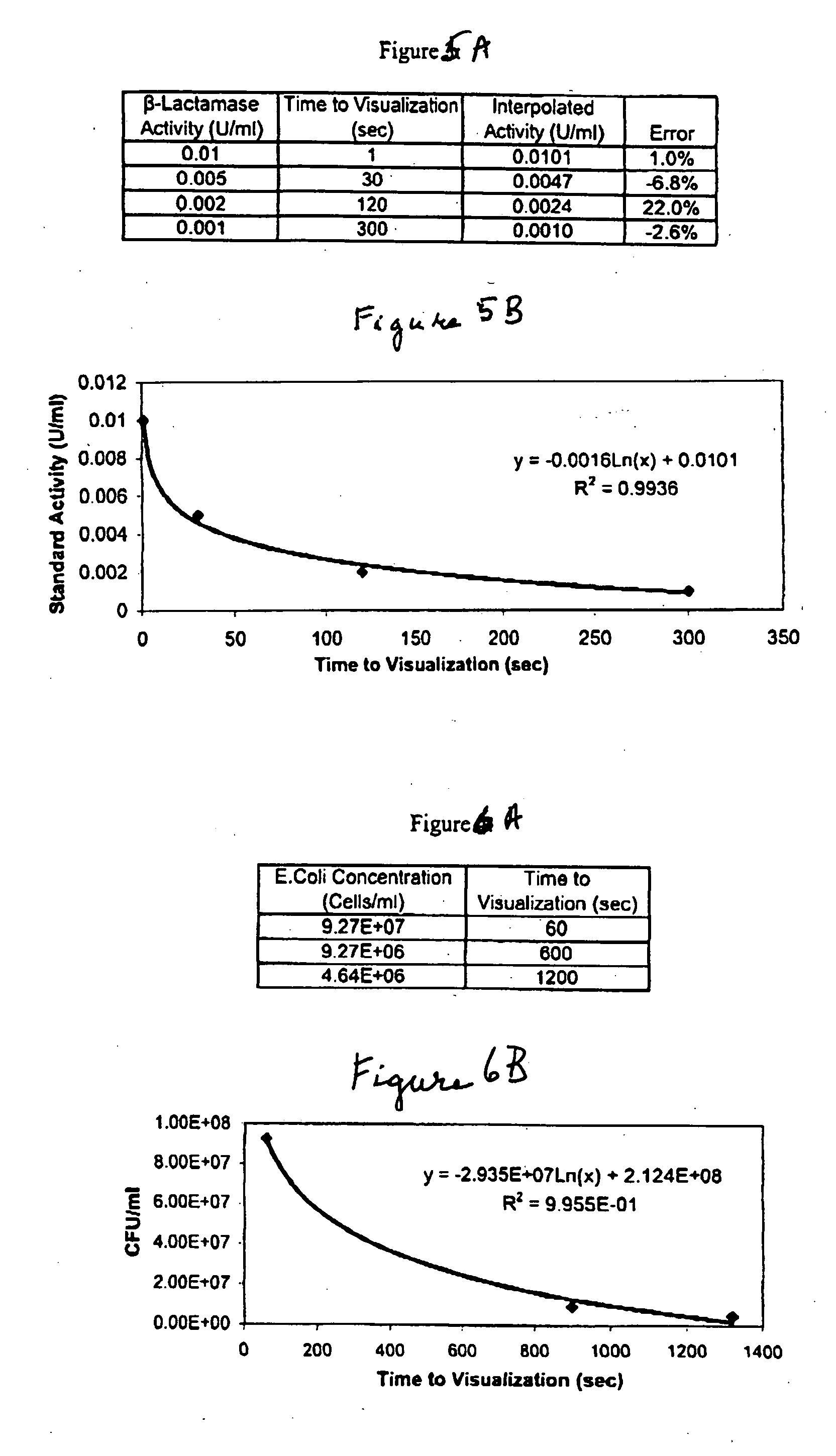
A lateral flow chromatographic assay format for the performance of rapid enzyme-driven assays is described. A combination of components necessary to elicit a specific enzyme reaction, which are either absent from the intended sample or insufficiently present therein to permit completion of the desired reaction, are predeposited as substrate in dry form together with ingredients necessary to produce a desired color upon occurrence of the desired reaction. The strip is equipped with a sample pad placed ahead of the substrate deposit in the flowstream, to which liquid sample is applied. The sample flows from the sample pad into the substrate zone where it immediately reconstitutes the dried ingredients while also intimately mixing with them and reacting with them at the fluid front. The fluid front moves rapidly into the final “read zone” wherein the color developed is read against predetermined color standards for the desired reaction. Pretreatment pads for the sample, as needed, (e.g. a lysing pad for lysing red blood cells in whole blood) are placed in front of the sample pad in the flow path as appropriate. The assay in the format of the invention is faster and easier to perform than analogous wet chemistry assays.Specific assays for glucose-6-phosphate dehydrogenase (“G-6PD”), total serum cholesterol, β-lactamase activity and peroxidase activity are disclosed.
Owner:ABBOTT DIAGNOSTICS SCARBOROUGH INC
Gynaecologic multi-item dry chemical united detection test paper strip and its measuring method
InactiveCN101320040AReflect cleanlinessComprehensive detectionMaterial analysis by observing effect on chemical indicatorBiological testingWhite blood cellPaper tape
The invention relates to a test paper tape which can be used in the gynecological multiprogramming dry-chemistry joint detection. The test paper tape provided by the utility model comprises a dry-chemistry multiprogramming detection test paper tape which consists of a plastic substrate tape and various solidified regent blocks, and sample diluent. The dry regent blocks include combinations formed by increasing or decreasing at least three or more regent blocks of a Ph test regent block, a lactic acid regent block, a hydrogen peroxide concentration regent block, a leukocyte esterase concentration regent block, a neuraminidase activity regent block, an amine test regent block, a proline aminopeptidase substrate reagent block, an oxidase substrate reagent block, a N-acetylamine hexosidase substrate reagent block, a trichomonas specific protease substrate hydrolysis reagent block. the gynecological multiprogramming dry-chemistry joint detection test paper test provided by the utility model can detect Ph, lactic acid, hydrogen peroxide, leukocyte esterase, neuraminidase, amine test, proline aminopeptidase, oxidase, N-acetylamine hexosidase and trichomonas specific protease which are contained in leucorrhea sample at the same time, and can accurately reflect the microorganism environment of a women reproductive tract, the cleanness of leucorrhea secretion and the conditions of Candida albicans, trichomonas, gonococcus and the pathogens of bacterial vaginosis, thereby making the gynecological trichomonas detection more comprehensive; besides, the test paper tape provided by the utility model can be used easily and conveniently, and results can be obtained quickly. If the test paper tape is used together with a gynecological dry-chemistry analyzer, the operation can become easier and the result can be obtained more quickly.
Owner:杭州健宝医疗器械有限公司
Noninvasive Transdermal Systems for Detecting an Analyte in a Biological Fluid and Methods
InactiveUS20100049016A1Low costEasy to useInvestigating moving sheetsDiagnostic recording/measuringTransdermal patchColor changes
The present invention relates to noninvasive transdermal systems comprised of a noninvasive transdermal patch and a reflectometer. The noninvasive transdermal patches are comprised of a wet chemistry component and a dry chemistry component. The wet chemistry component is a liquid transfer medium in the form of a gel layer for the extraction and liquid bridge transfer of the analyte of interest from the biological fluid within or beneath the skin to the dry chemistry component. The dry chemistry component is a reagent system for interacting with the analyte of interest (glucose) to generate a color change. The reflectometers include a modulated light source for emitting light to illuminate a target surface which possesses a certain color and shade of color for detection by an optical detector. The output signal is processed for determining a corresponding quantity of quality measurement.
Owner:ARONOWITZ JACK L +3
Dry chemistry, lateral flow-reconstituted chromatographic enzyme-driven assays
ActiveUS20040203086A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPeroxidaseAdditive ingredient
A lateral flow chromatographic assay format for the performance of rapid enzyme-driven assays is described. A combination of components necessary to elicit a specific enzyme reaction, which are either absent from the intended sample or insufficiently present therein to permit completion of the desired reaction, are predeposited as substrate in dry form together with ingredients necessary to produce a desired color upon occurrence of the desired reaction. The strip is equipped with a sample pad placed ahead of the substrate deposit in the flowstream, to which liquid sample is applied. The sample flows from the sample pad into the substrate zone where it immediately reconstitutes the dried ingredients while also intimately mixing with them and reacting with them at the fluid front. The fluid front moves rapidly into the final "read zone" wherein the color developed is read against predetermined color standards for the desired reaction. Pretreatment pads for the sample, as needed, (e.g. a lysing pad for lysing red blood cells in whole blood) are placed in front of the sample pad in the flow path as appropriate. The assay in the format of the invention is faster and easier to perform than analogous wet chemistry assays. Specific assays for glucose-6-phosphate dehydrogenase ("G-6PD"), total serum cholesterol, beta-lactamase activity and peroxidase activity are disclosed.
Owner:ABBOTT DIAGNOSTICS SCARBOROUGH INC
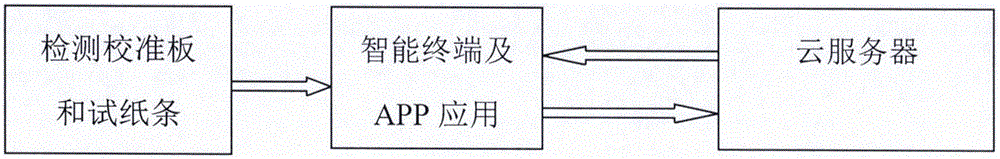
Color-discrimination-based rapid detection system and rapid dry chemistry detection method
ActiveCN105973885AAvoid Accuracy ImpactLow costMaterial analysis by observing effect on chemical indicatorColor recognitionColor changes
The invention discloses a color-discrimination-based rapid detection system and a rapid dry chemistry detection method. The rapid detection system comprises a detection calibration plate, a cloud server and an intelligent terminal with the photographing function. The intelligent terminal photographs the detection calibration plate and uploads photos to the cloud server. The rapid dry chemistry detection method comprises the following steps that 1, initial environment information is calibrated; 2, a to-be-tested paper slip is used, photographing is performed, and photos are uploaded to the cloud server; 3, the cloud server analyzes the to-be-tested paper slip and feeds back the result to a user according to data uploaded by the user. According to the color-discrimination-based rapid detection system and the rapid dry chemistry detection method, the detection time and cost can be lowered, test paper or reagent color changes can be rapidly and accurately detected, and an analysis result is given.
Owner:宋秀龙 +1
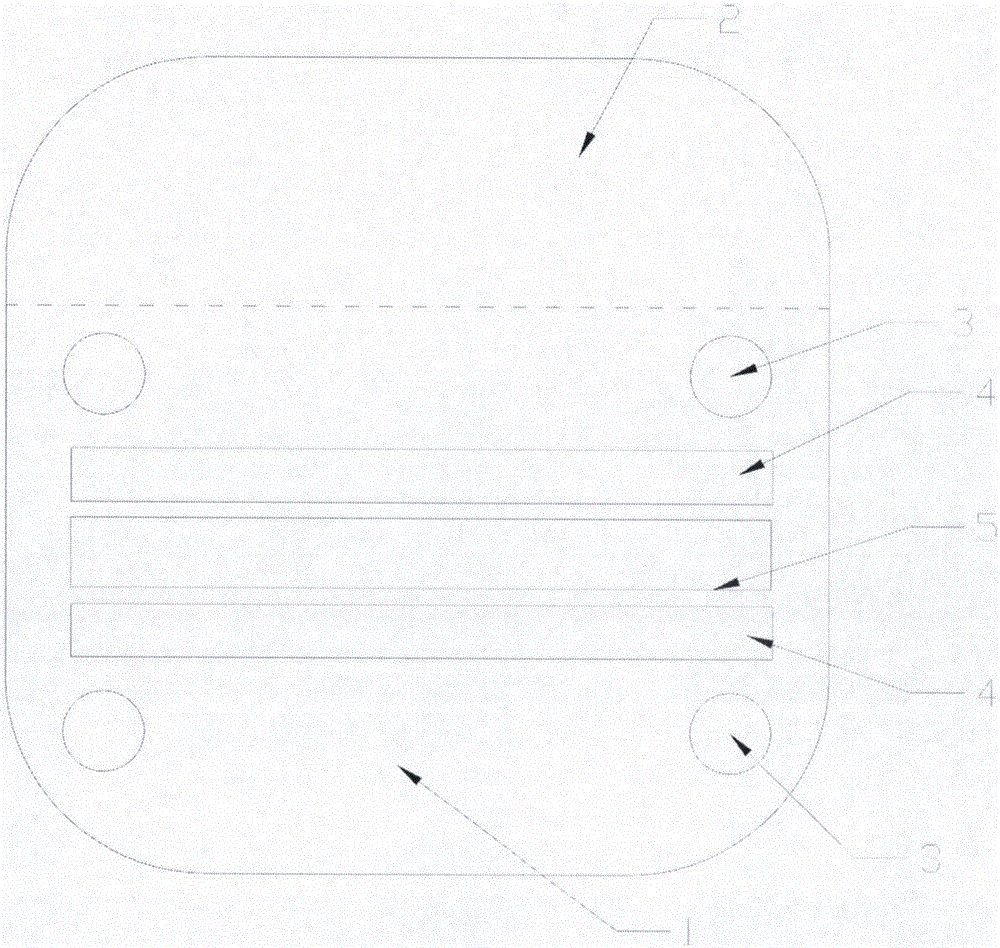
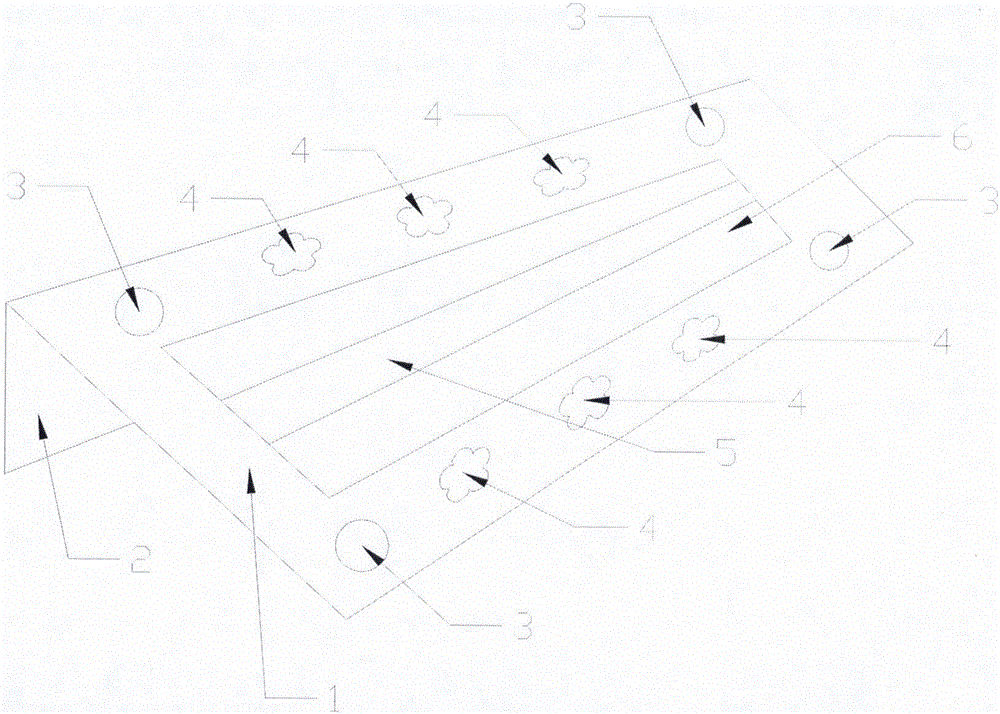
Dry chemical test paper for quantitatively determining human alanine aminotransferase
InactiveCN101105491AImprove stabilityHigh color intensityMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementChemical reactionAdditive ingredient
The invention belongs to the in vitro clinical diagnosis reagent field and specifically relates to a dry chemistry test paper which quantitatively determines the activity of alanine aminotransferase in human body; the dry chemistry test paper consists of an upper and a lower supporting layers in long-belt-shapes; a loading hole, a substrate layer, a visualization layer and a test hole are provided in a test area from top to bottom; the substrate layer can adopt the materials such as glass fibre, filter paper and non-woven fabrics and is soaked with reagents such as a substrate; the visualization layer is composed of various filter papers, non-woven fabrics or synthetic film and is soaked with reagents such as chromogen. A sample penetrates into the substrate layer through the loading hole and flows to a sample surface of the visualization layer; a liquid with detected ingredients penetrates to a detecting surface; the color of chromogen is changed under chemical reaction; the liquid penetrates through the test hole on the lower supporting layer and reaches the detecting surface of the visualization layer; the photodensity of the liquid can be determined on the detecting surface. The test paper is used to rapidly test the activity of alanine aminotransferase in whole blood, serum or plasma in the human body, which is one of important indexes of the detection of liver function.
Owner:SHANGHAI KEHUA BIO ENG
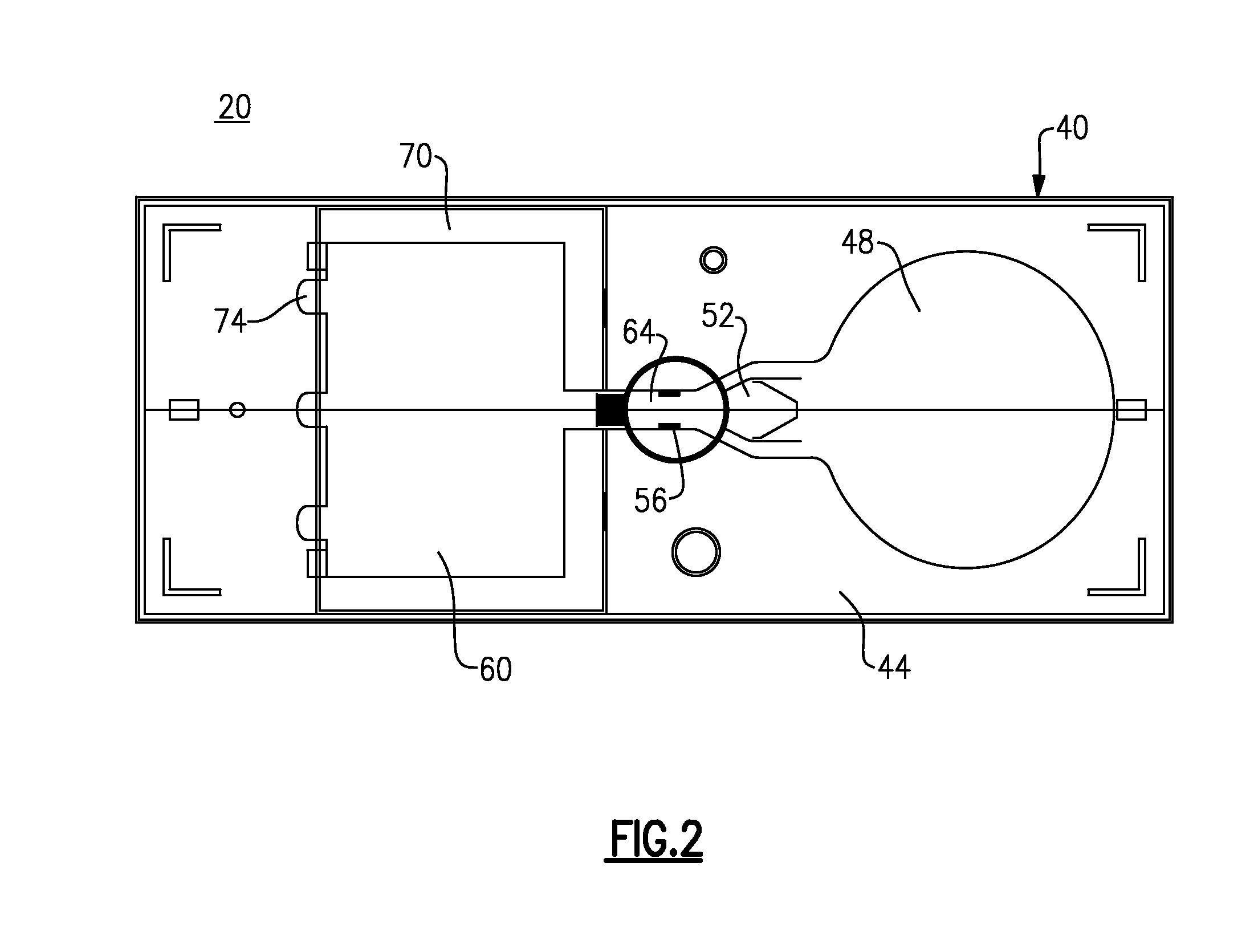
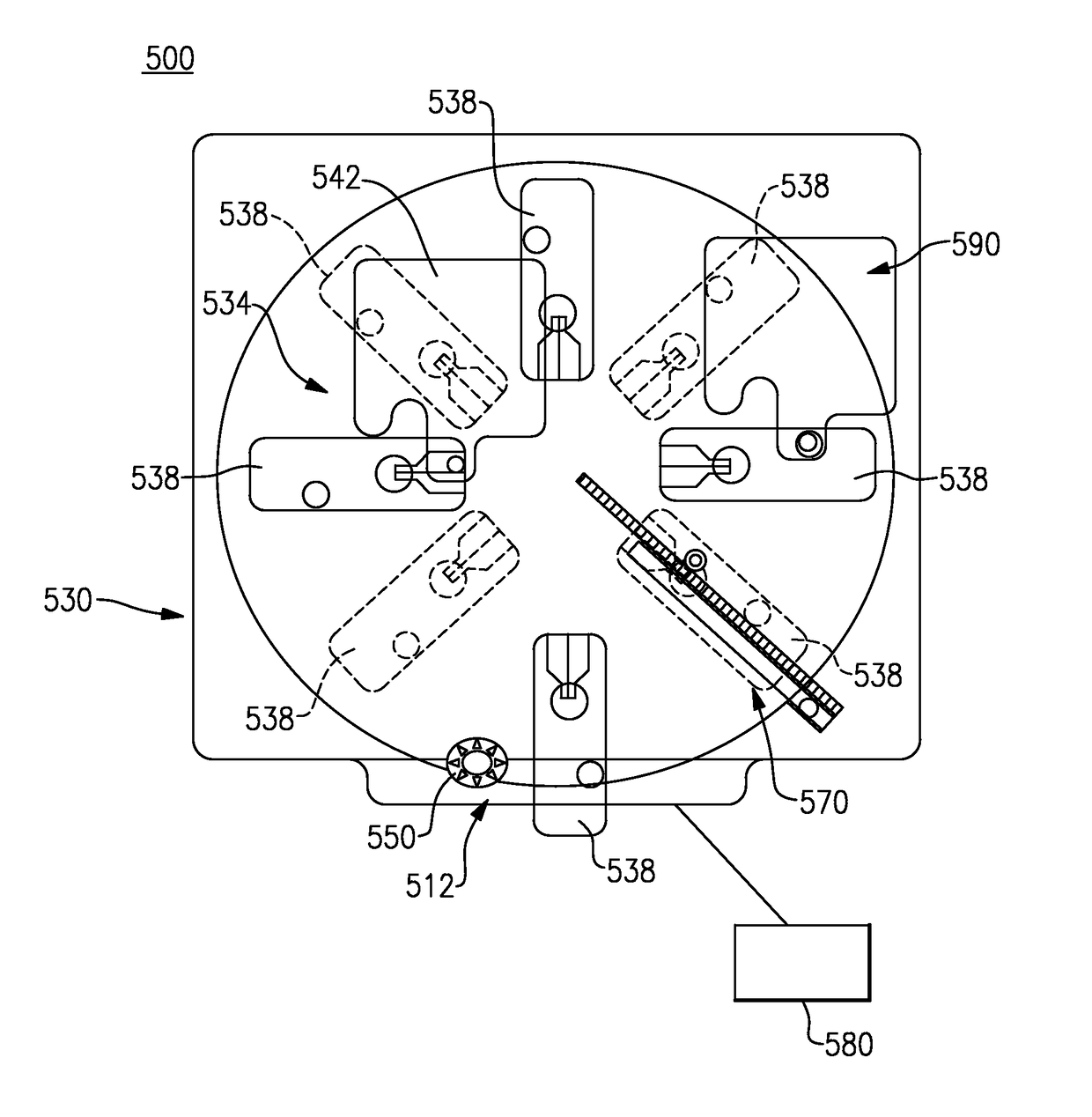
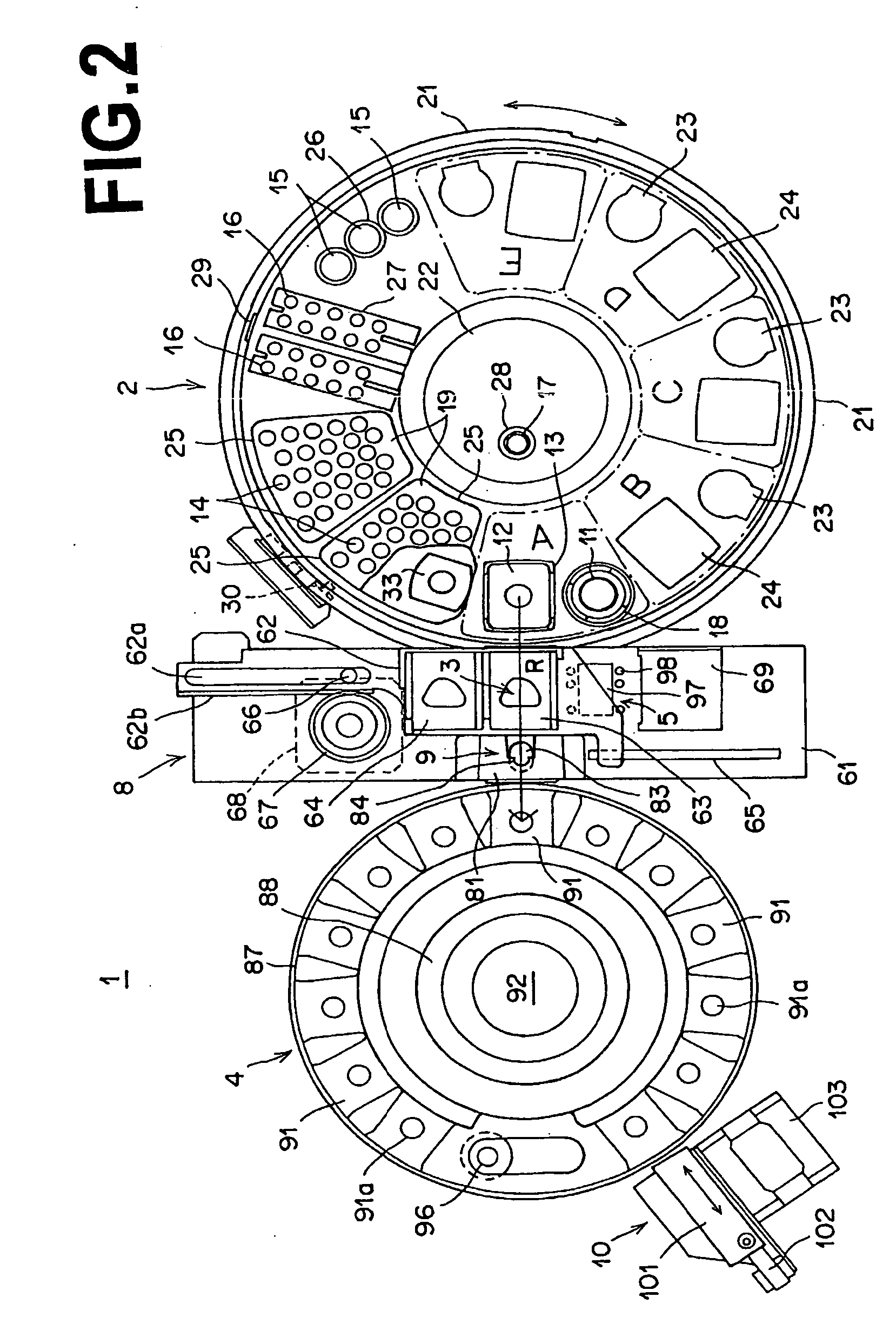
Point of care analytical processing system
ActiveUS20160025639A1Efficient workflowIncrease turnaround timeBioreactor/fermenter combinationsBiological substance pretreatmentsMultiplexingPoint of care
A point of care testing system includes a reader having an incubator disposed within a reader housing, the incubator having a rotor supported for rotation and having a plurality of circumferentially disposed slots. A drive mechanism is configured to rotate the rotor about a center axis A plurality of analytical test elements are sized for fitting in the slots of the incubator either manually or on demand. Each analytical test element commonly includes a support within a cartridge. The support retains at least one of a dry chemistry chip comprising a porous spreading layer disposed in stacked relation with at least one reagent layer or a lateral flow assay device wherein the plurality of test elements can assume a common form factor with multiplexed capability, and in which cartridges are preferably gated to enable random access processing.
Owner:ORTHO-CLINICAL DIAGNOSTICS
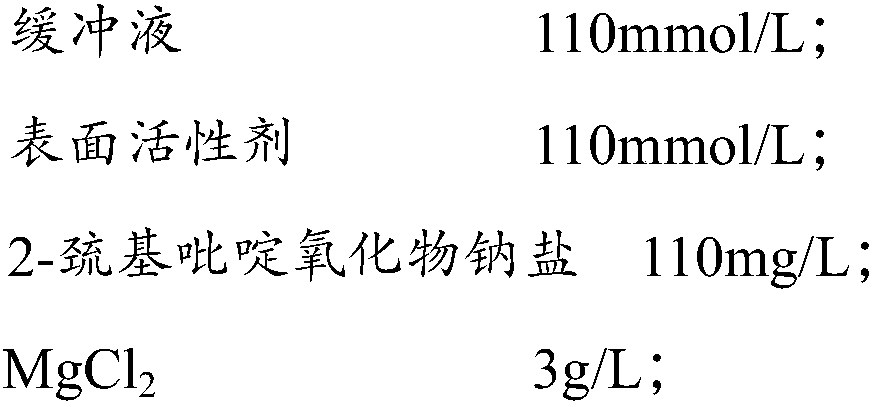
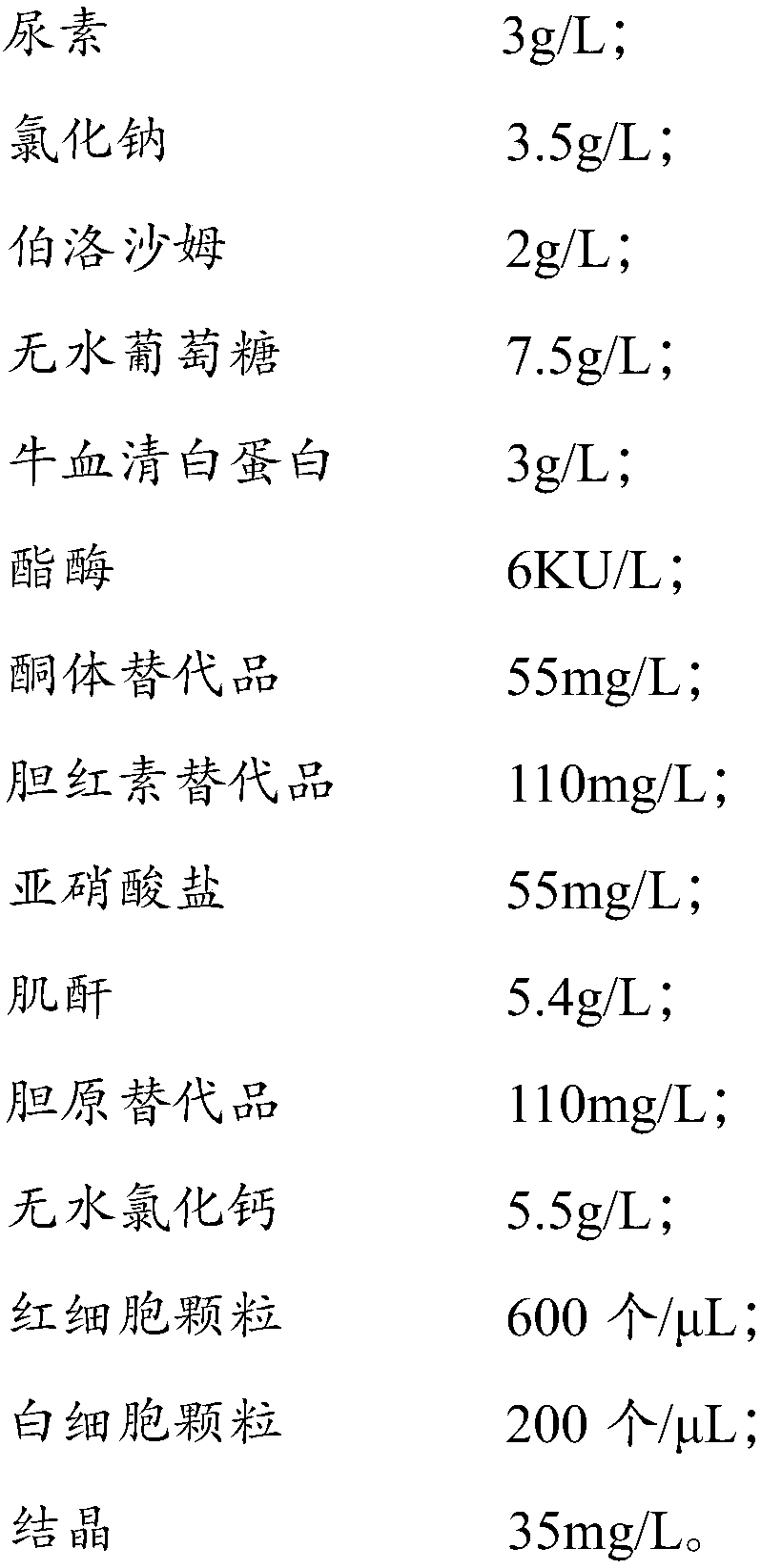
Multi-item composite quality control liquor for urine analysis
ActiveCN106771112AGood real-time stabilityLow toxicityBiological testingWhite blood cellAdditive ingredient
The invention discloses multi-item composite quality control liquor for urine analysis. The multi-item composite quality control liquor comprises a buffering solution, a surface active agent, 2-pyridinethiol 1-oxide sodium salt, MgC12, urea, sodium chloride, poloxamer, anhydrous dextrose, bovine serum albumin, esterase, a ketone body substitute, a bilirubin substitute, nitrite, creatinine, a urobilinogen substitute, anhydrous calcium chloride, red blood cell particles, white blood cell particles and crystals. Compared with existing quality control liquor, the multi-term composite quality control liquor can carry out quality control over urine dry chemistry and formal ingredients, and is good in real-time stability, small in toxicity, high in sensitiveness, accurate in detection result, low in production cost and capable of being applied to external quality assessment.
Owner:DIRUI MEDICAL TECH CO LTD
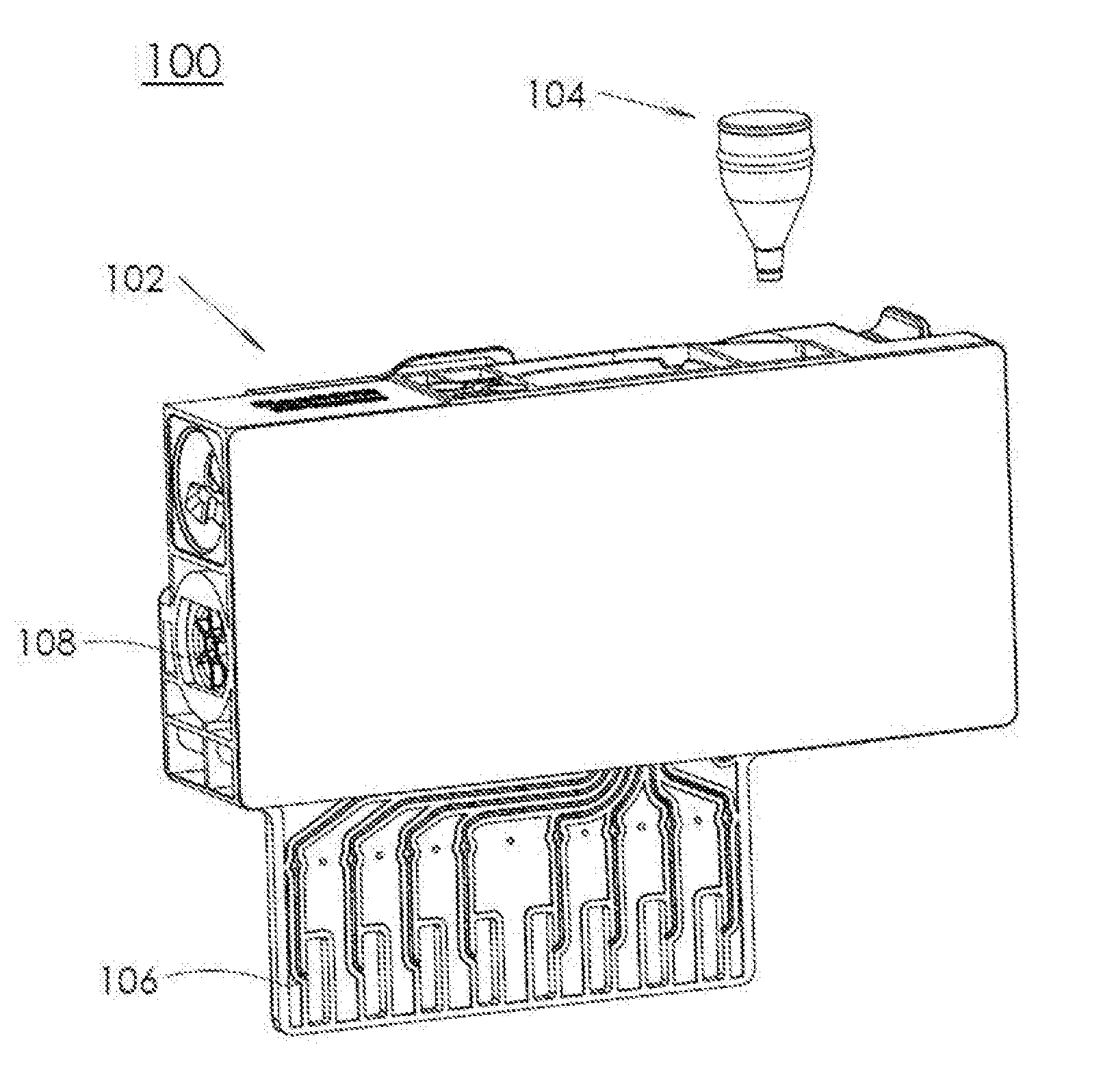
Dry Chemistry Container
ActiveUS20170014826A1Drying solid materials without heatPreparing sample for investigationCompound (substance)Engineering
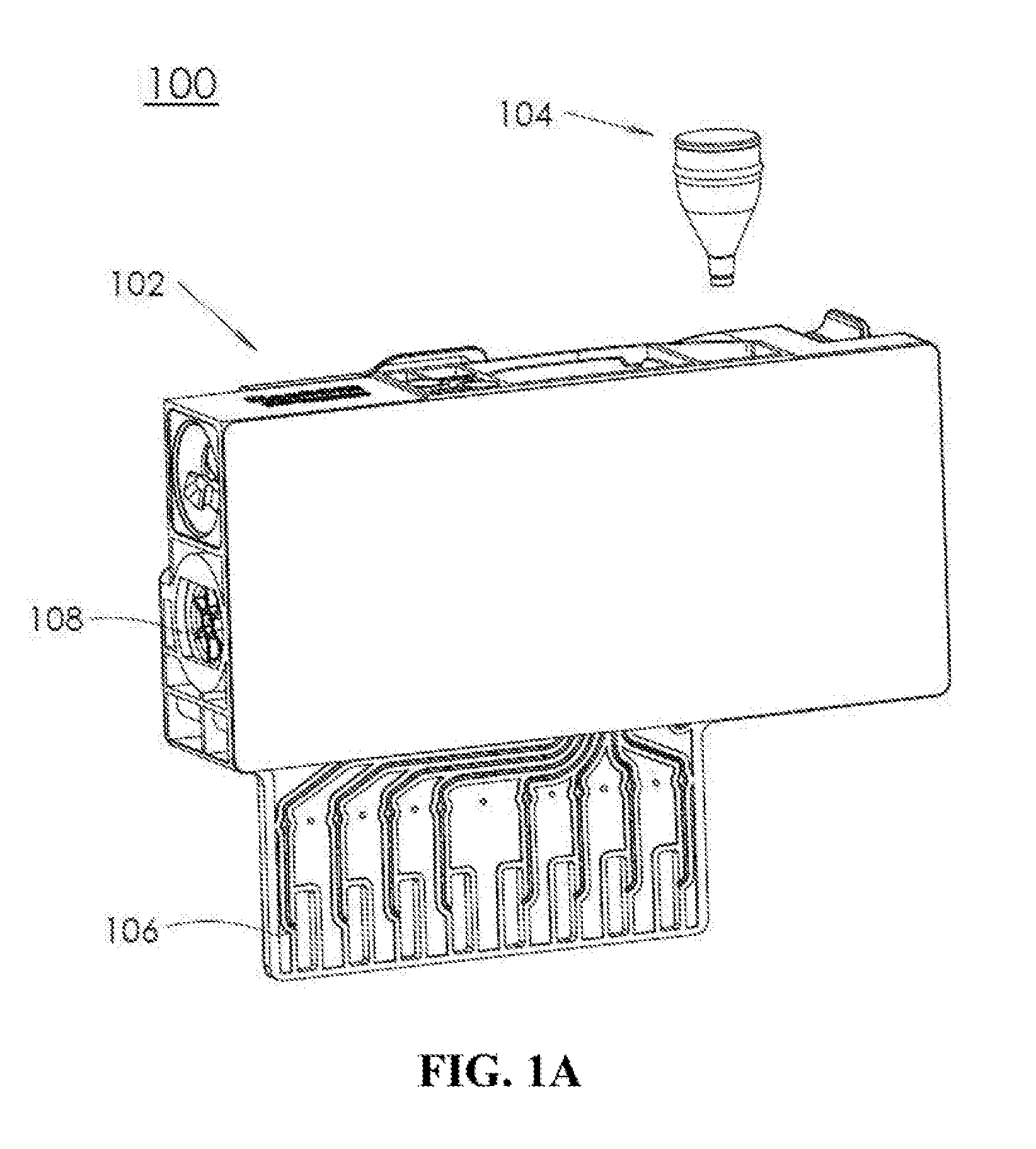
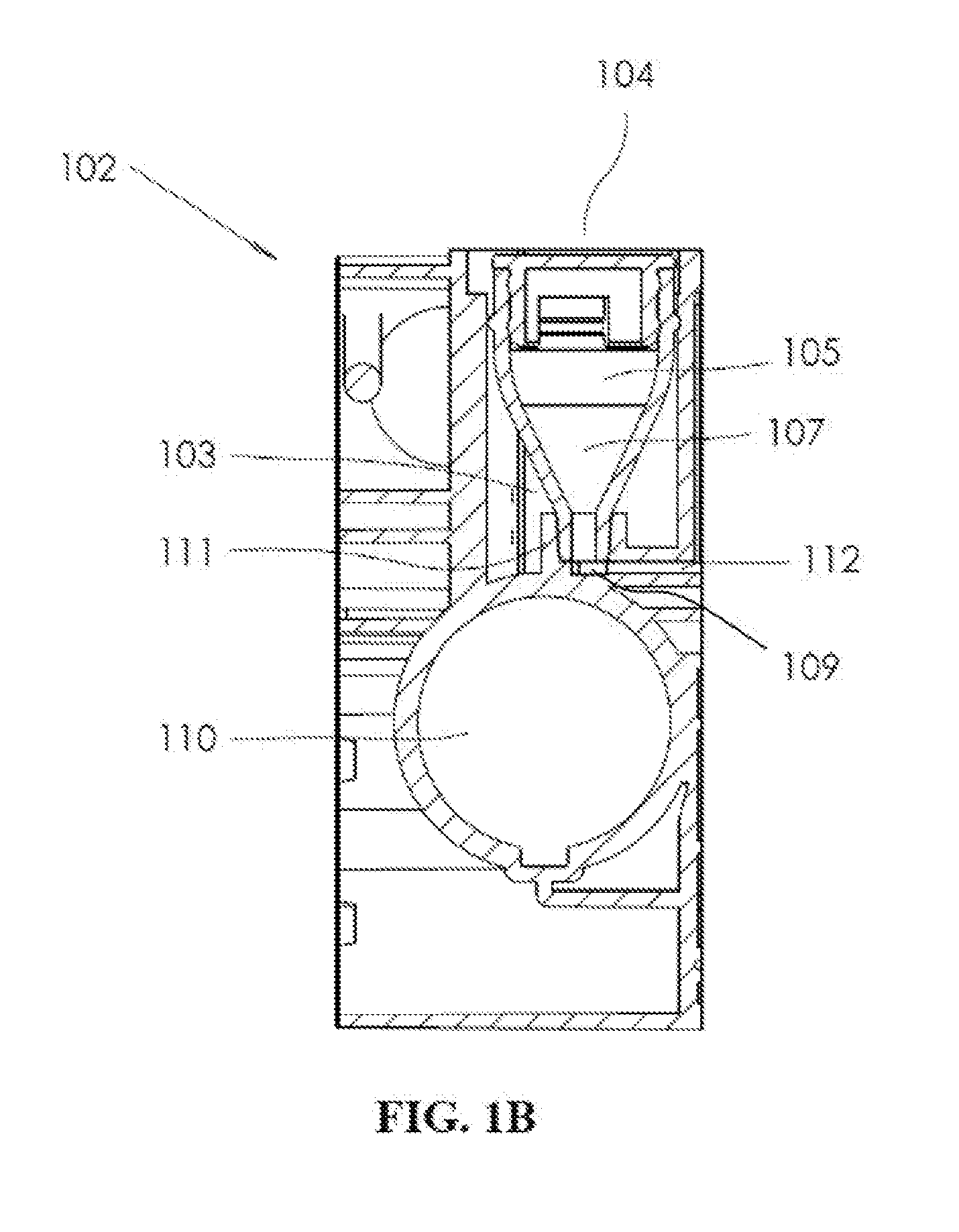
A microfluidic system is presented that includes a cartridge and a container. The cartridge includes a plurality of microfluidic channels coupled to one or more chambers. The container holds dry chemicals and includes a housing with a first opening and a second opening smaller than the first opening. The container is designed to be inserted into an opening of the cartridge, such that the container is independently secured within the opening. The insertion of the container allows for the container to be fluidically coupled with a microfluidic channel of the plurality of microfluidic channels via the second opening.
Owner:STAT DIAGNOSTICA & INNOVATION
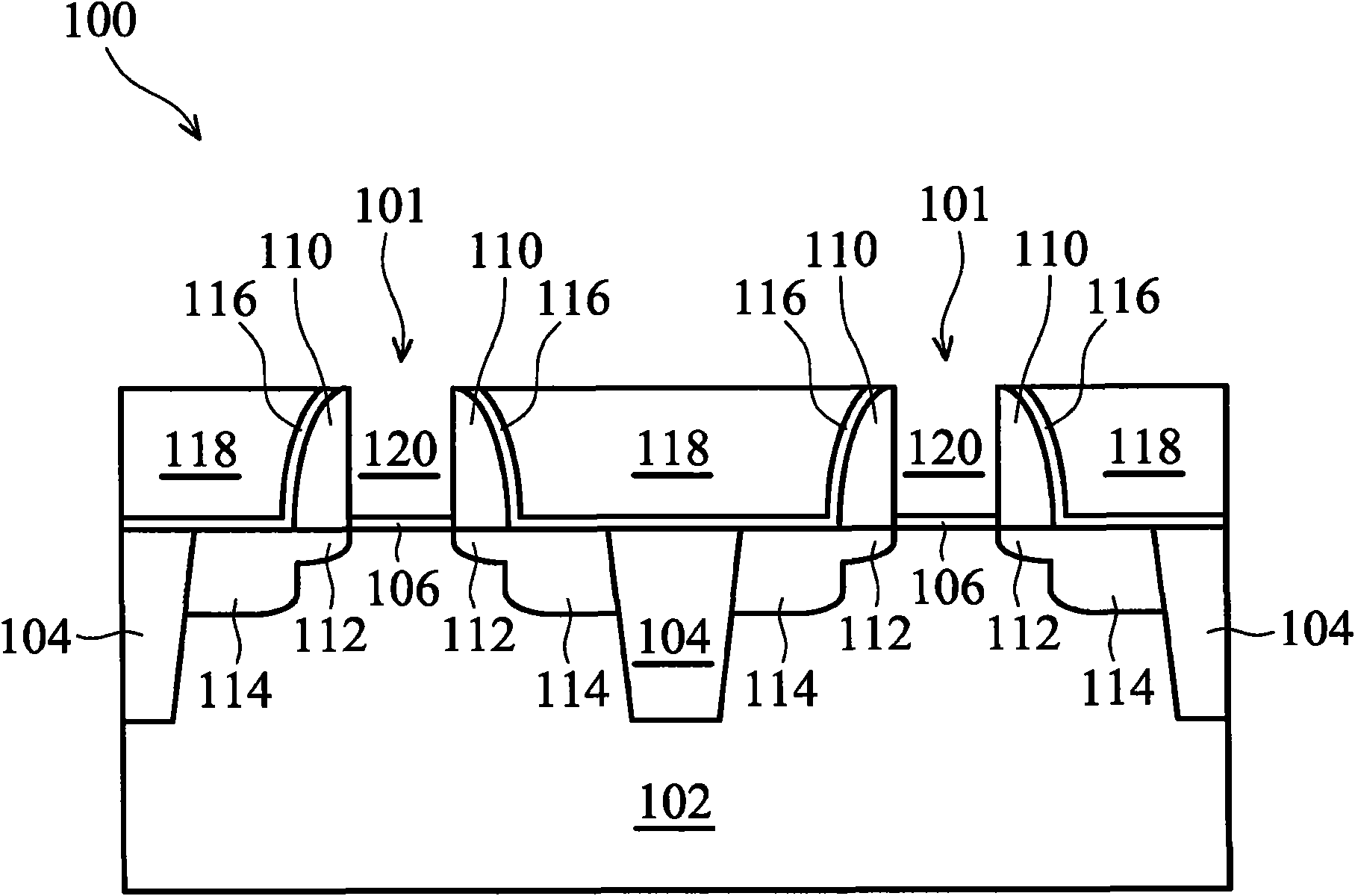
Self-limited metal recess for deep trench metal fill
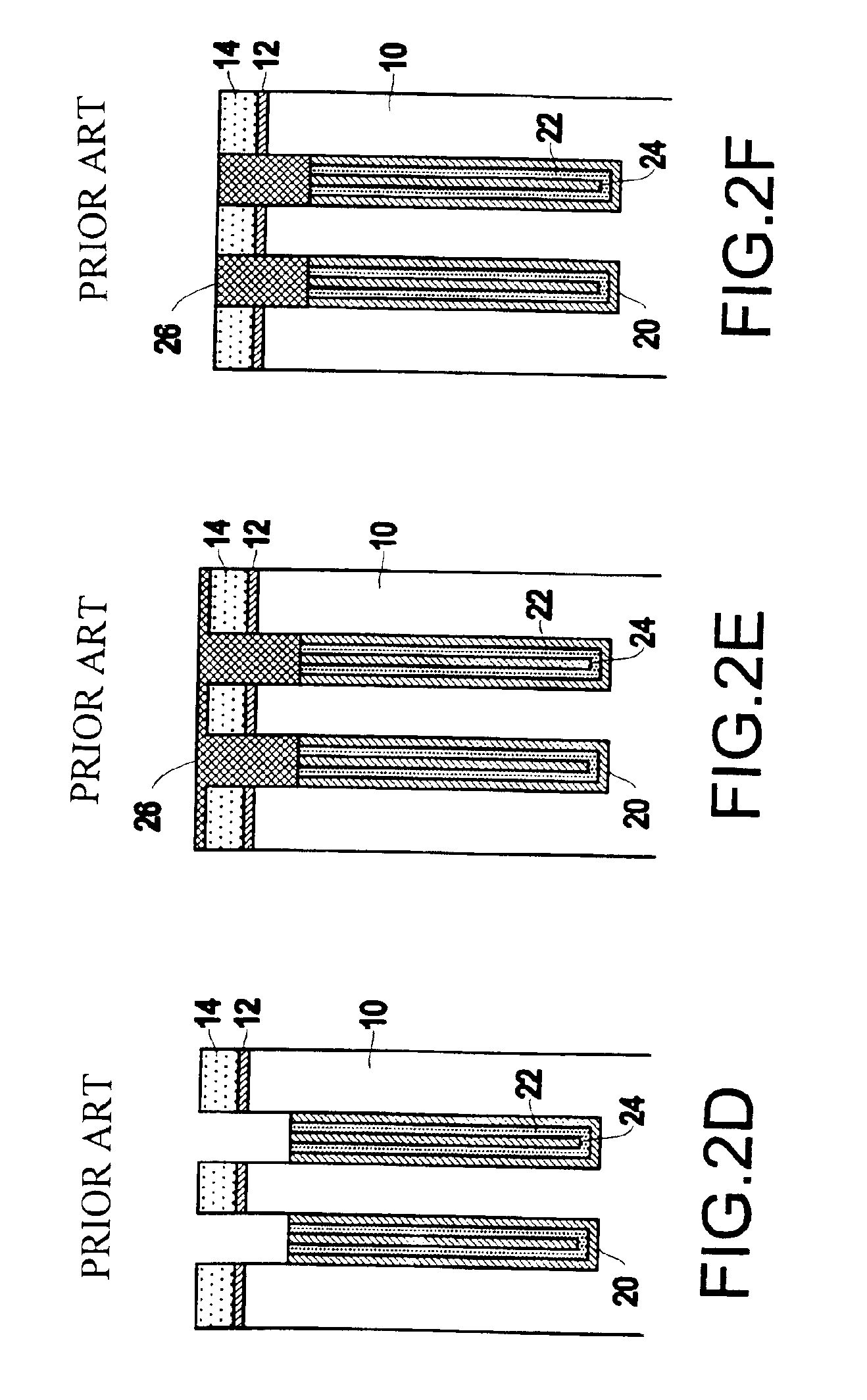
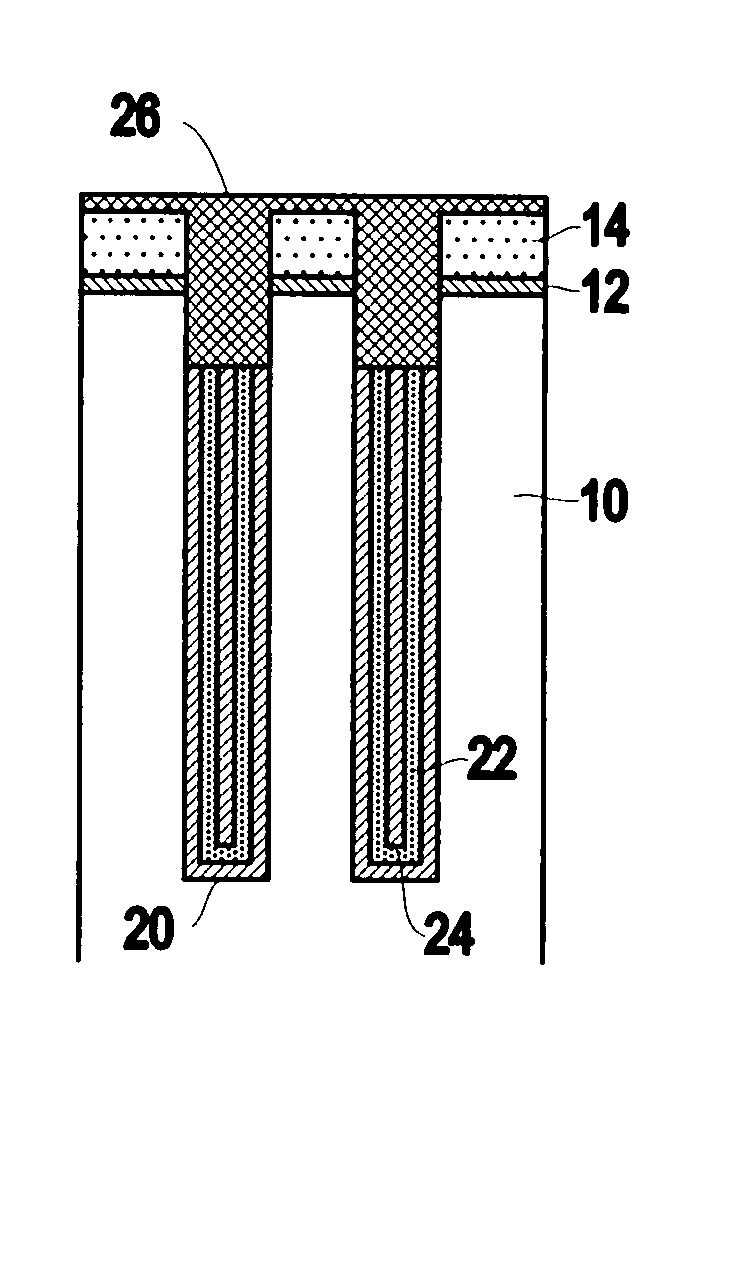
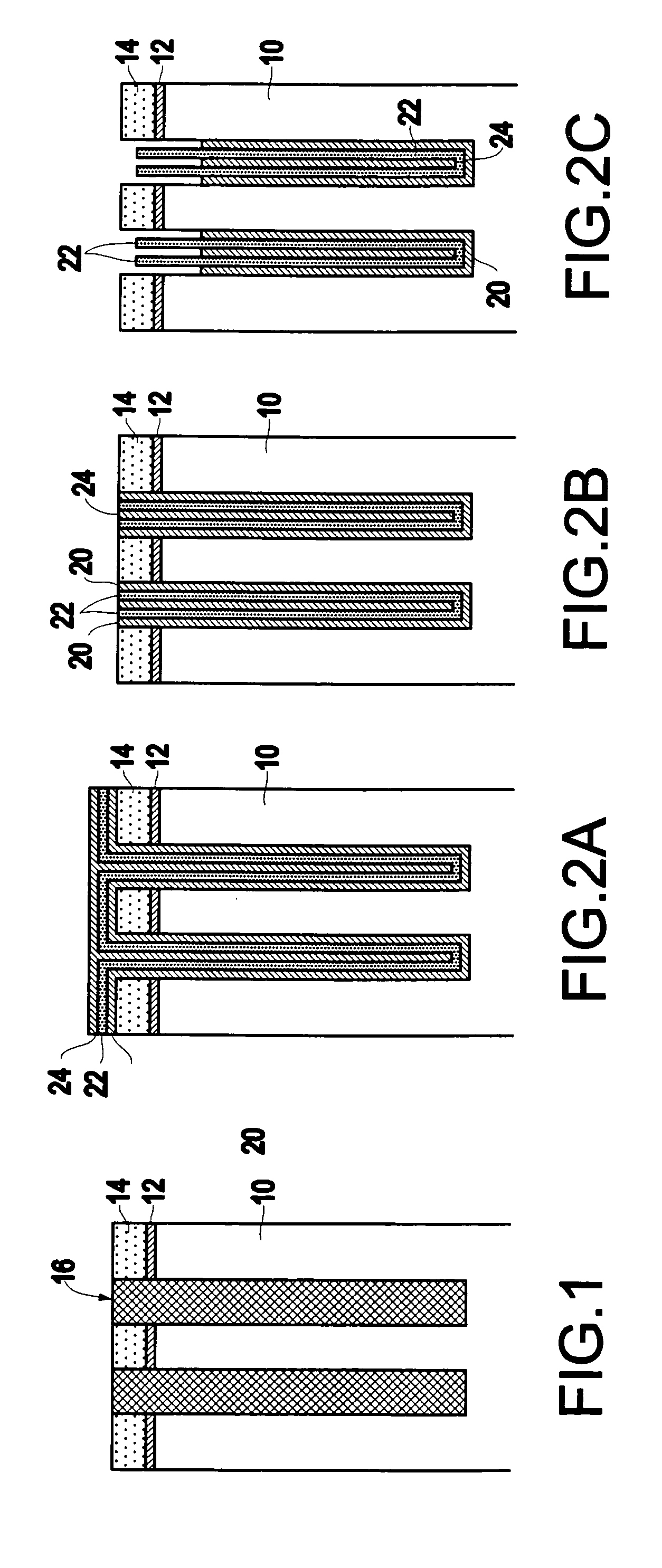
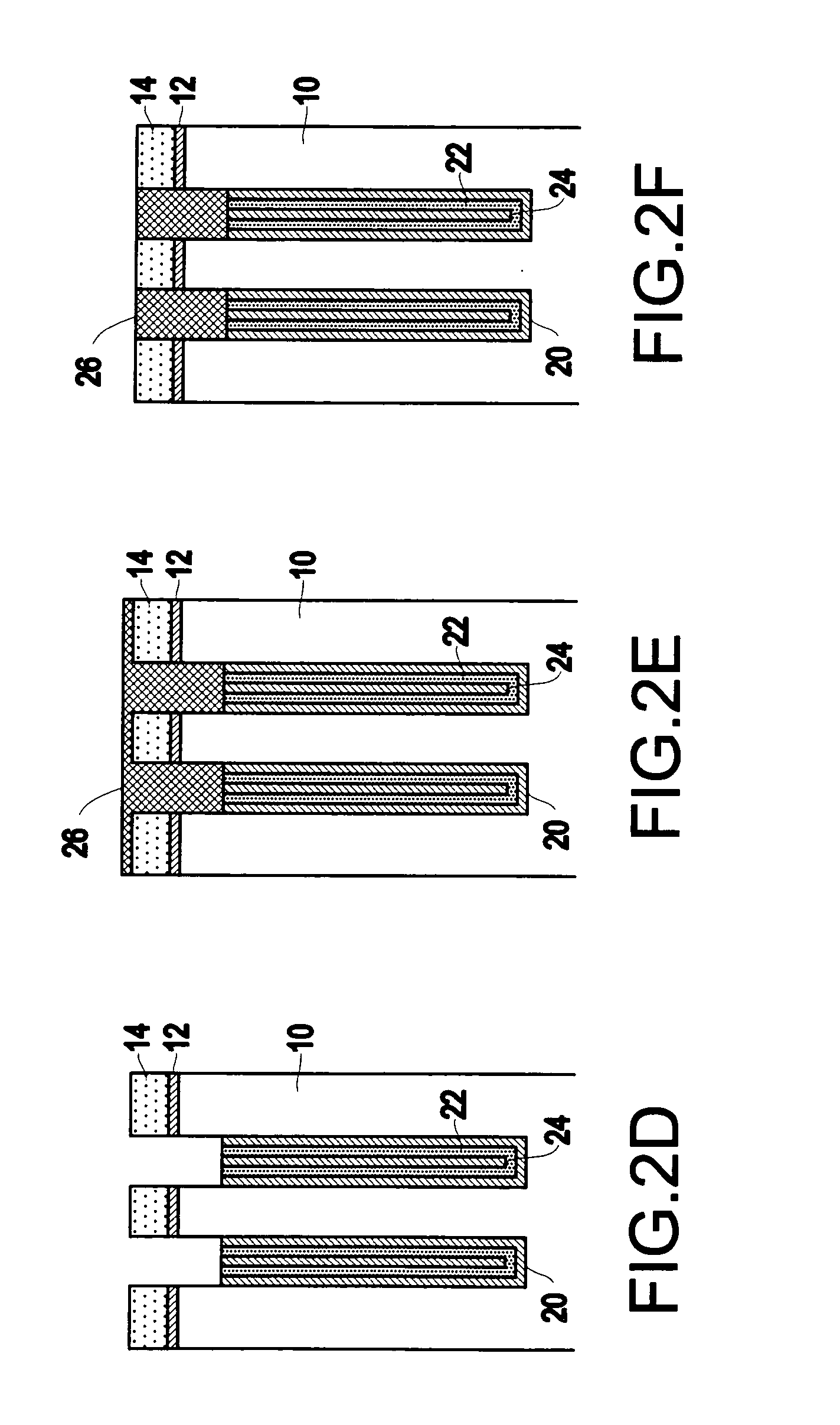
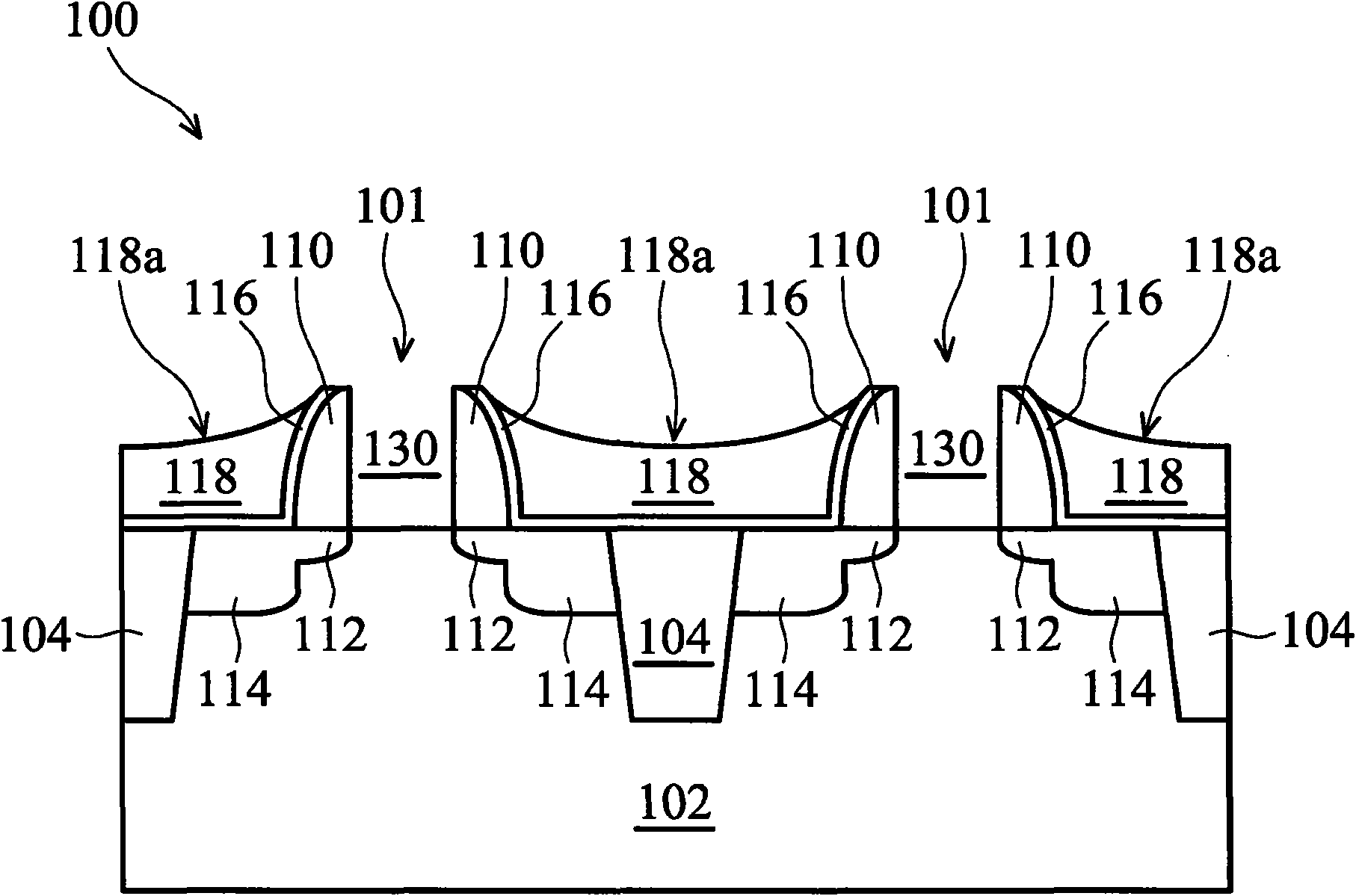
InactiveUS6953724B2Weaken energyIncrease etch rateSolid-state devicesSemiconductor/solid-state device manufacturingTitanium nitrideMetal
Disclosed is a method of manufacturing a deep trench capacitor structure that forms a trench in a substrate, lines the trench with a polysilicon liner, and forms titanium nitride columns along the polysilicon liner. The method etches the titanium nitride columns using chlorine-based dry chemistry that is substantially isotropic. This etching process removes the upper portion of the titanium nitride columns without affecting the polysilicon liner. The etching process attacks only in the uppermost portion of the titanium nitride columns such that, after the etching process is completed, the remaining lower portions of the titanium nitride columns are substantially unaffected by the etching process. Then, the method fills the space between the titanium nitride columns and the upper portion of the trench with additional polysilicon material. The process of filling the space simultaneously forms a polysilicon plug and polysilicon cap.
Owner:INT BUSINESS MASCH CORP
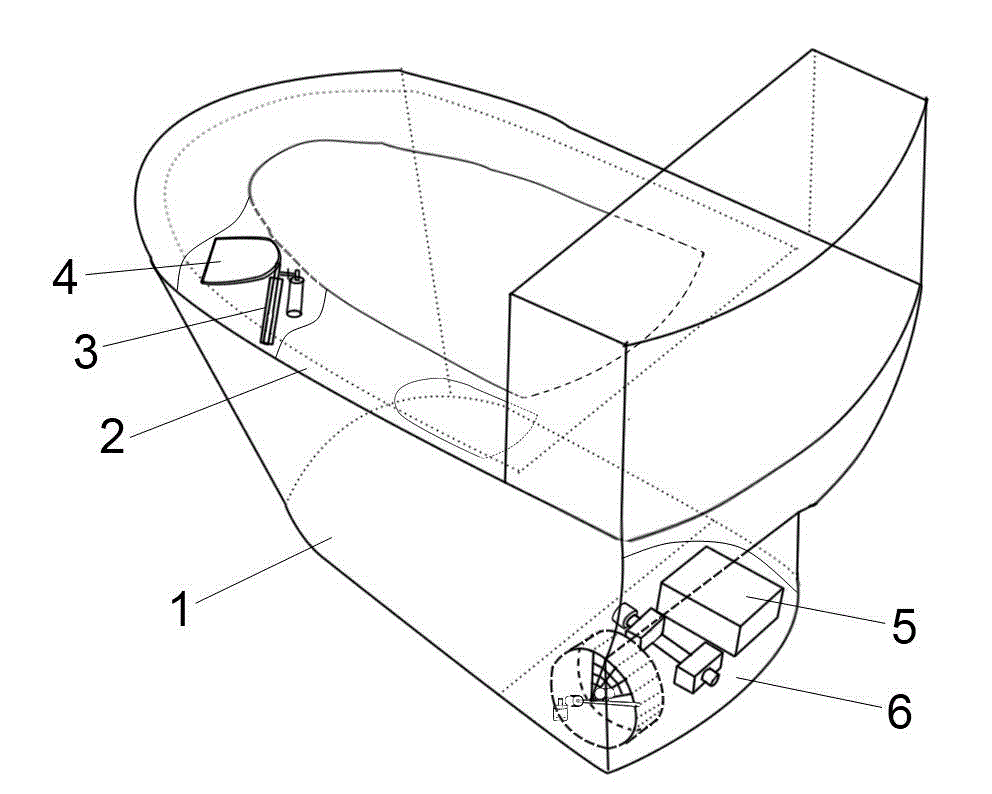
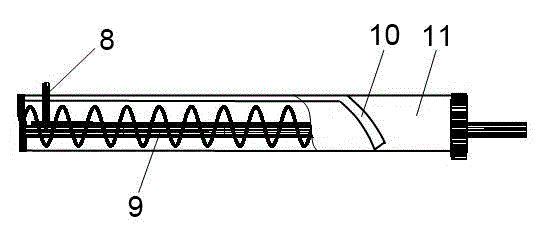
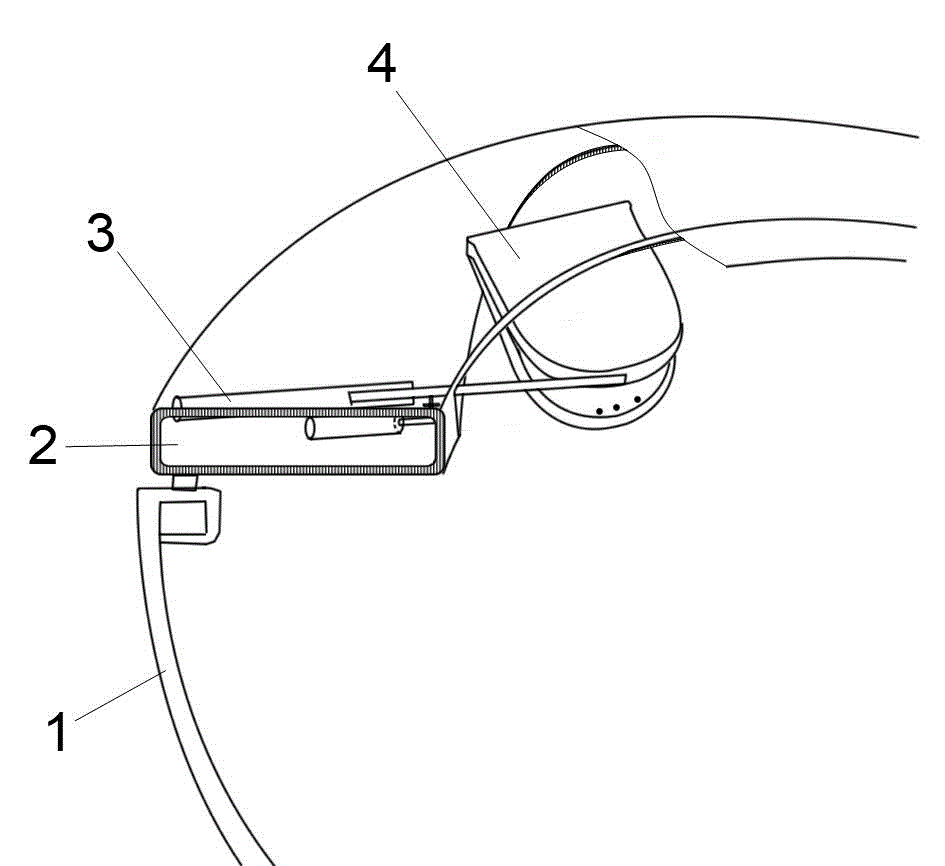
Dry chemistry analysis urine test toilet
InactiveCN103821213ARealize automatic controlReasonable functionLavatory sanitoryMaterial analysisAutomatic controlEngineering
The invention discloses a dry chemistry analysis urine test toilet and belongs to the technical field of sanitary wares. The technical problems that an existing technical structure is complex, and firmness and practicability are sufficient are solved. The dry chemistry analysis urine test toilet is characterized in that a urine collecting box is placed in a cavity in the front end of a toilet body and above the water level of a water seal. The urine collecting box is in a drawer shape, a panel of the drawer faces towards the inner side of the toilet body, and the shape of the drawer panel is in accordance with the arc-shaped obliquely erected curved surface of the inner side of the front end of the toilet body in a matched mode. A transmission lead screw is arranged below the urine collecting box, the arrangement direction of the transmission lead screw is in accordance with the outward extension or retraction direction of the urine collecting box, and the outward extension or retraction position of the urine collecting box is controlled by a step motor and is in linkage with the lead screw in the rotation direction. The dry chemistry analysis urine test toilet has the advantages of being simple in structure, convenient to use and better in firmness and practicability. The integrated concealed design is adopted, the whole process from urine collecting to urine test data output is automatically controlled by a microcomputer control unit, automation and domestication of urine test analysis are achieved, and the dry chemistry analysis urine test toilet has positive significance for health of all the people.
Owner:镡丰锦 +1
Toilet with automatic urine storage and dry chemistry urinalysis functions
InactiveCN102877526ARealize automatic controlSimple structureLavatory sanitoryMaterial analysisDiseaseUrinalysis
The invention provides a toilet with automatic urine storage and dry chemistry urinalysis functions and belongs to the technical field of sanitary ware. According to the toilet, urine of a person to be tested is automatically connected with a urine source of a packaging for inspection device. The toilet is characterized in that a cavity is arranged in front of a cushion ring, and an automatic urine storage device capable of extending outside and retracting is arranged in the cavity; the automatic urine storage device comprises a urine storage cup, an expansion link connected with the urine storage cup and an expansion transmission and turnover mechanism; a urine guide port is arranged at the bottom end of the urine storage cup and connected with an inlet of the urine source of the test paper strip batch packaging for inspection device. The toilet has the advantages that the toilet is combined with the automatic urine storage and dry chemistry urinalysis functions, simple in structure, convenient to use, and completely controlled by a control unit automatically from collecting of urine to be tested to urine analysis output; domestic self-urinalysis is facilitated, the positive significance for screening and preventing diseases and monitoring changes of physical functions by people is provided.
Owner:镡丰锦 +1
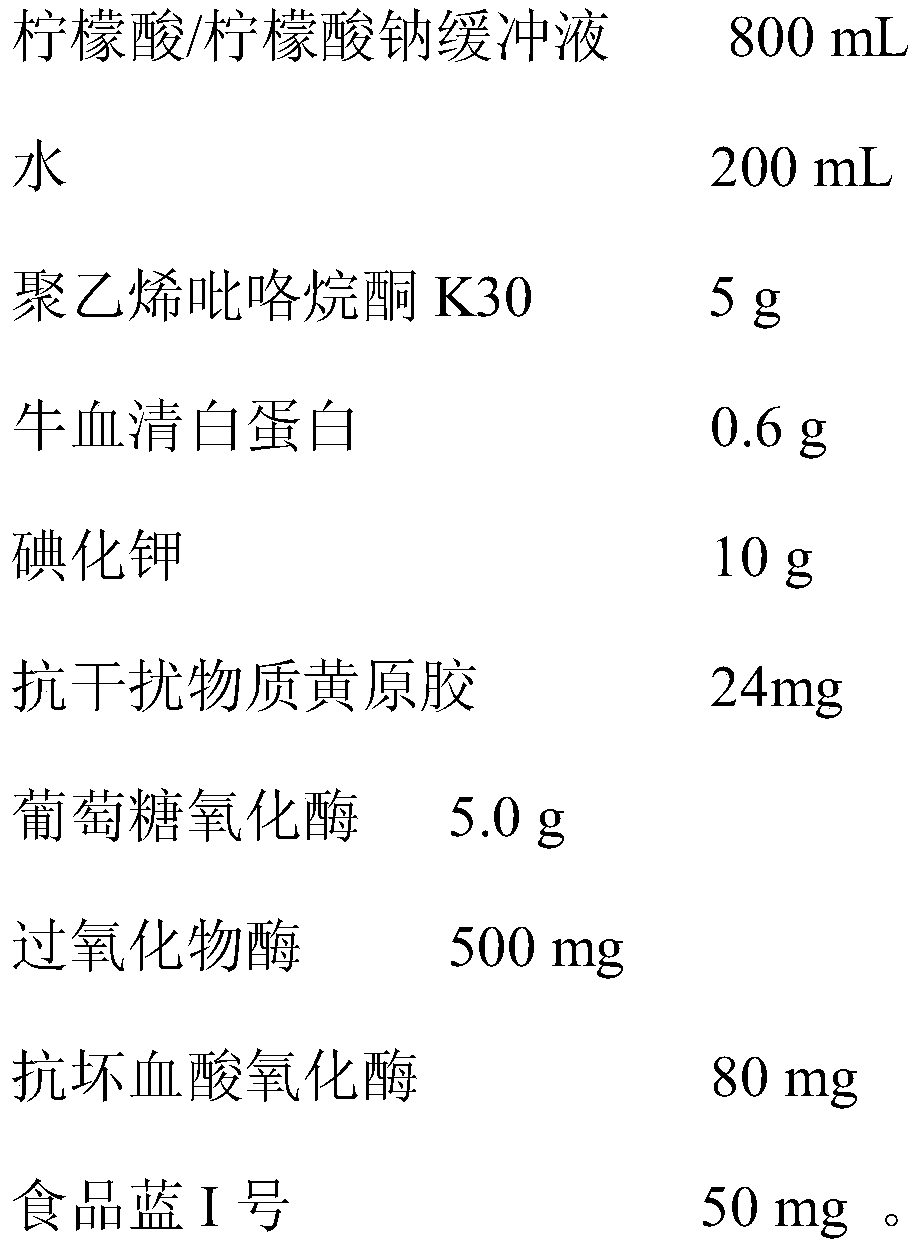
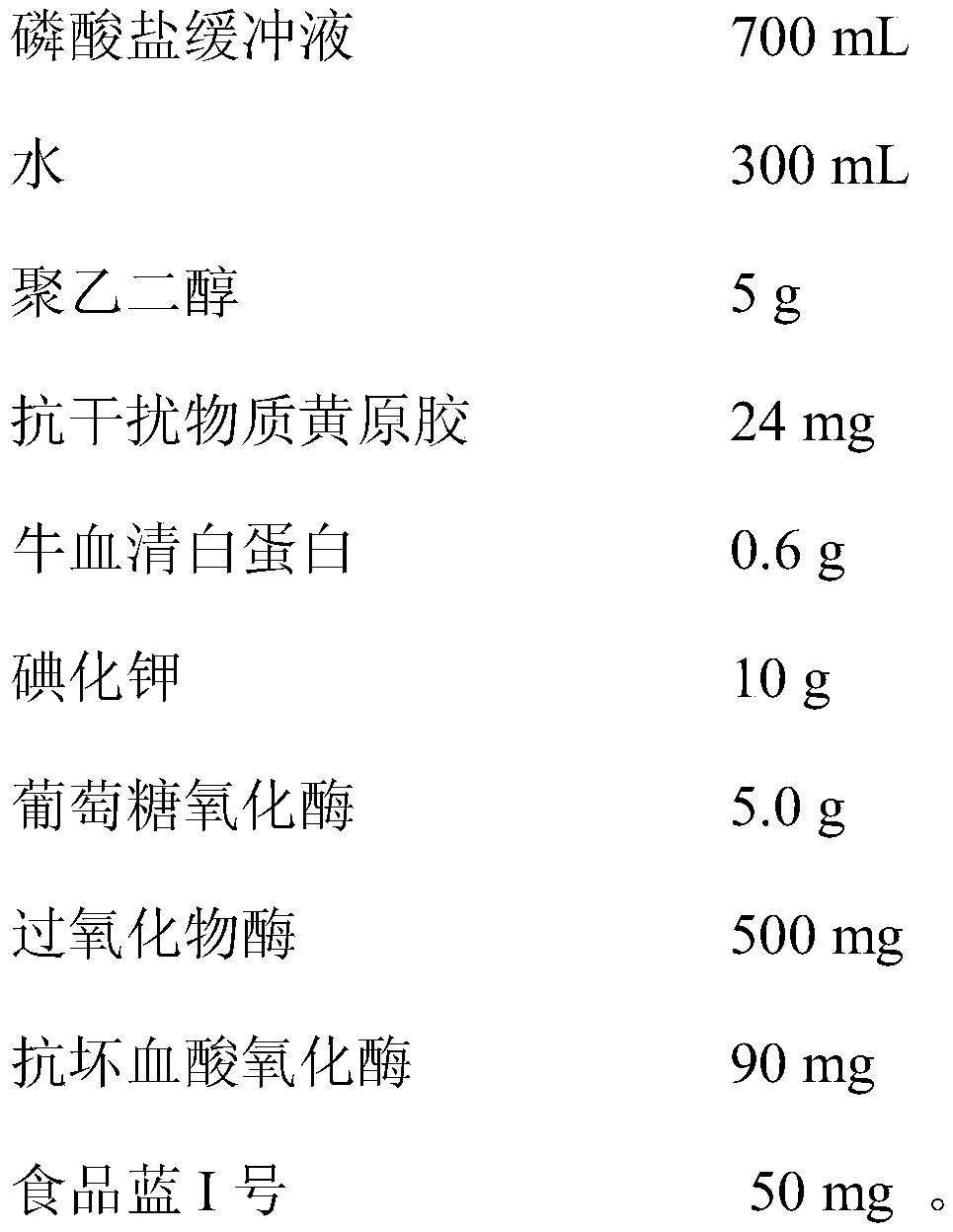
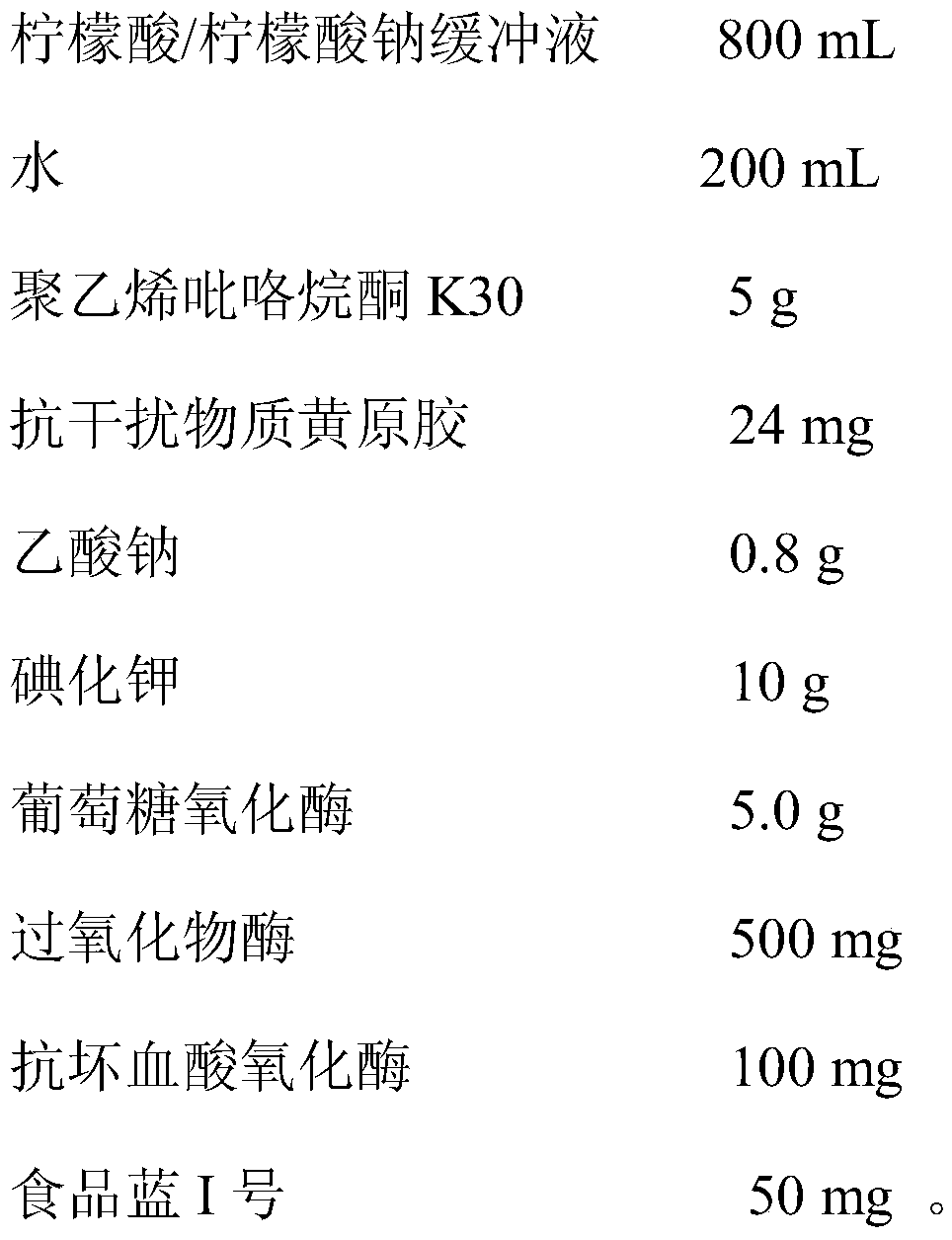
Urine glucose test paper capable of resisting ascorbic acid interference and preparation method thereof
InactiveCN109856128AImprove anti-VC interference abilityHigh sensitivityMaterial analysis by observing effect on chemical indicatorPeroxidasePotassium iodine
The invention belongs to the field of urine analysis and detection, relates to urine glucose test paper capable of resisting ascorbic acid interference and a preparation method thereof and solves thetechnical problem of existence of ascorbic acid interference in the detection of urine glucose in an existing dry chemistry test paper. The urine glucose test paper capable of resisting ascorbic acidinterference is formed by a substrate and filter paper fixedly arranged on the substrate. The test paper is obtained by being immersed into immersion liquid consisting of a buffer solution, glucose oxidase, peroxidase, potassium iodide, ascorbic acid oxidase, anti-interference substance xanthan gum, a surfactant, an enzyme stabilizer and a blue dye substance, so that anti-VC interference capability of the glucose test paper with potassium iodide as the substrate can be significantly improved, and sensitivity and accuracy of the detection result are enhanced; and the glucose detection result isnot influenced when the content of the VC in the sample does not exceed 3.5mmol / L. The preparation method of the urine glucose test paper capable of resisting ascorbic acid interference is simple andeasy to operate, stable in performance and accurate in test result.
Owner:DIRUI MEDICAL TECH CO LTD
A kind of compound urinalysis quality control solution
ActiveCN106771112BGood real-time stabilityLow toxicityBiological testingAdditive ingredientWhite blood cell
Owner:DIRUI MEDICAL TECH CO LTD
Method for quantitatively analyzing dry chemical detection reagent through mobile terminal
InactiveCN106226291AEasy accessEasy to collectMaterial analysis by observing effect on chemical indicatorQualitative analysisAnalysis method
The invention relates to a mobile terminal analysis method of quantitatively processing detection results of a dry chemical detection reagent, and provides a method for quantitatively analyzing the dry chemical detection reagent through a mobile terminal. The method includes the steps that the mobile terminal is used for collecting and reading information from the dry chemical detection reagent and synchronizing the information to a cloud server; the cloud server receives the information and starts calculation and analysis, results obtained after analysis are synchronized to the mobile terminal, and therefore a user can know the detection results and meanings fast and accurately. The problems that an existing method can carry out qualitative analysis instead of quantitative analysis and is high in subjectivity and large in misjudgement rate, and the detection results can only be recorded manually and not prone to storage. By implementing the mobile terminal analysis method, the user can obtain the detection results and meanings fast, easily and conveniently.
Owner:江苏戴格诺思生物技术有限公司
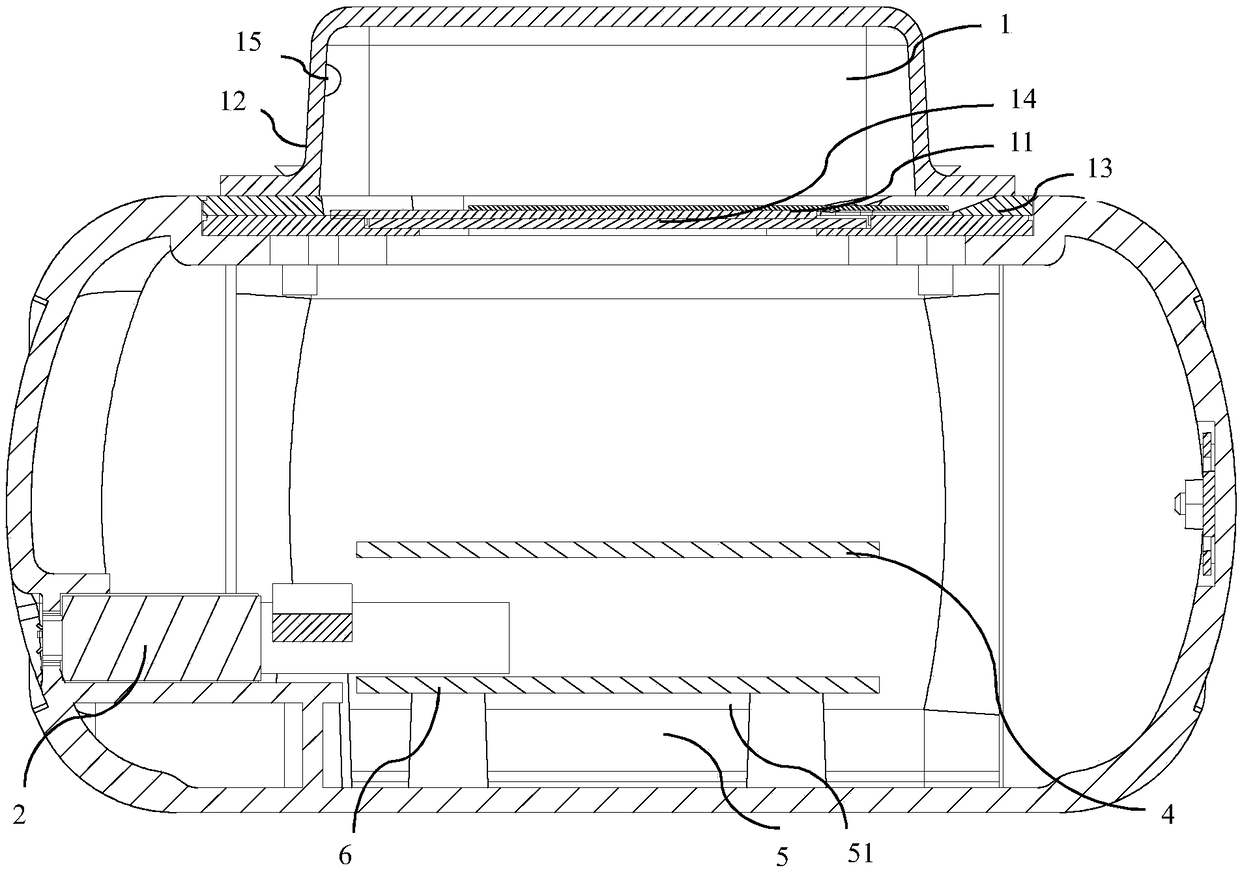
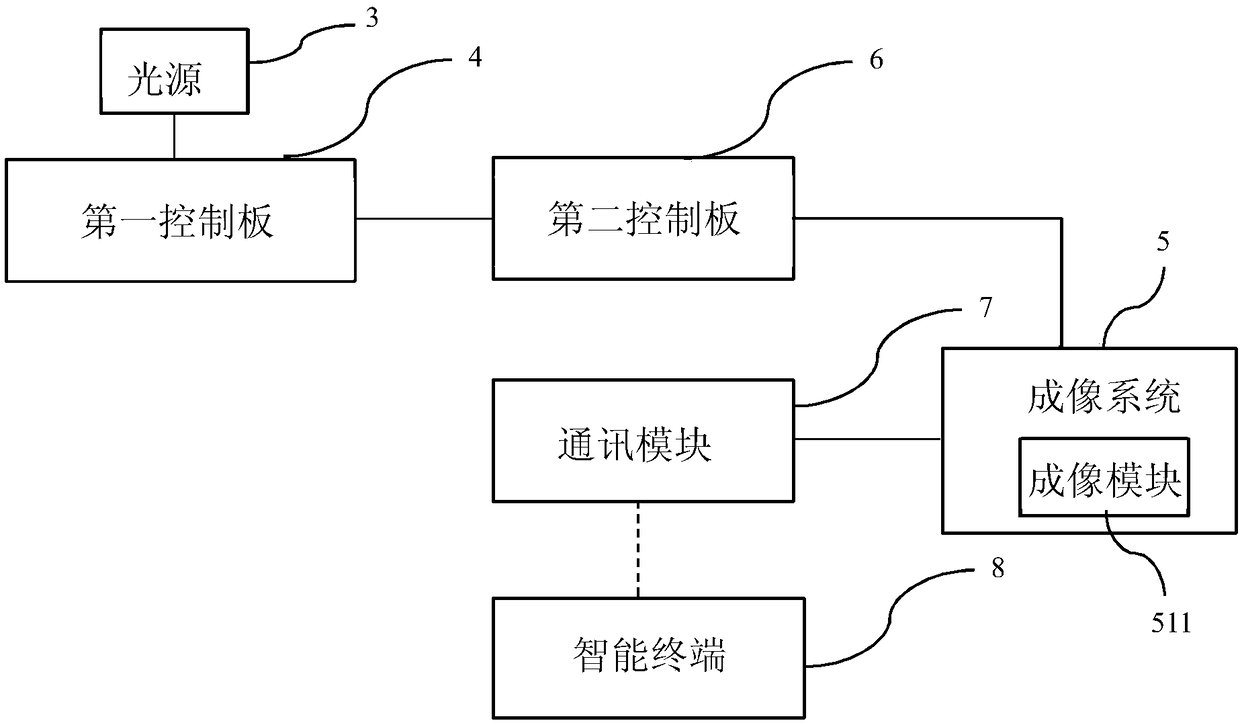
Multifunctional dry type POCT (Point-Of-Care Testing) device and detection method
PendingCN109100341ARealize automatic identificationRealize joint detectionMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescencePoint-of-care testingComputer module
The invention provides a multifunctional dry type POCT (Point-Of-Care Testing) device and detection method. The POCT device integrates dry chemistry and dry immunoassay; furthermore, the POCT device comprises a device body and an intelligent terminal; a detection bin, a power supply, a light source, a first control panel, an imaging system, a second control panel and a communication module are arranged in the device body; the light source is connected to the first control panel, and emits pre-set light to a to-be-detected object in the detection bin under the control of the first control panel; the second control panel is separately connected with the first control panel and the imaging system, and used for identifying the type of the to-be-detected object and performing corresponding detection according to the type of the to-be-detected object; the communication module is connected to the imaging system, and sends an imaging result to the intelligent terminal; and the imaging result is analyzed, so that a detection result is obtained. According to the multifunctional dry type POCT device and detection method provided in the invention, automatic identification of detection items and joint detection of multiple indexes can be realized through the light-adjustable light source and imaging system.
Owner:绿嘉图(北京)科技有限公司
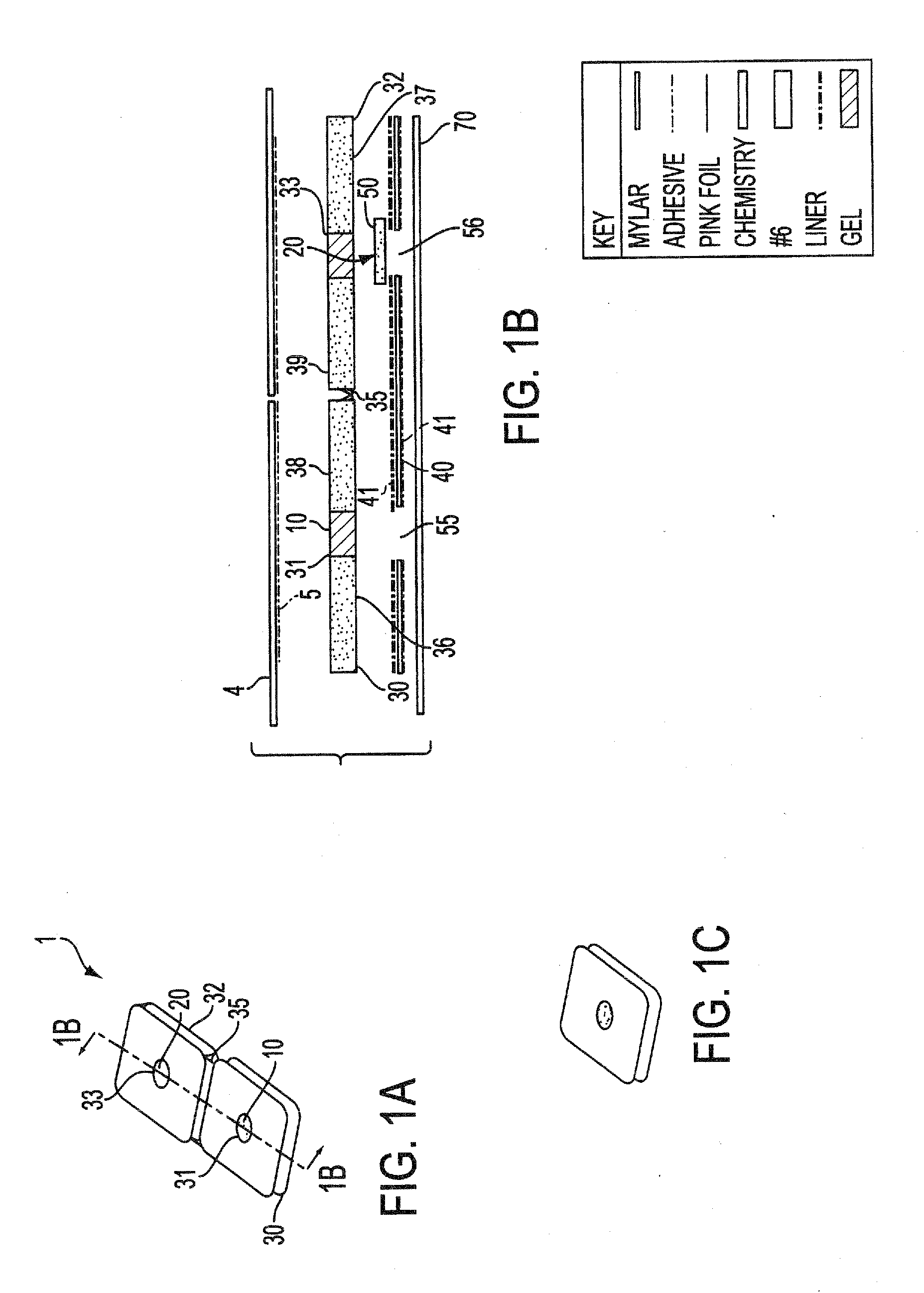
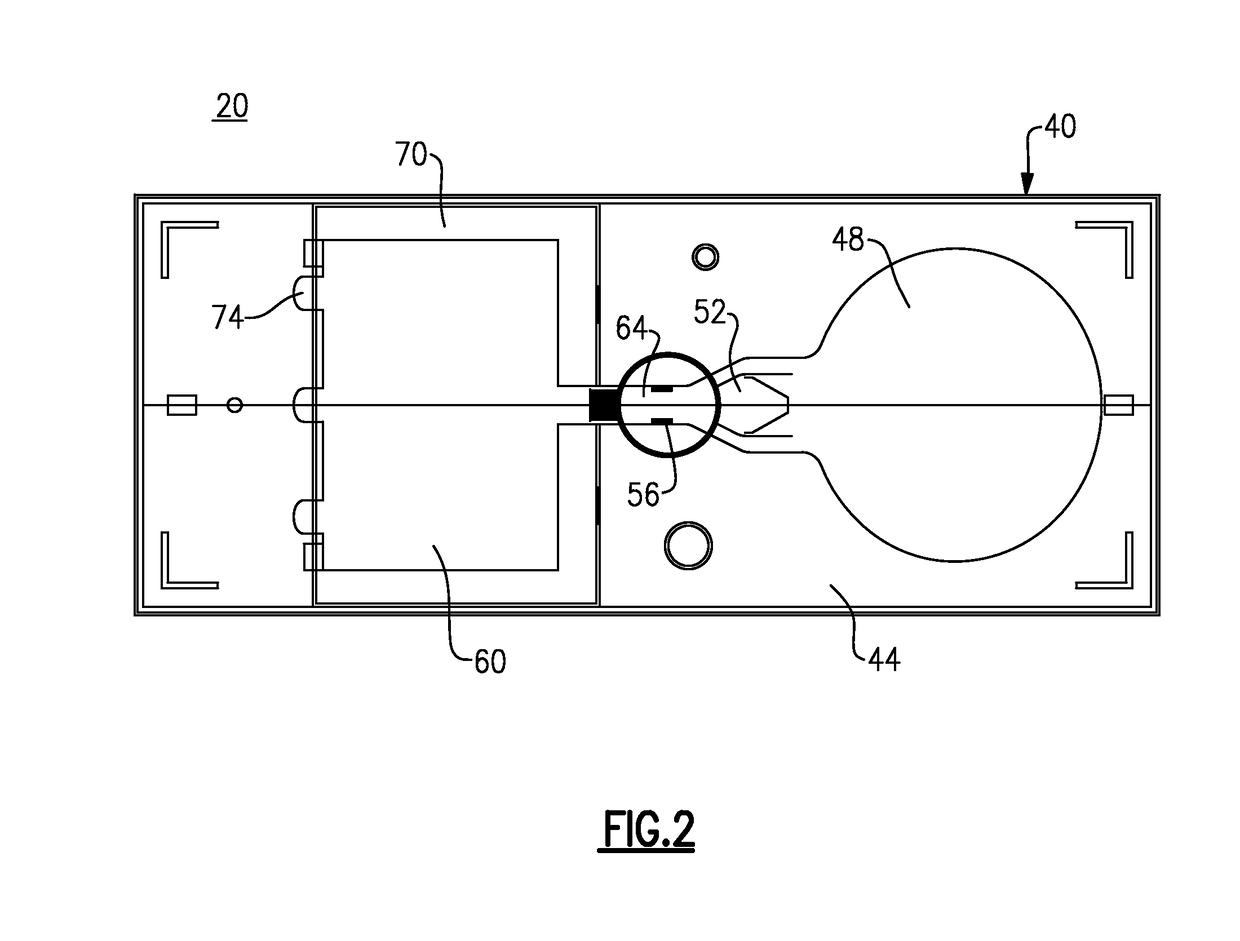
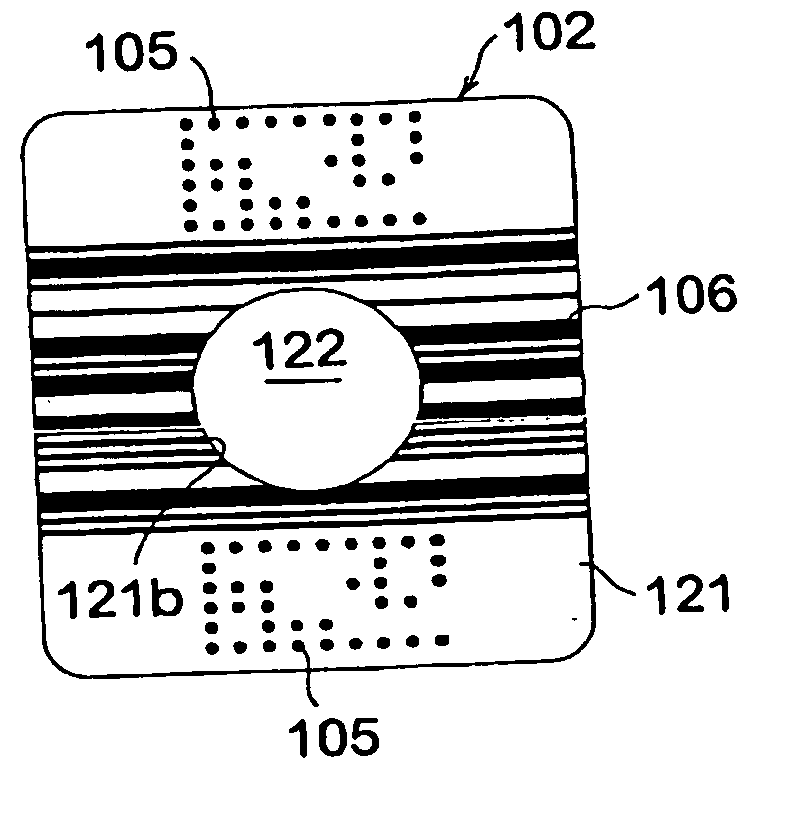
Immunoassay test slide
An immunoassay test slide for use in a dry chemistry analytical instrument includes a slide housing or case formed from two matable sections—a slide cover piece and a slide bottom piece. The slide housing defines an interior cavity in which is situated a sheet-like porous carrier matrix. The slide cover piece has an opening formed through the thickness thereof to expose a central portion of the fluid flow matrix so that a precise volume of fluid sample of blood, serum or the like, preferably pre-mixed with a conjugate reagent, and precise volumes of a wash reagent and a substrate (detector reagent), may be deposited on the matrix through the cover opening by a metering device of the analytical instrument. The bottom piece of the immunoassay test slide is transparent, and the slide is moved by a transport mechanism of the analytical instrument over a reflectometer or a fluorometer for performing reflectance or fluorescence measurements.
Owner:IDEXX LABORATORIES
Self-limited metal recess for deep trench metal fill
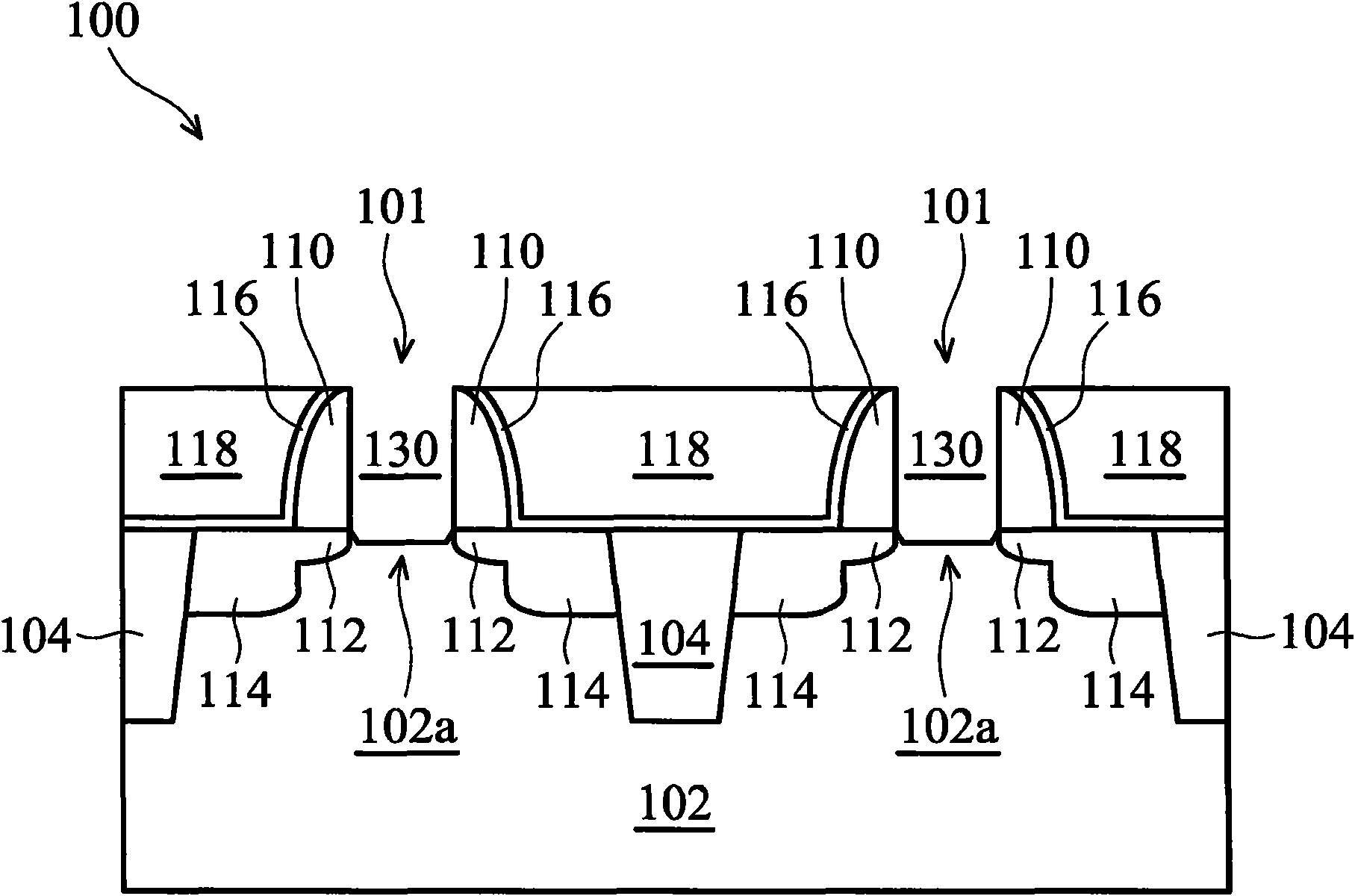
InactiveUS20050070064A1Weaken energyIncrease etch rateSolid-state devicesSemiconductor/solid-state device manufacturingTitanium nitrideMetal
Disclosed is a method of manufacturing a deep trench capacitor structure that forms a trench in a substrate, lines the trench with a polysilicon liner, and forms titanium nitride columns along the polysilicon liner. The method etches the titanium nitride columns using chlorine-based dry chemistry that is substantially isotropic. This etching process removes the upper portion of the titanium nitride columns without affecting the polysilicon liner. The etching process attacks only in the uppermost portion of the titanium nitride columns such that, after the etching process is completed, the remaining lower portions of the titanium nitride columns are substantially unaffected by the etching process. Then, the method fills the space between the titanium nitride columns and the upper portion of the trench with additional polysilicon material. The process of filling the space simultaneously forms a polysilicon plug and polysilicon cap.
Owner:IBM CORP
Point of care analytical processing system
ActiveUS10031085B2Efficient workflowQuick turnaround timeMaterial analysis by observing effect on chemical indicatorLaboratory glasswaresMultiplexingPoint of care
A point of care testing system includes a reader having an incubator disposed within a reader housing, the incubator having a rotor supported for rotation and having a plurality of circumferentially disposed slots. A drive mechanism is configured to rotate the rotor about a center axis A plurality of analytical test elements are sized for fitting in the slots of the incubator either manually or on demand. Each analytical test element commonly includes a support within a cartridge. The support retains at least one of a dry chemistry chip comprising a porous spreading layer disposed in stacked relation with at least one reagent layer or a lateral flow assay device wherein the plurality of test elements can assume a common form factor with multiplexed capability, and in which cartridges are preferably gated to enable random access processing.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Method for fabricating a gate structure
InactiveCN102044423ASolve known problemsTransistorSemiconductor/solid-state device manufacturingGate dielectricNitrogen
A method of fabricating the gate structure comprises: sequentially depositing and patterning a dummy oxide layer and a dummy gate electrode layer on a substrate; surrounding the dummy oxide layer and the dummy gate electrode layer with a nitrogen-containing dielectric layer and an interlayer dielectric layer; removing the dummy gate electrode layer; removing the dummy oxide layer by exposing a surface of the dummy oxide layer to a vapor mixture comprising NH3 and a fluorine-containing compound at a first temperature; heating the substrate to a second temperature to form an opening in the nitrogen-containing dielectric layer; depositing a gate dielectric; and depositing a gate electrode. The gate structure of the invention can be etched in the interlayer dielectric layer or the substrate without notches by dry chemistry.
Owner:TAIWAN SEMICON MFG CO LTD
Dry chemistry hydrogen peroxide detecting method based on heme hexapeptide
InactiveCN109507176AFast responseHigh sensitivityMaterial analysis by observing effect on chemical indicatorPolyesterMicrosphere
The invention discloses a dry chemistry hydrogen peroxide detecting method based on heme hexapeptide, and belongs to the technical field of the biochemical detection. The method comprises the following steps: spraying a heme hexapeptide mixture reagent layer on a baseplate polyester film of a detecting card, spraying a titanium dioxide diffusion layer on the reagent layer, wherein the reagent layer is a mixture of a polymer microsphere, the heme hexapeptide, 4-AAP-chlorophenol, phosphatebuffer solution, a surfactant and a water-soluble binder. The beneficial effect is that the dry chemistry H2O2 detecting method is provided by using a principle that DhHP-6 has a peroxidase function, and a quantity of H2O2 in a liquid sample to be detected is calculated through detecting a change of diffusereflection light after a reaction; the method has the characteristics of a rapid response rate, high sensitivity, a wide linearity range, a small system volume, easy carrying, and no external reagent; and the method can be extensively applied to the field of monitoring the H2O2 quantity in the liquid sample by medicine health and food production enterprises, agricultural trade markets, quality supervision and environment monitoring departments and the like.
Owner:CHANGCHUN UNIV OF SCI & TECH
Dry chemical method TORCH detection reagent kit and method of manufacture
The invention relates to a TORCH detection kit using a dry chemistry method and a preparation method of the kit. The detection kit comprises a detection unit consisting of a PVC strip for supporting a reaction system, a cellulose nitrate membrane reaction solution system for adsorbing reactants, a protein stabilizer and sodium azide; an enhancement solution containing sodium chloride and sodium azide; a binding solution containing alkaline phosphatase dissolved in a buffer solution and combined with goat anti human body, a protein stabilizer and sodium azide; and a developing solution containing a developing substrate dissolved in a buffer solution. The entire kit also includes a disposable tube for allowing the test strip to react therein and an anti-moisture drying agent. The kit of the invention has high detection speed, low detection cost, and no need for any auxiliary equipment as well as a great amount of expensive detection equipment in the detection process. The kit helps to develop rapid TORCH screening in various occasions in clinic units and family planning systems of various levels, thus promoting national health.
Owner:WUXI SHENRUI BIO PHARMA
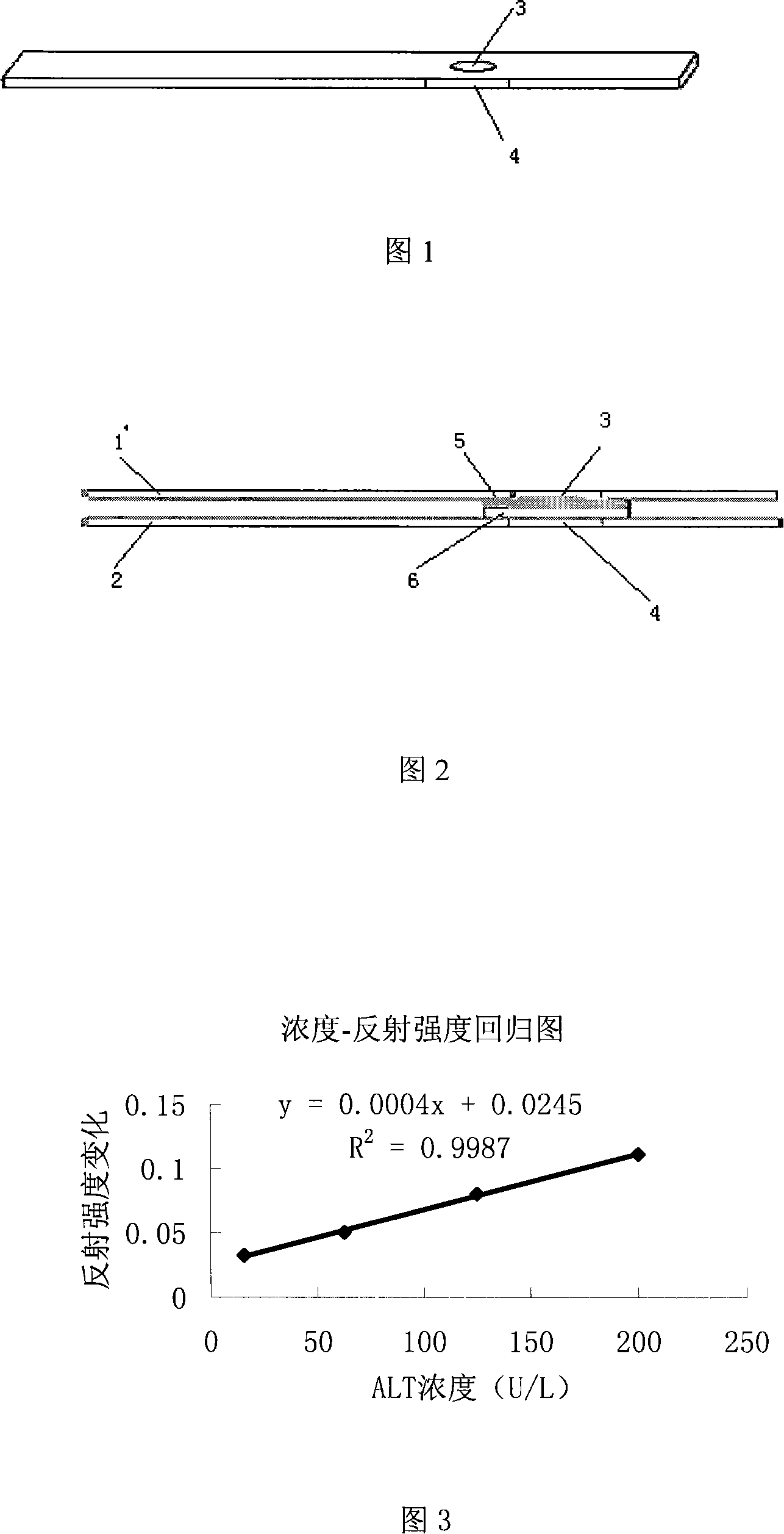
Dry chemistry test paper for quantitative determination of urea content in human blood
The invention belongs to the technical field of in-vitro clinical diagnostic reagent, in particular to a dry chemistry test paper for quantitative determination of urea content in human blood. The test paper comprises an upper, a middle and a lower support layers and testing layers thereof and is separated into a hand-held area and a test area. The test area is composed of a diffusion layer, a reagent layer and a color reagent layer from the top down. A loading hole is arranged on the upper support layer, a test sample is introduced into the diffusion layer from the loading hole to penetrate into the reagent layer, then enzymatic reaction is carried out on the reagent layer, ammonia is generated by catalyzing urea by using urease, and under the alkaline environment, the generated ammonia spreads to the color reagent layer to perform color reaction with the indicator of ammonia. The variation of optical density can be measured with a reflectance spectrophotometer through a test hole onthe lower support layer. The test paper can be used to quantitatively test the urea content in human blood, provides basis for the diagnosis of renal function, and is suitable to be used in emergencyrooms of the hospitals.
Owner:SHANGHAI KEHUA BIO ENG
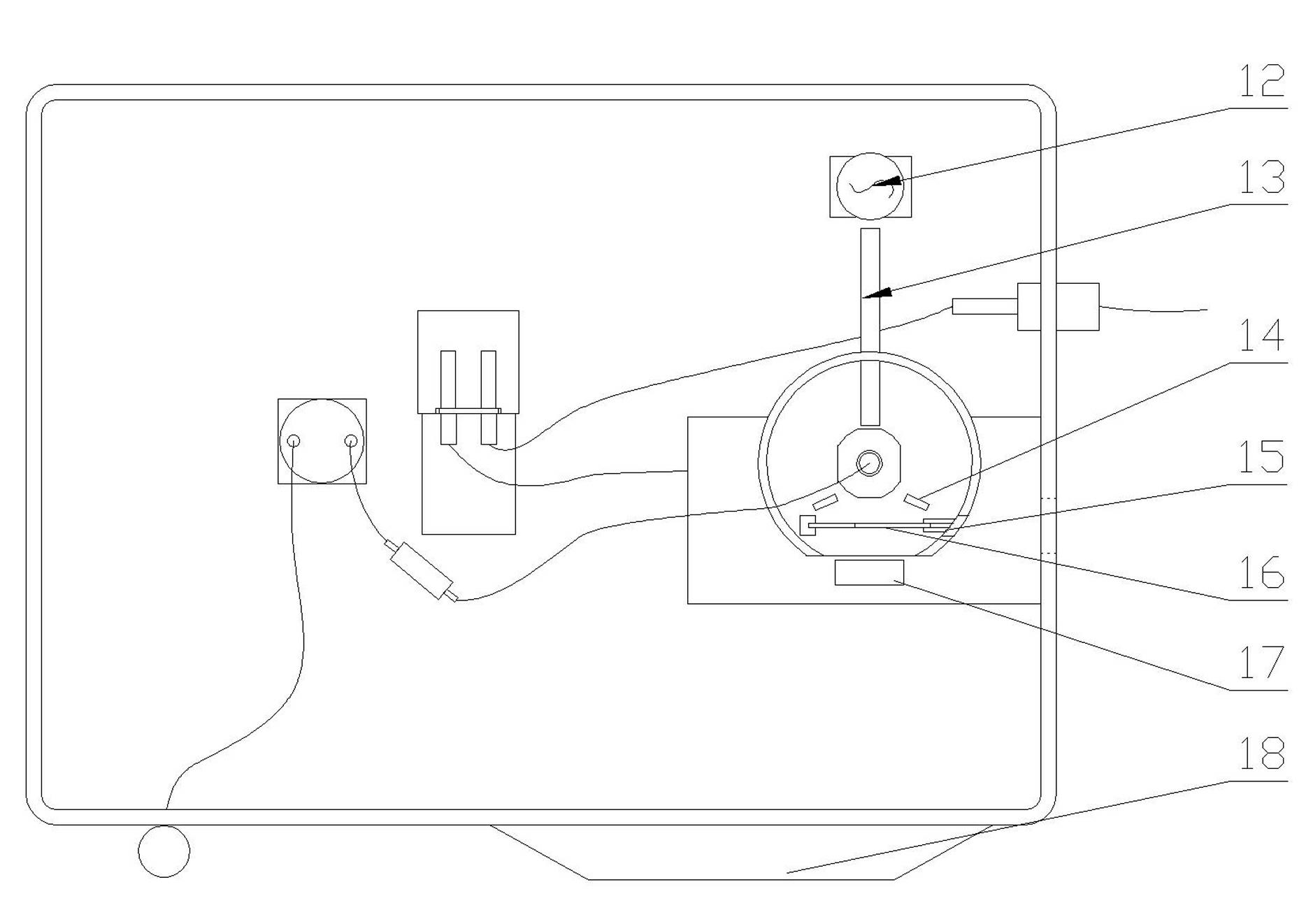
A conventional whole blood transaminase testing instrument
InactiveCN102279180AReduce usageLong storage timeMaterial analysis by observing effect on chemical indicatorEngineeringInstrumentation
The invention discloses a conventional whole blood transaminase testing instrument. The invention comprises a casing, a temperature control incubator, a cuvette, a test strip bayonet, a light source, a transmitted light photoelectric connector and a reflected light photoelectric receiver arranged in the casing. The cuvette, cuvette and test strip bayonet are set in the shell of the temperature-controlled incubator, the cuvette is connected to the liquid delivery system to be tested and the waste liquid discharge system, and the transmitted light photoelectric connector is used to receive the light from the light source and pass through the cuvette After the transmitted light, the reflected light photoelectric receiver is used to receive the reflected light after the light from the light source is transmitted through the cuvette and then reflected by the test strip in the test strip bayonet. The invention not only has the advantages of high precision of dry chemical detection, fast test speed, less sample volume, saving reagents, and long storage time of reagents, but also has the advantages of low cost of wet chemical detection and low test interference. At present, the defects in the dry and wet biochemistry of the biochemical instrument are complemented.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
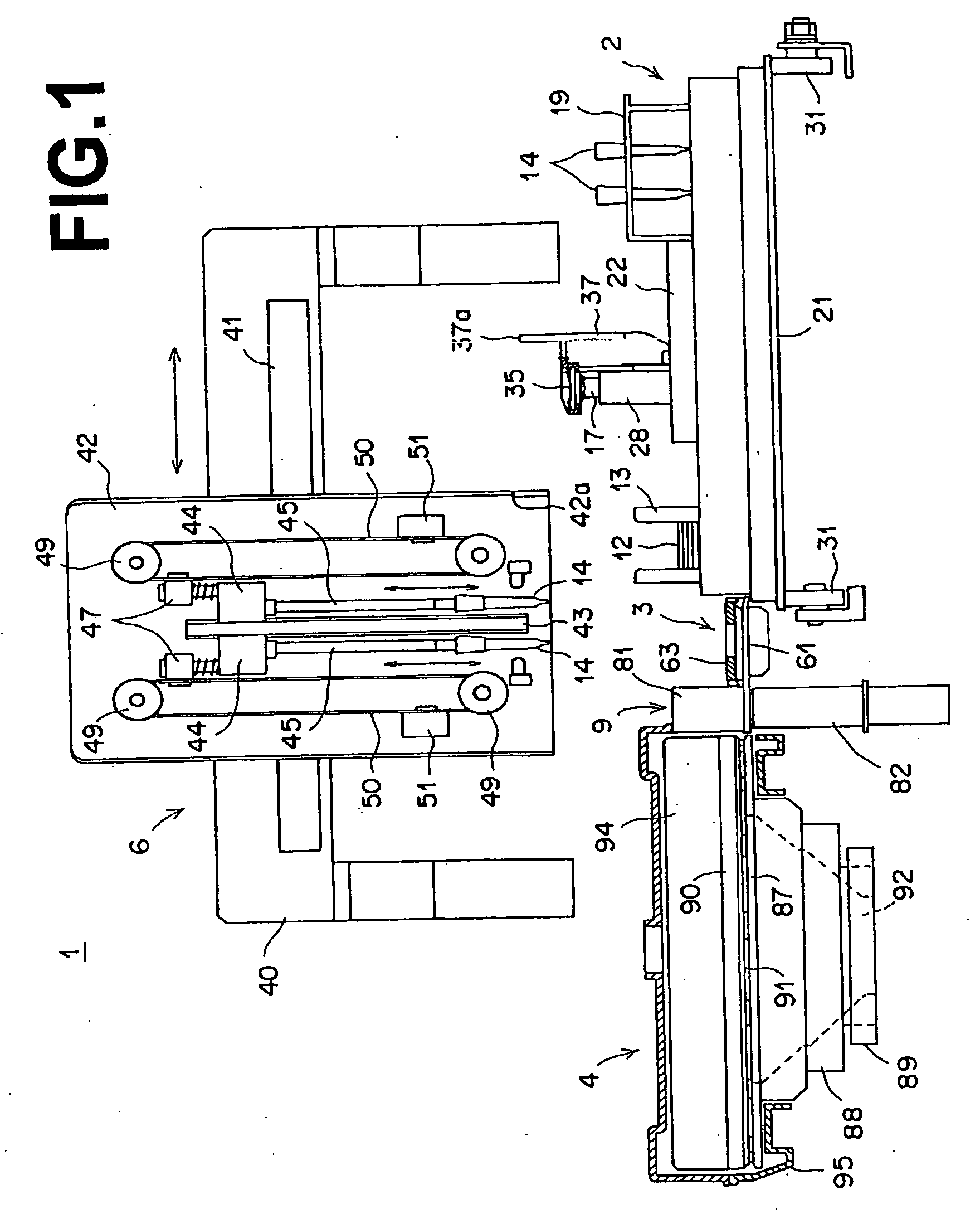
Biochemical analysis method and apparatus
InactiveUS20070041868A1Efficient executionSequence control being simplifiedAnalysis using chemical indicatorsCharacter and pattern recognitionCompound (substance)Engineering
Samples and dry chemical analysis elements, which are necessary for analyses of the samples, are loaded on a sample tray. Each sample is sucked with a spotting nozzle of a spotting unit and spotted onto one dry chemical analysis element. Analysis information, which contains information representing a type of analysis, is appended to each dry chemical analysis element. The analysis information is read with a reading device located such that, when a certain sample is located at a position for sample suction by an operation of the sample tray, the reading device reads the analysis information, which has been appended to a next dry chemical analysis element to be used for the analysis of the certain sample, at a position at which the next dry chemical analysis element is located.
Owner:FUJIFILM CORP
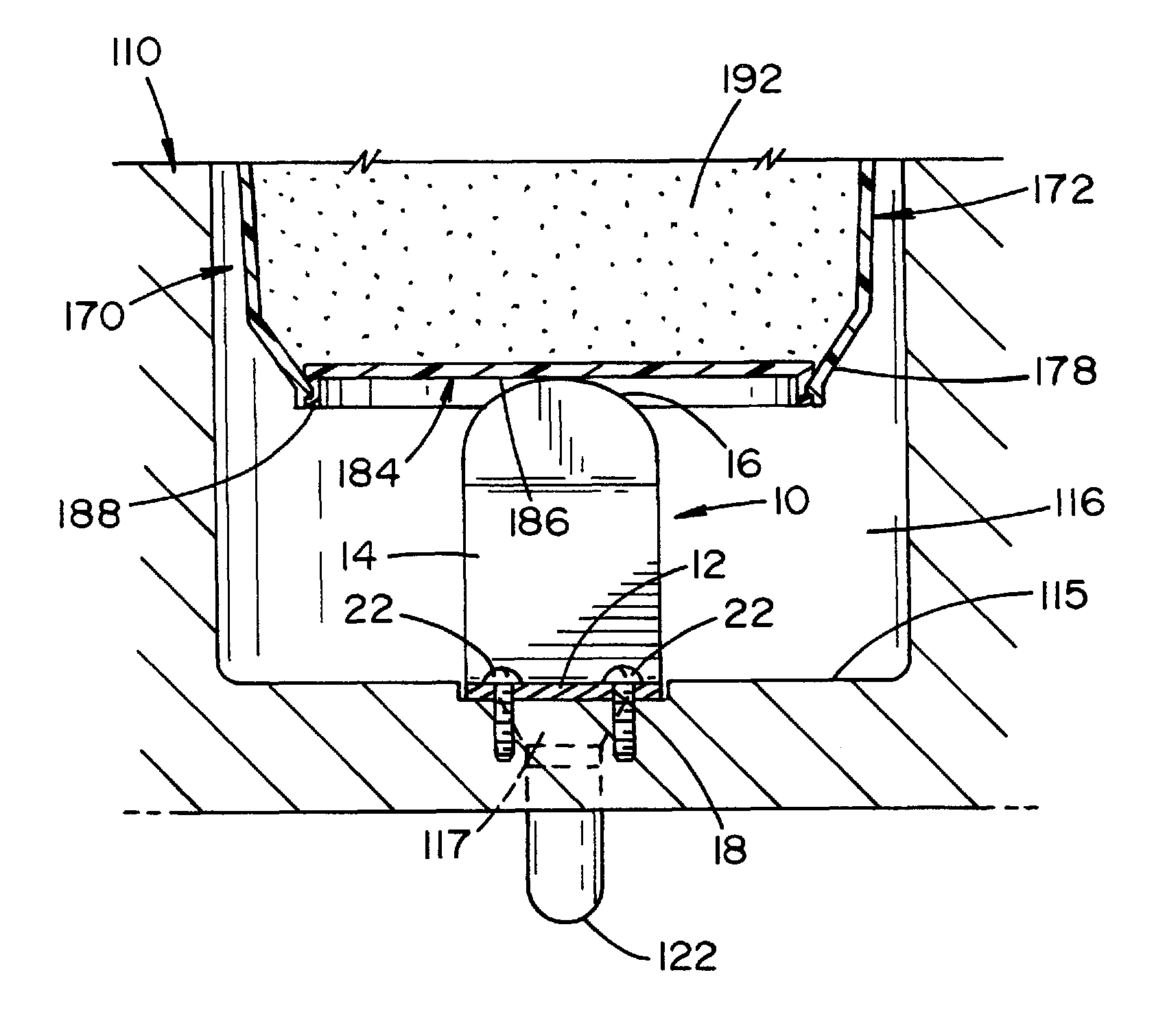
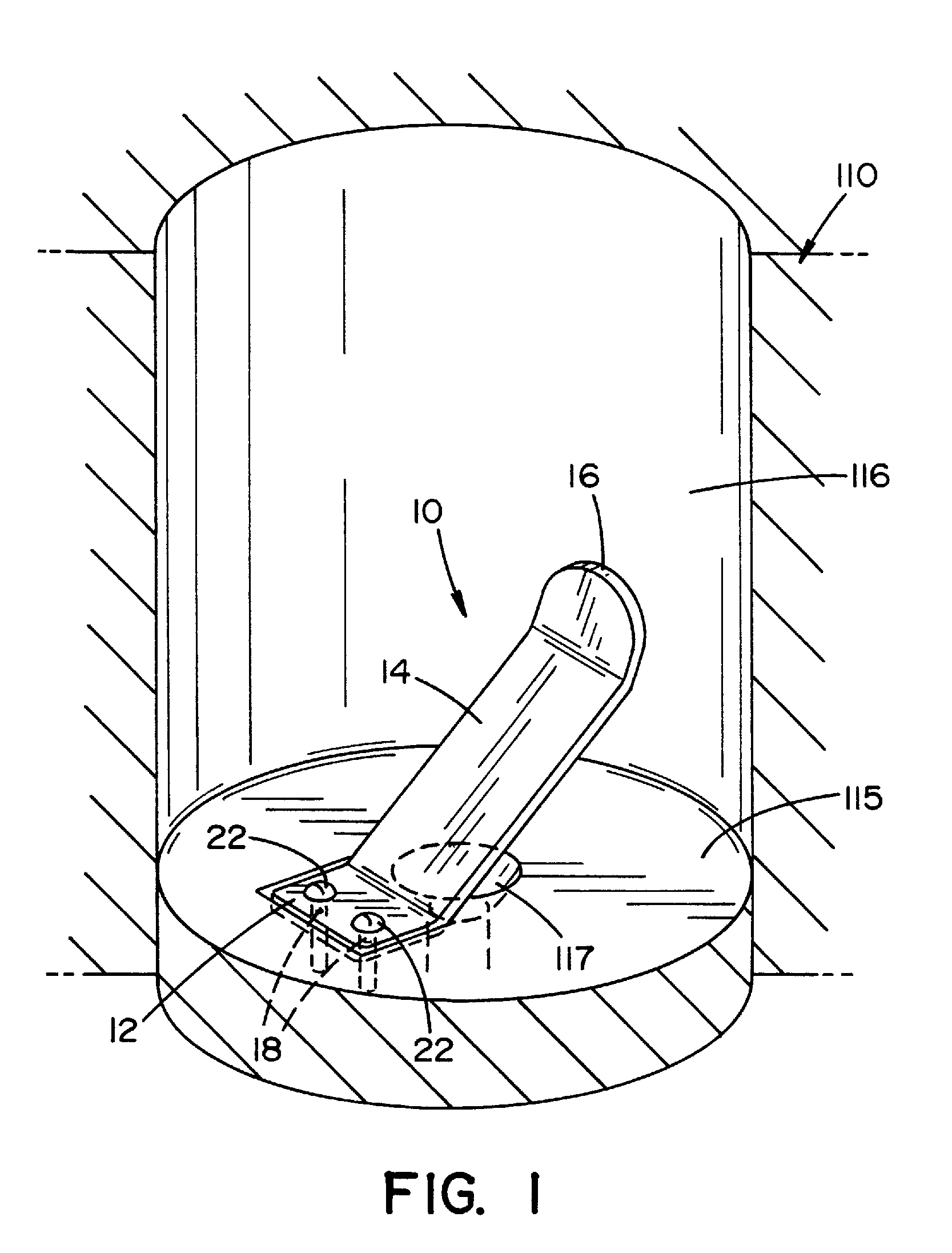
Apparatus for releasing a dry chemistry into a liquid sterilization system
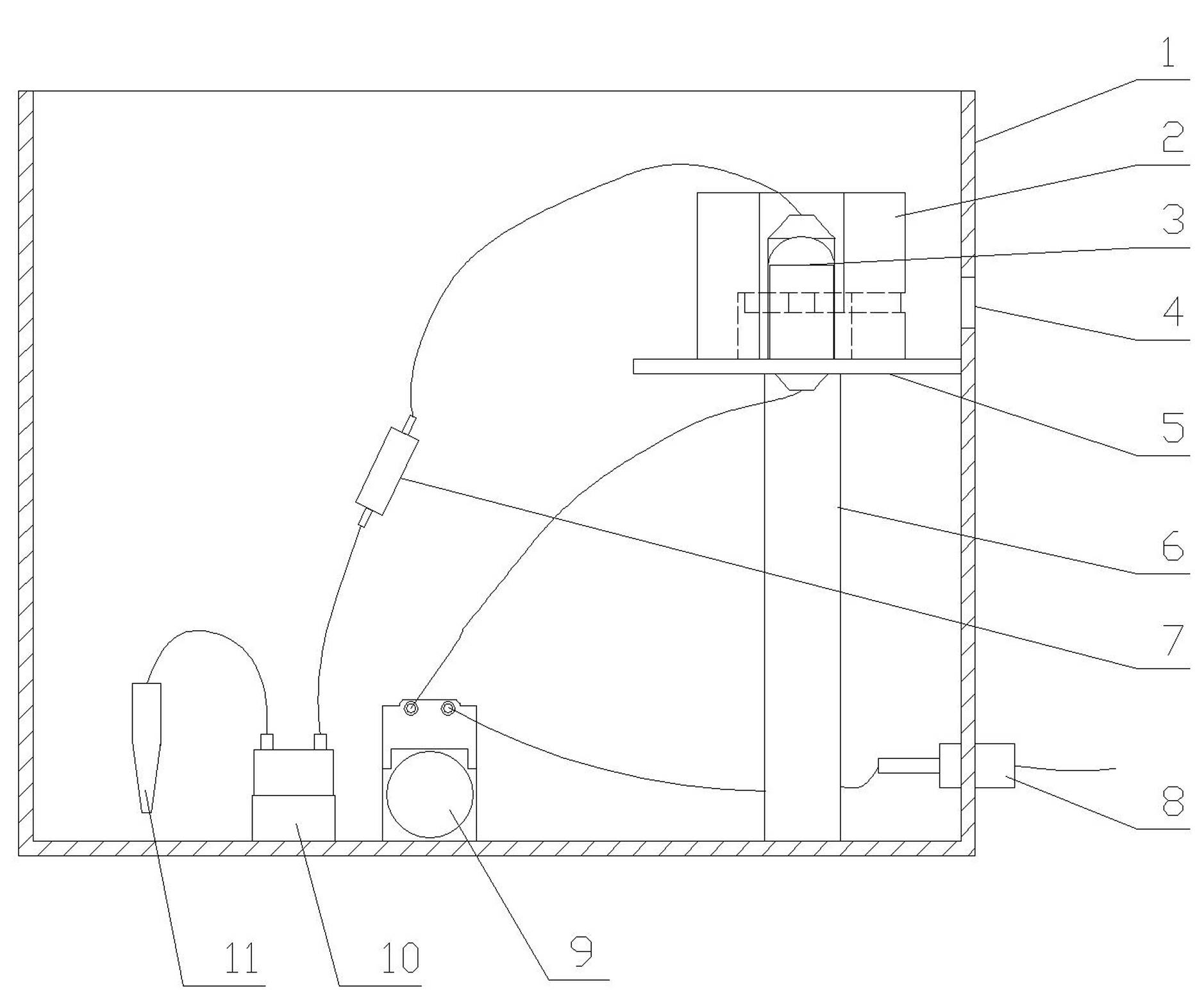
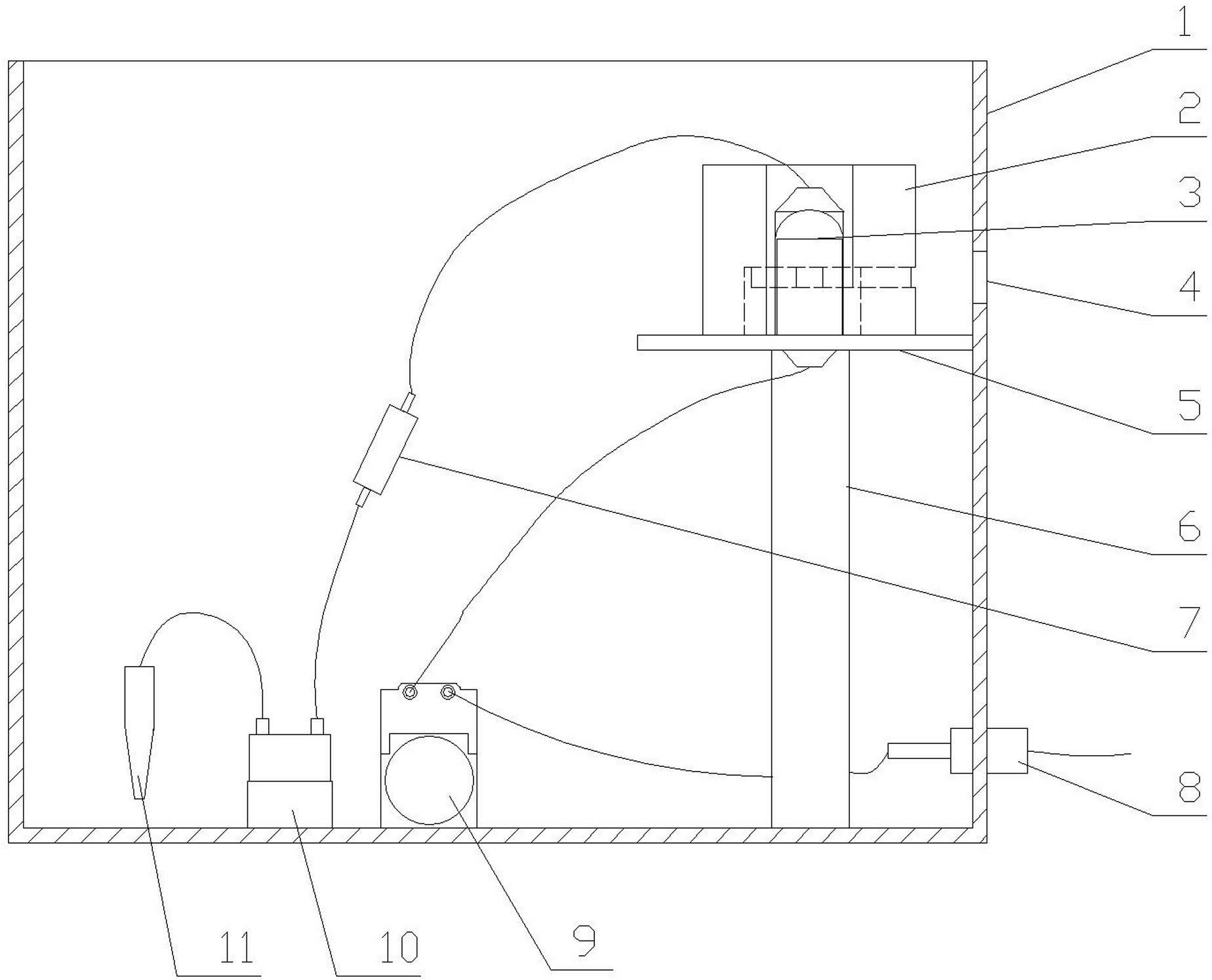
The present invention provides an apparatus that inhibits microbial viability on a medical instrument. The apparatus has a sterilization chamber for receiving the medical instrument. A circulation system is fluidly connected to the sterilization chamber to circulate a fluid through the sterilization chamber. A well is provided to receive a chemistry container that includes a removable base portion. A device is provided to detach the removable base portion from the chemistry container. The device includes a mounting end that is mountable within the well such that the device extends into a lower portion of the well. An elongated intermediate section extends from the mounting end at a first angle. A free end extends from the elongated intermediate section at a second angle. The free end is dimensioned to matingly engage and apply a force to a mating feature on the chemistry container or on the removable base portion as the chemistry container is inserted into the well.
Owner:AMERICAN STERILIZER CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com