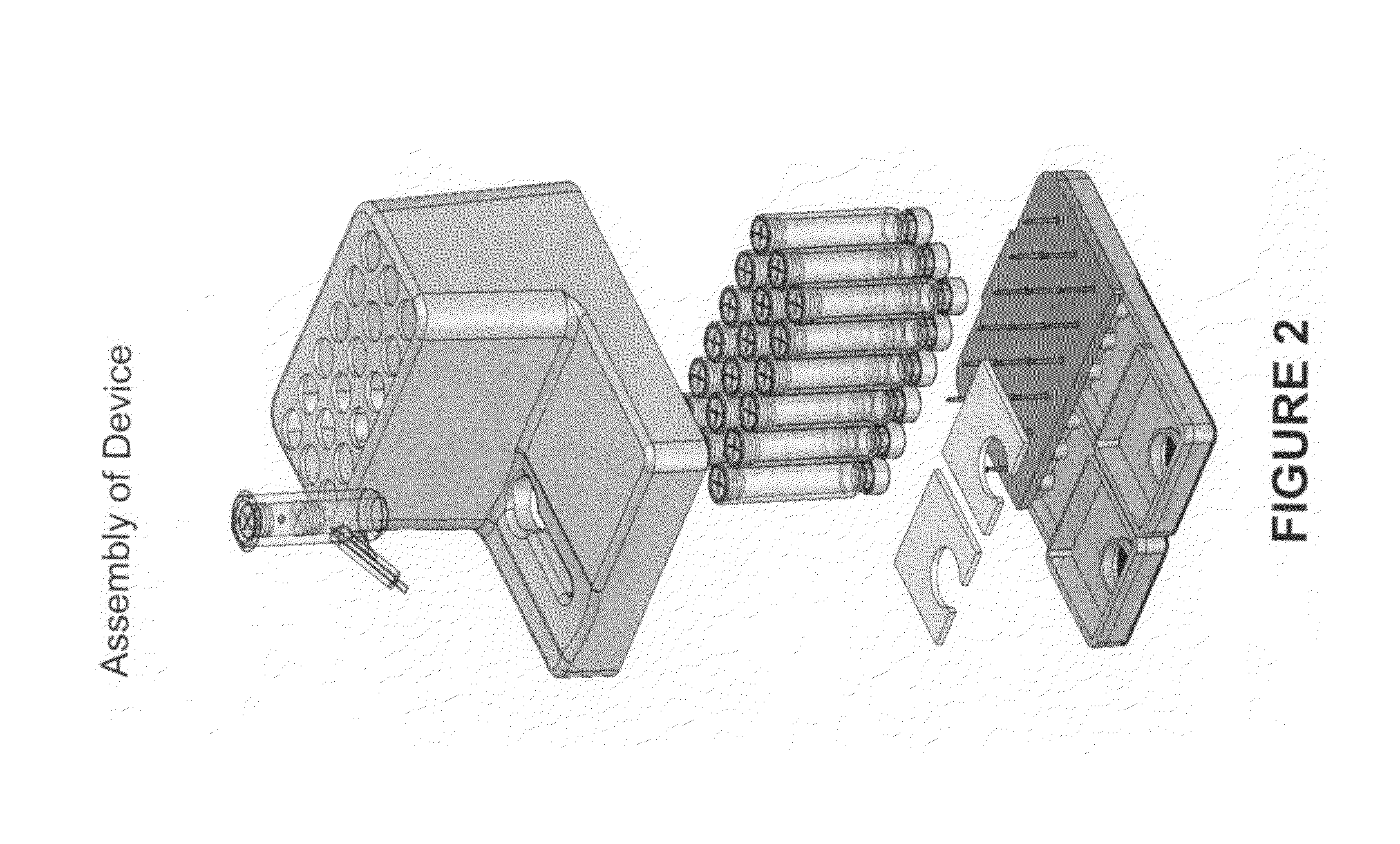Patents
Literature
181 results about "Point-of-care testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Point-of-care testing (POCT or bedside testing) is defined as medical diagnostic testing at or near the point of care—that is, at the time and place of patient care. This contrasts with the historical pattern in which testing was wholly or mostly confined to the medical laboratory, which entailed sending off specimens away from the point of care and then waiting hours or days to learn the results, during which time care must continue without the desired information.
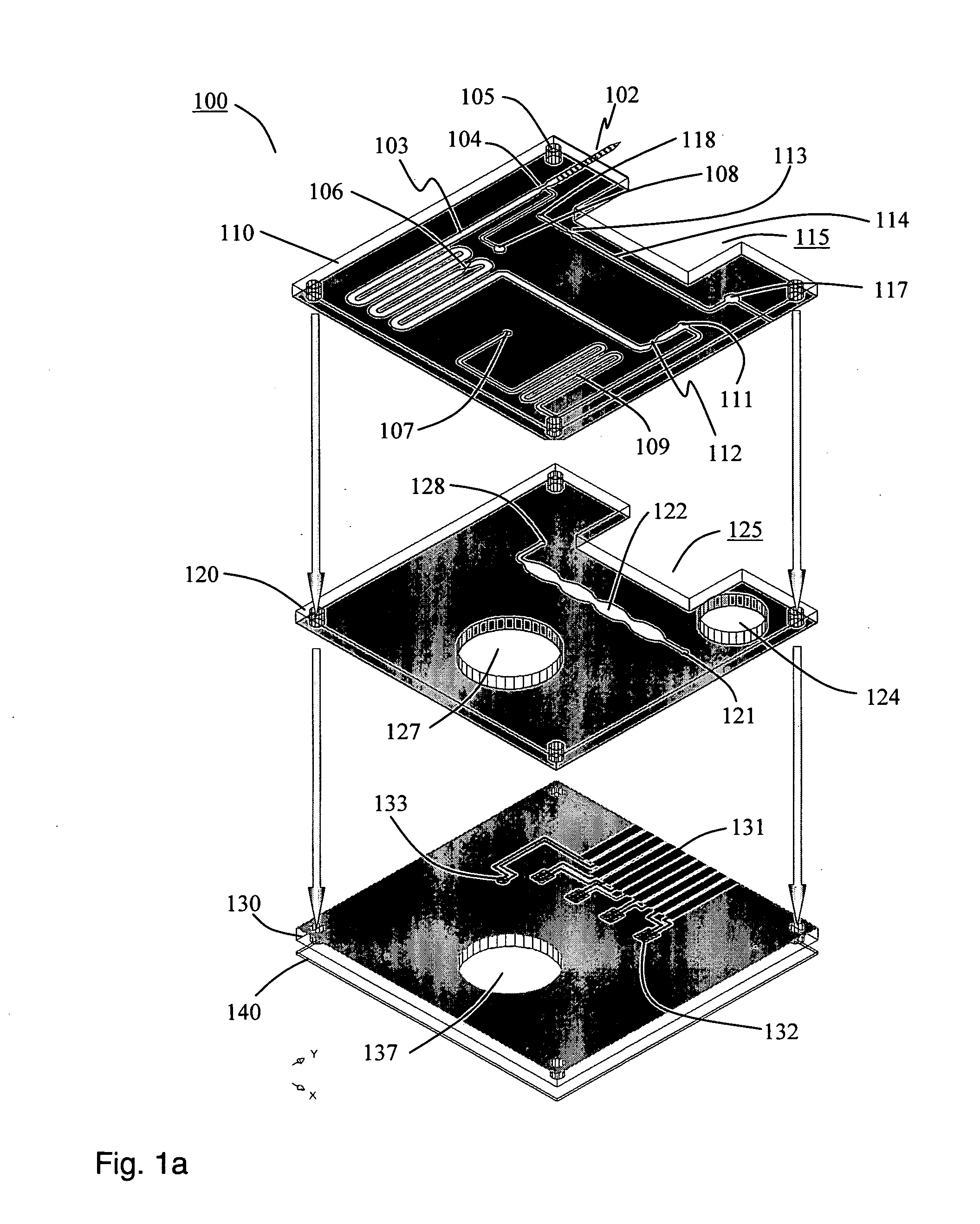
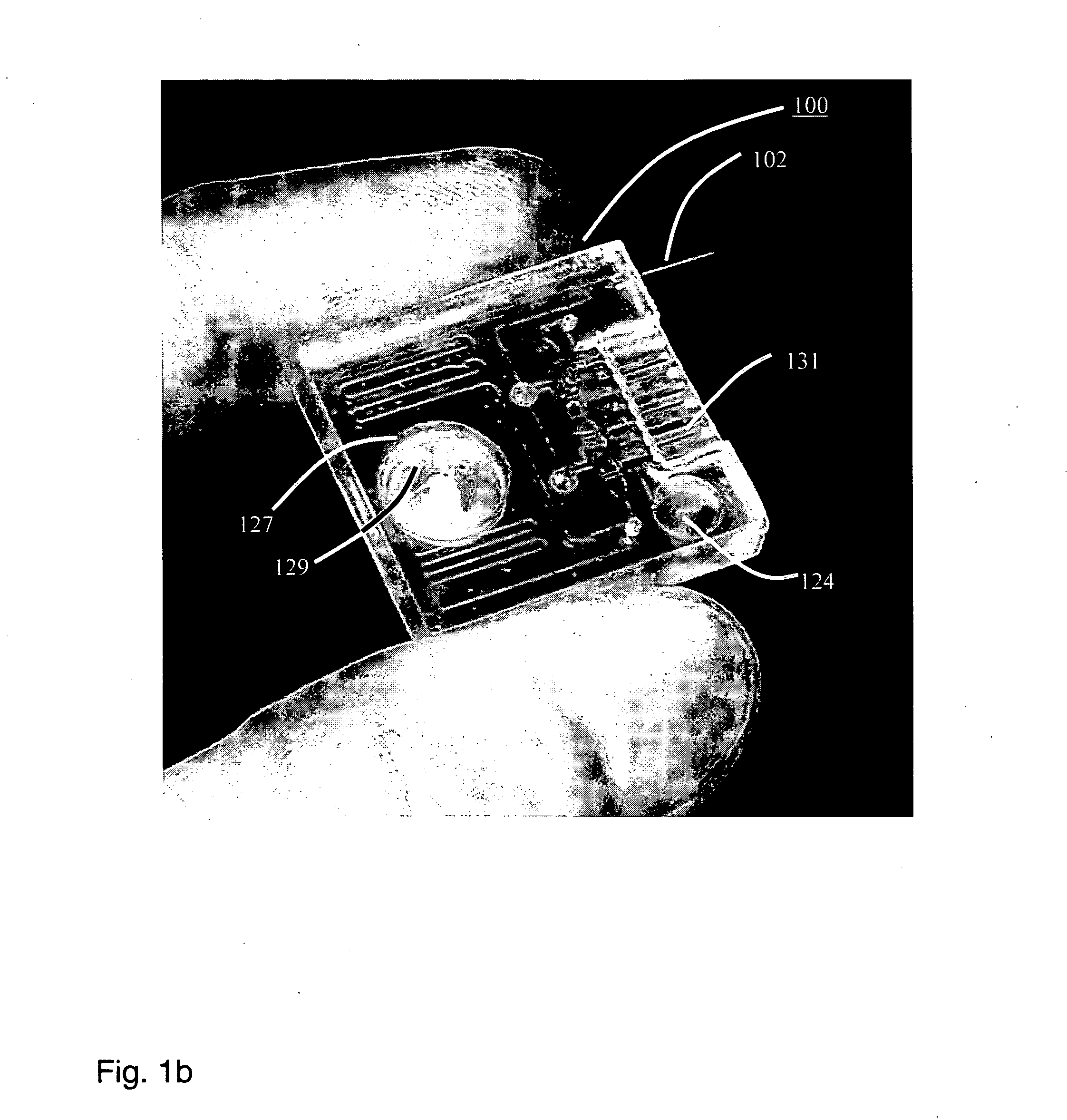
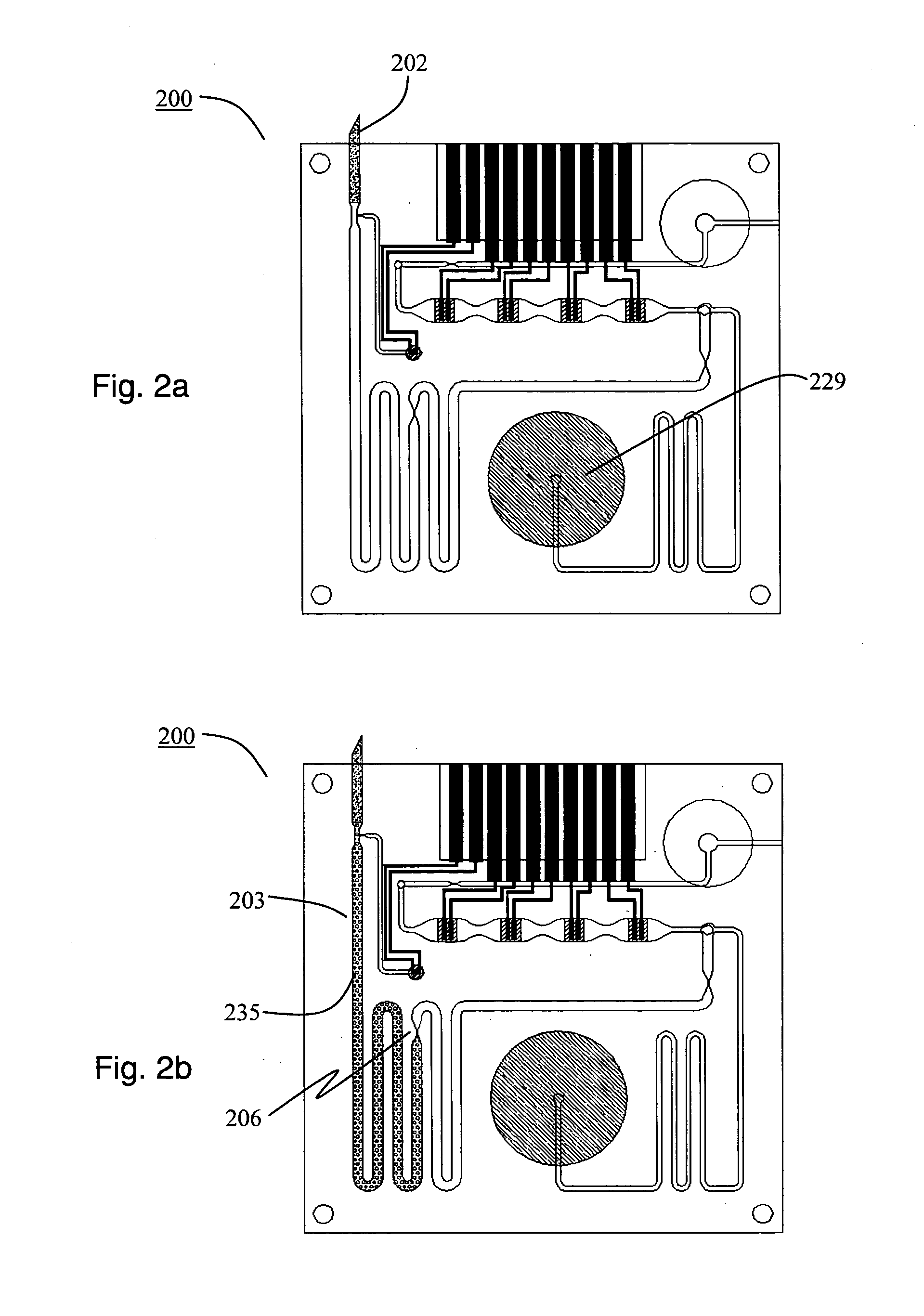
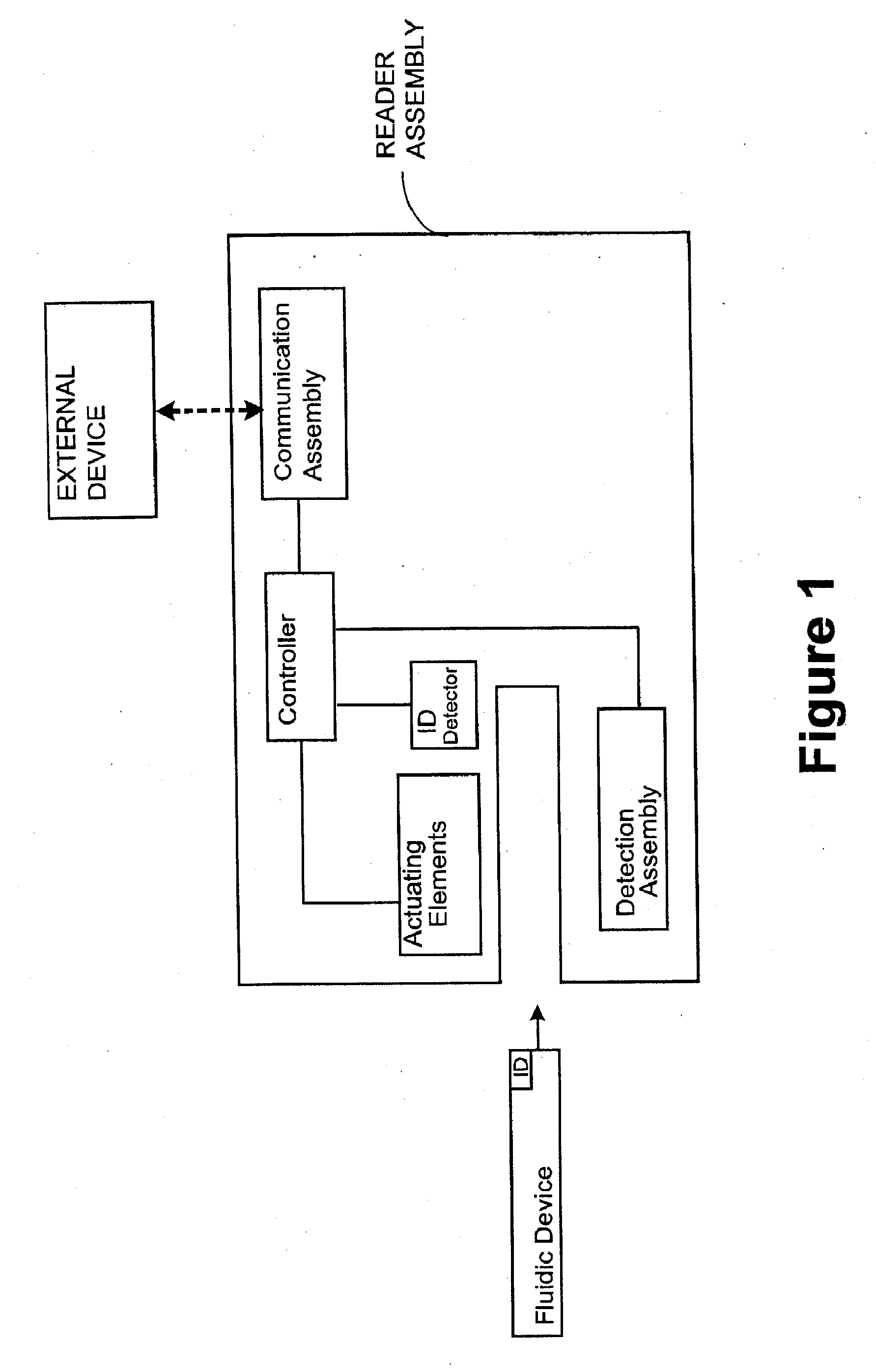
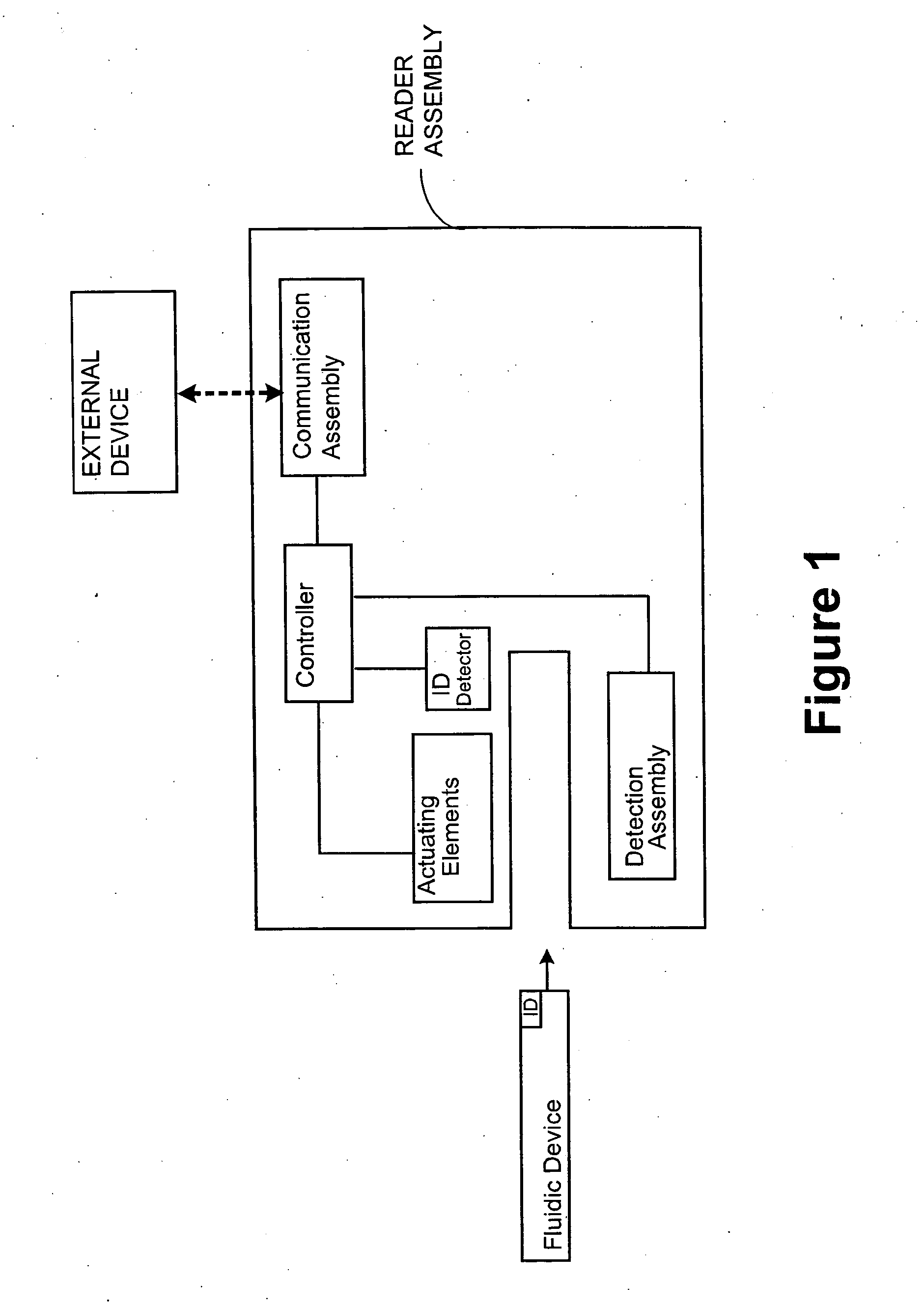
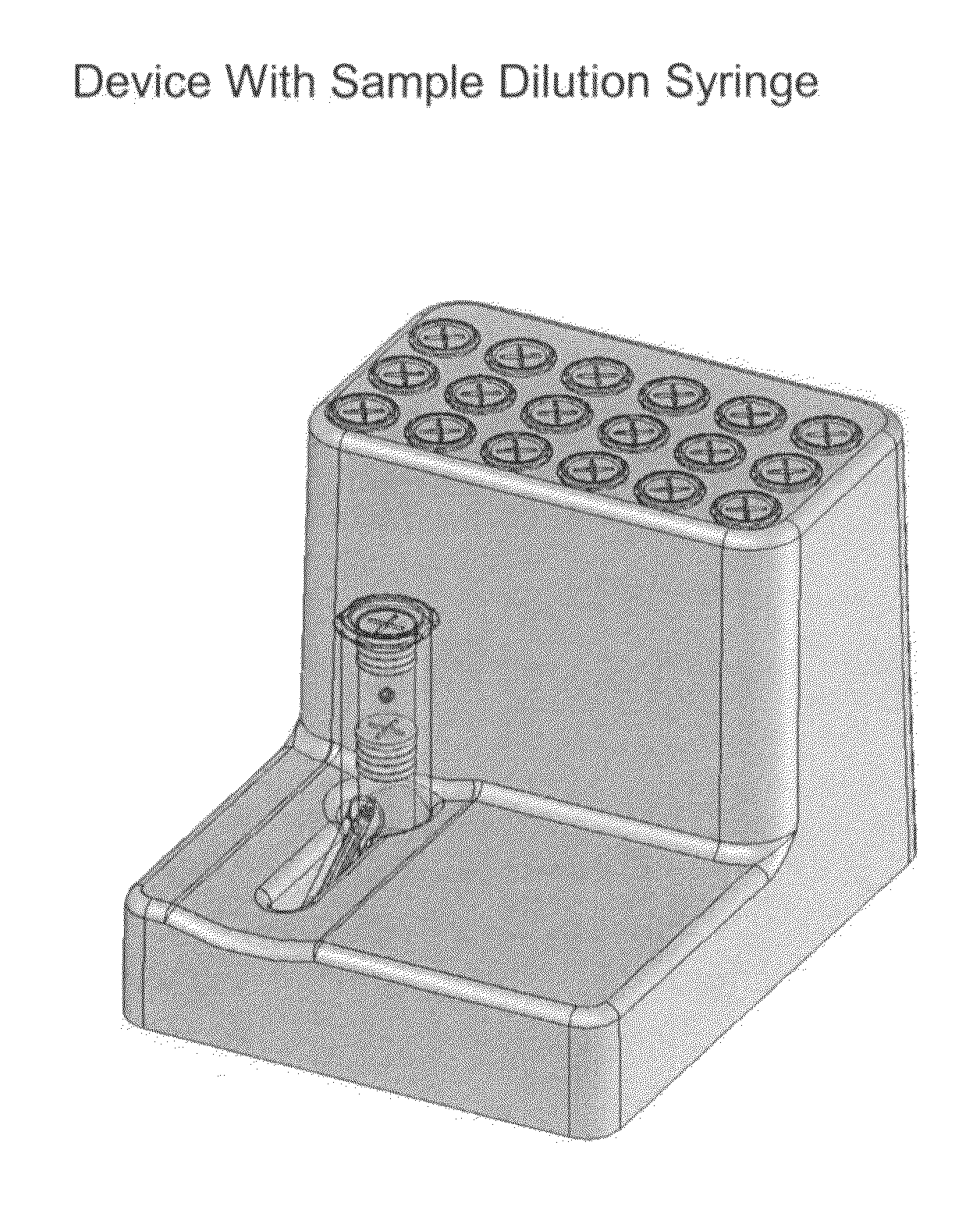
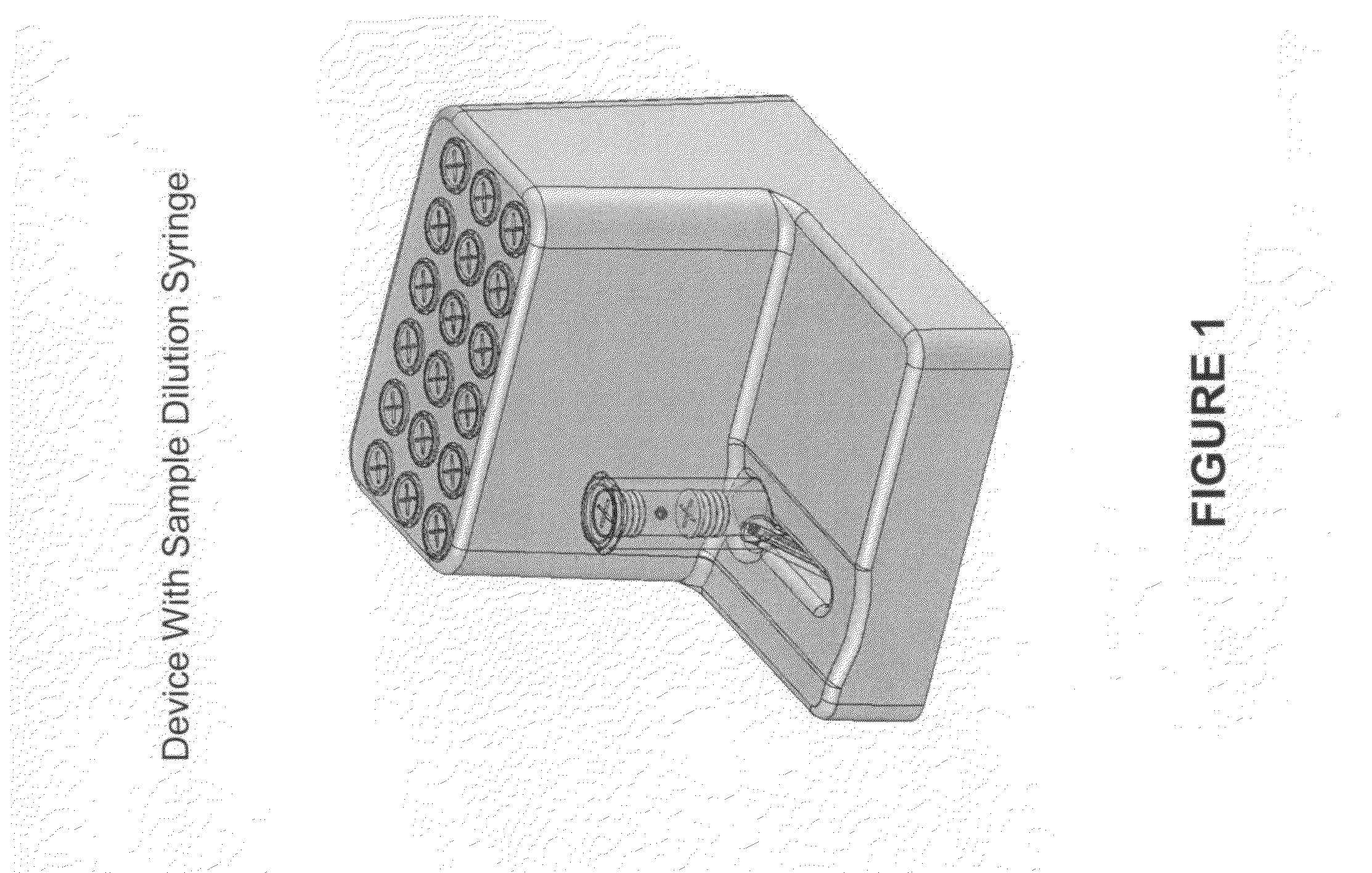
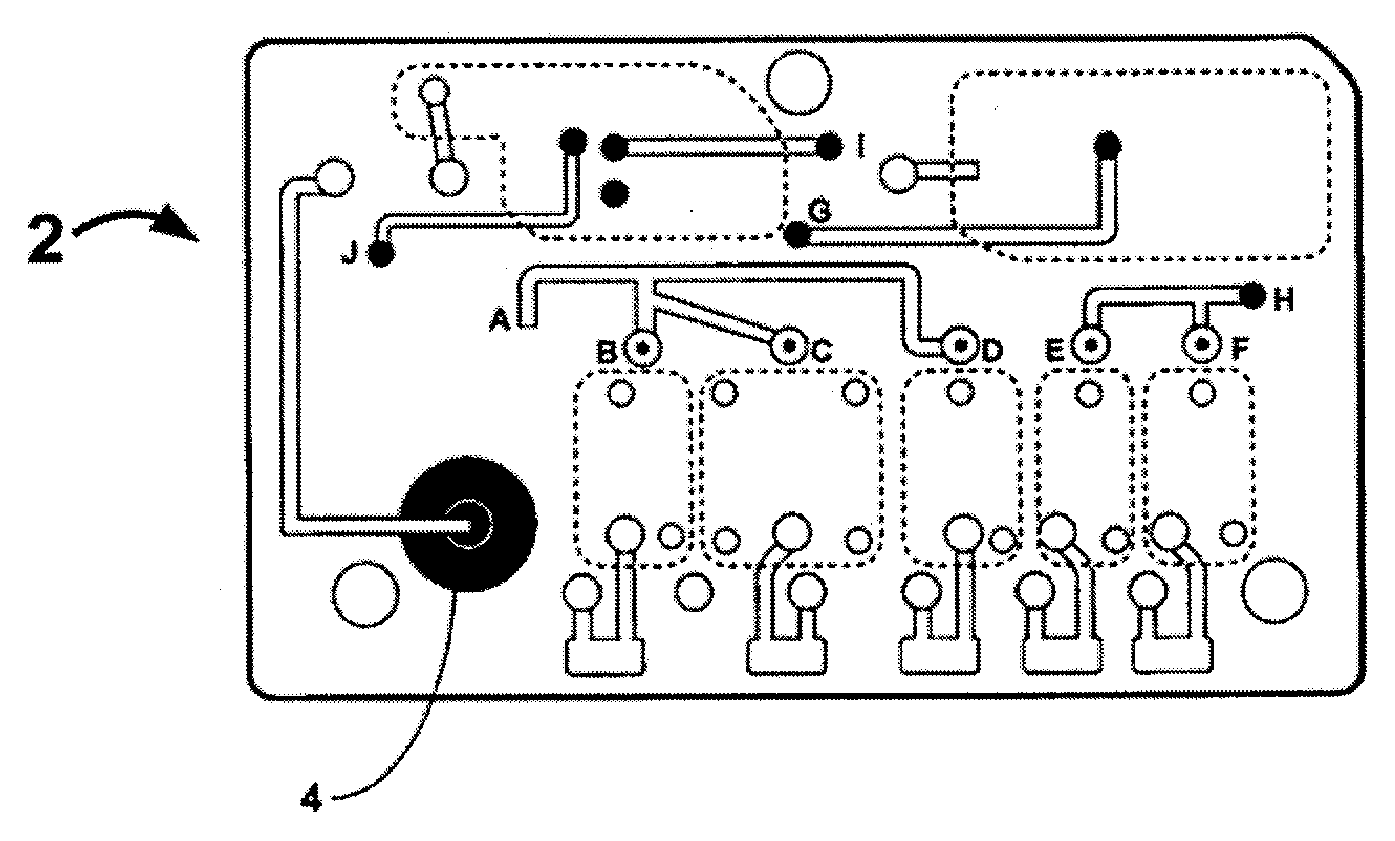
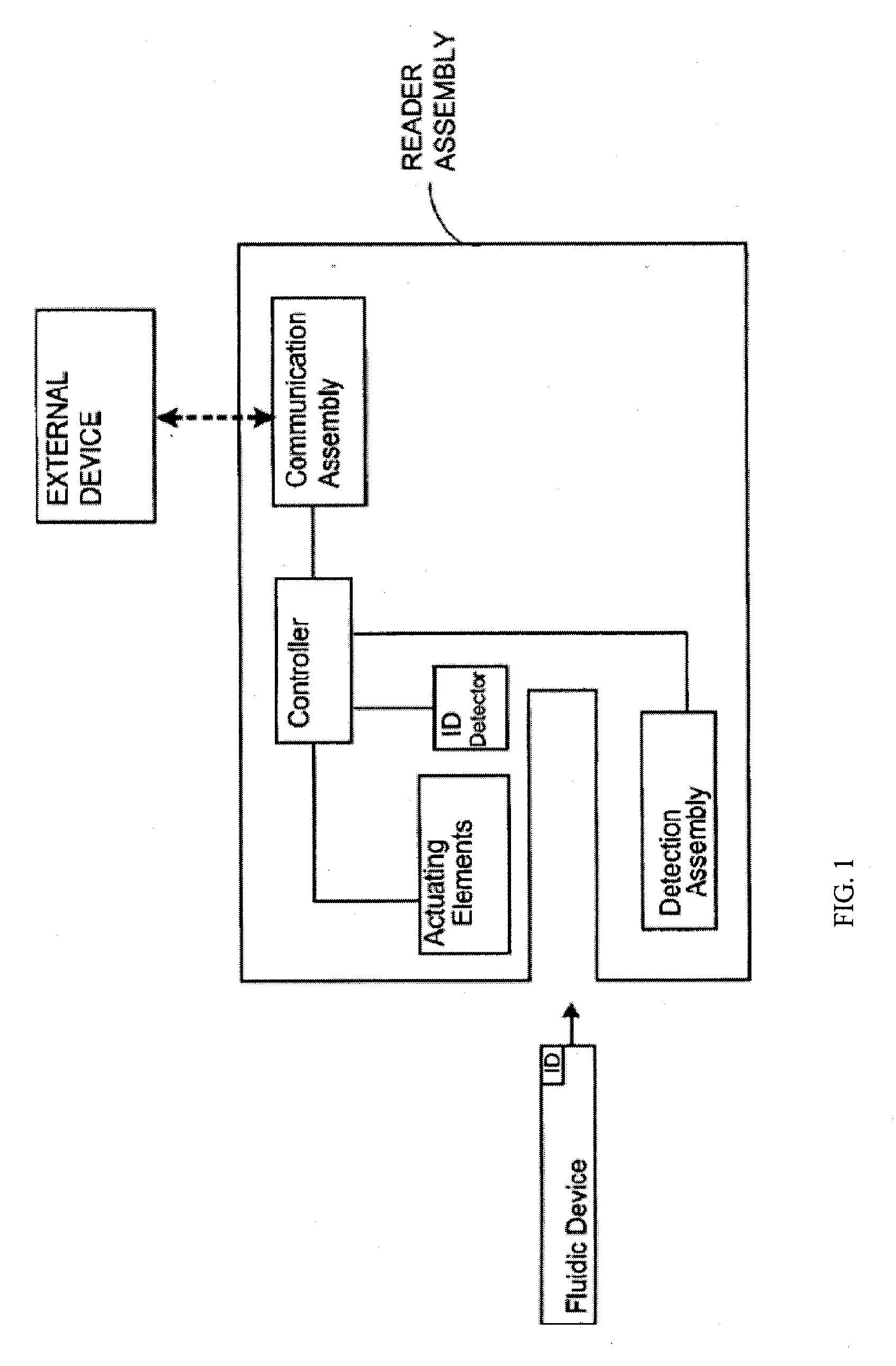
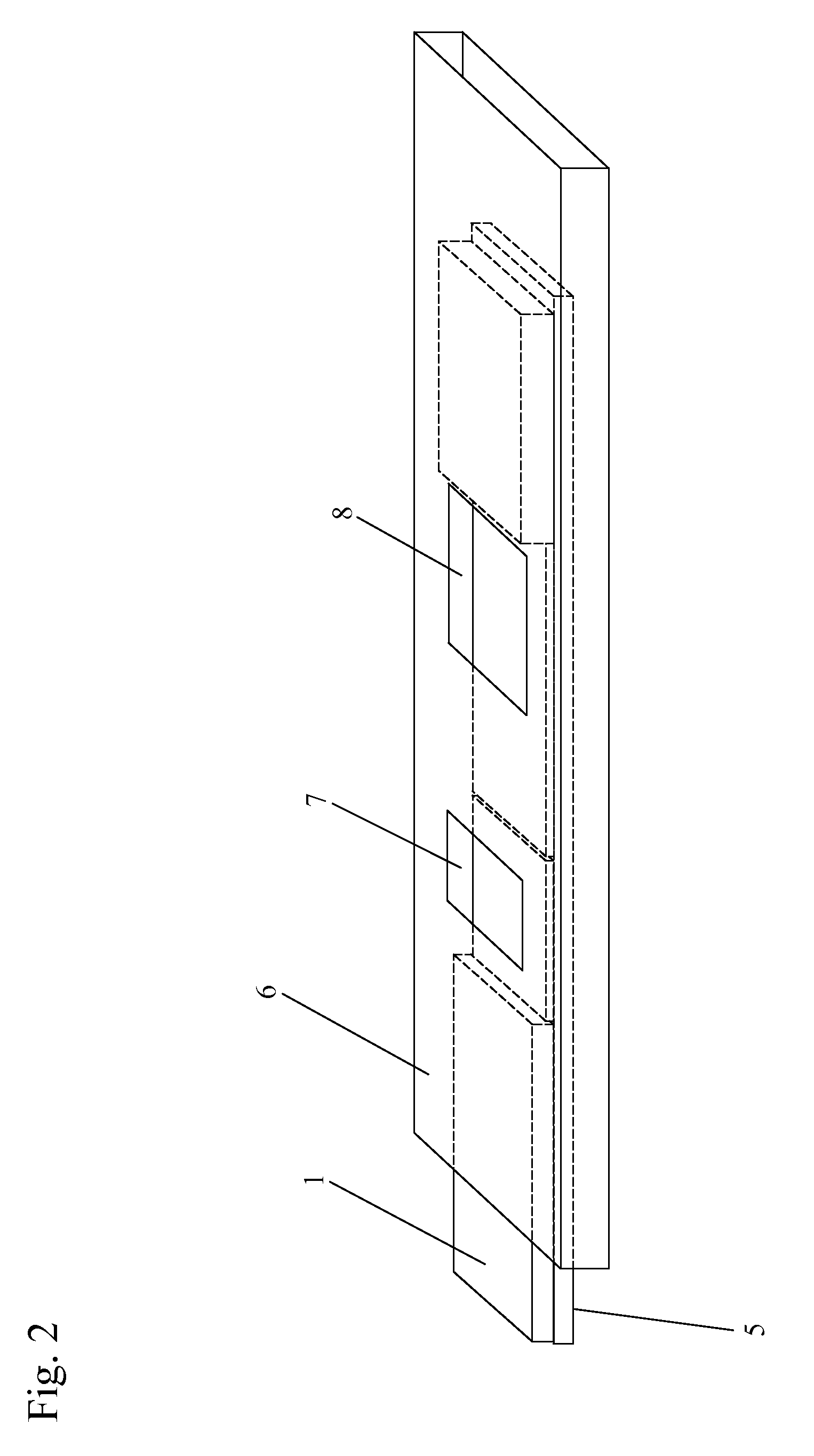
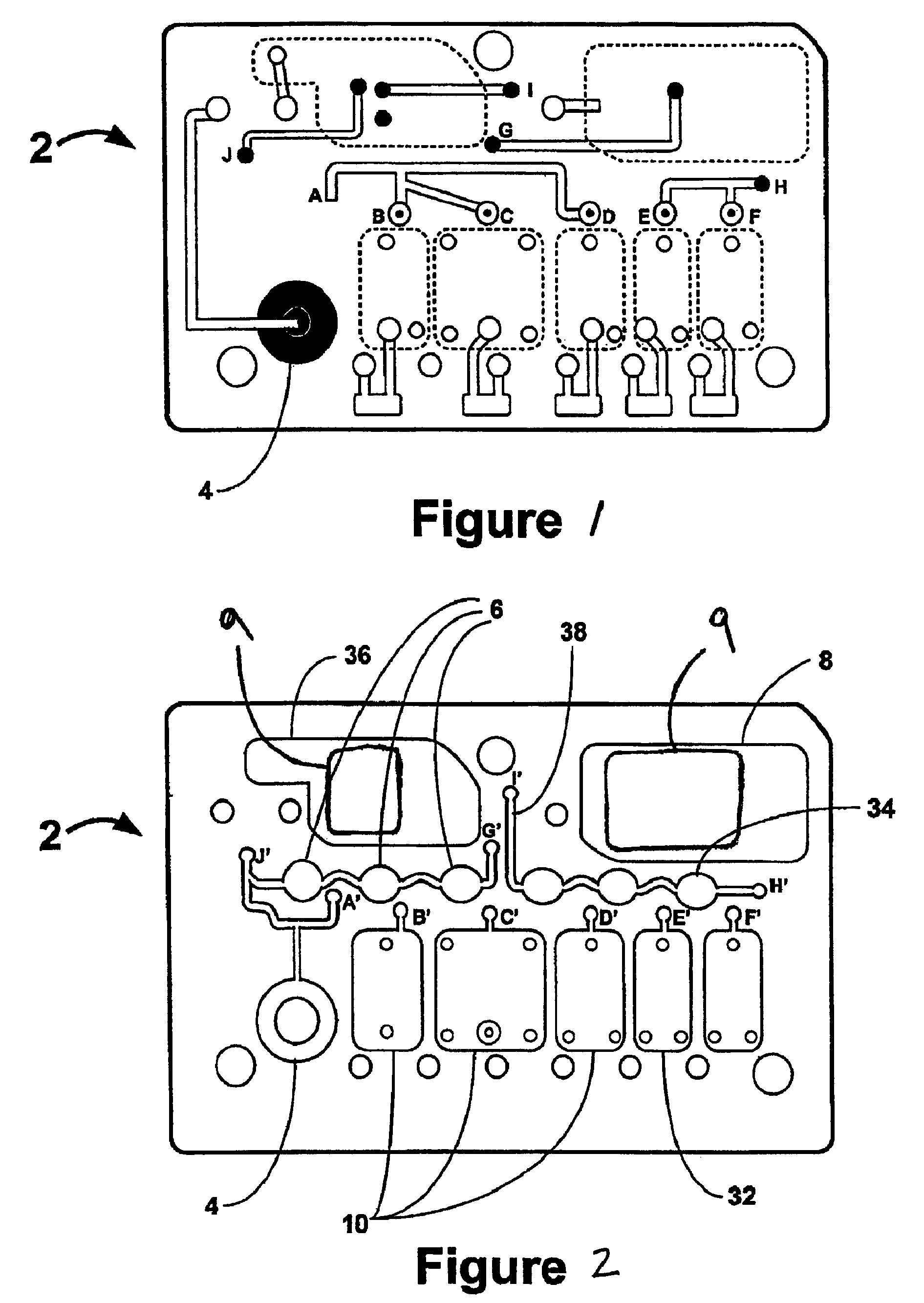
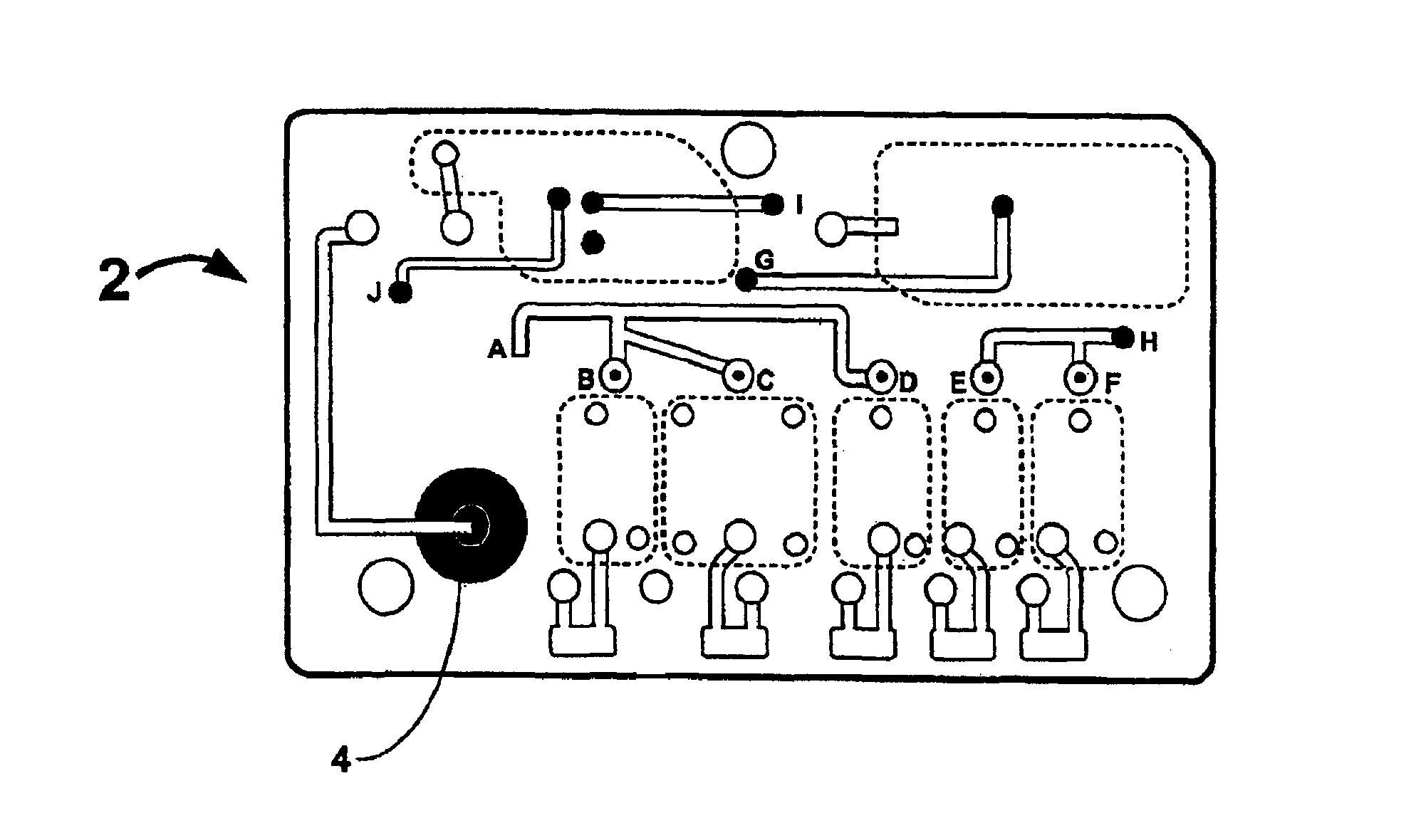
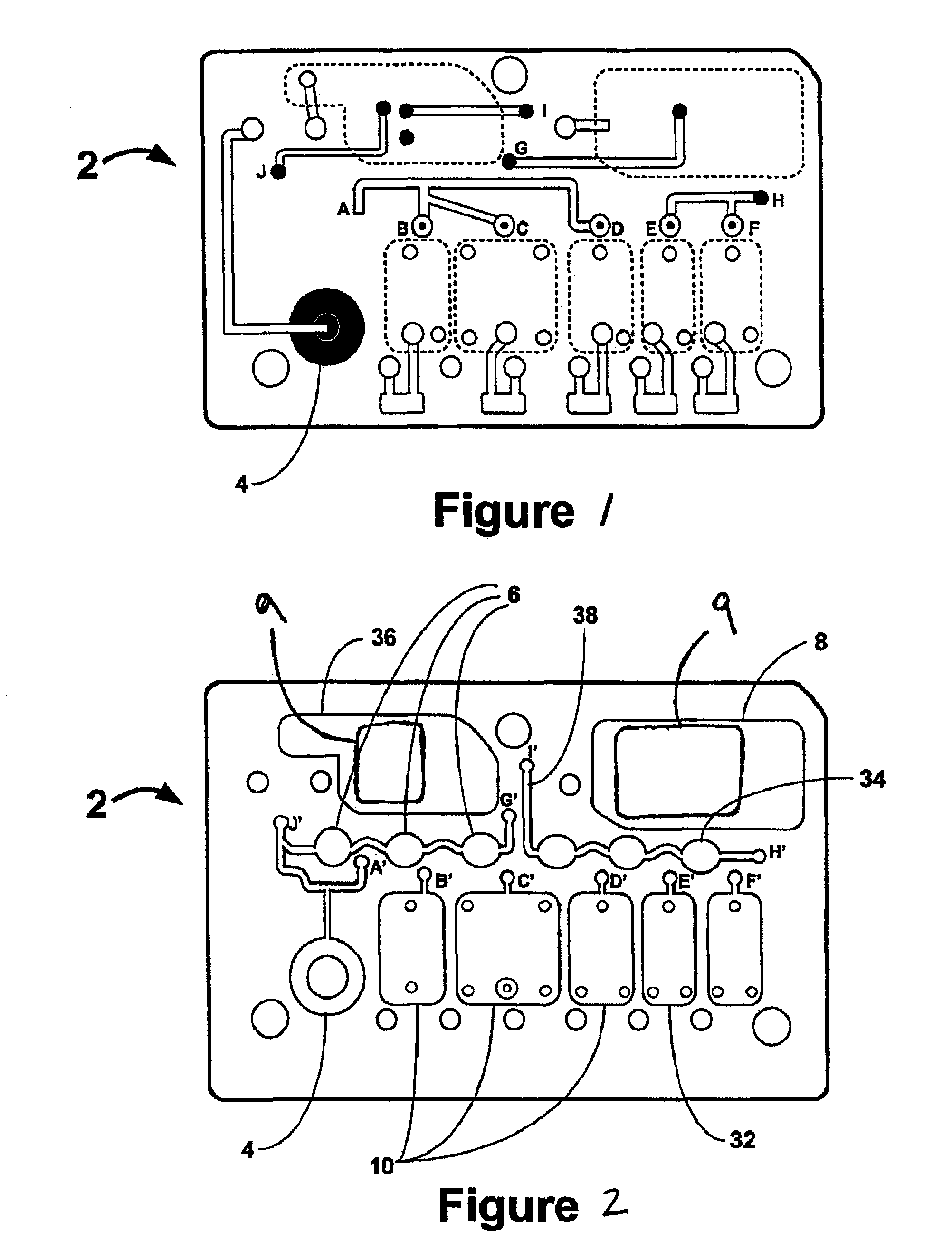
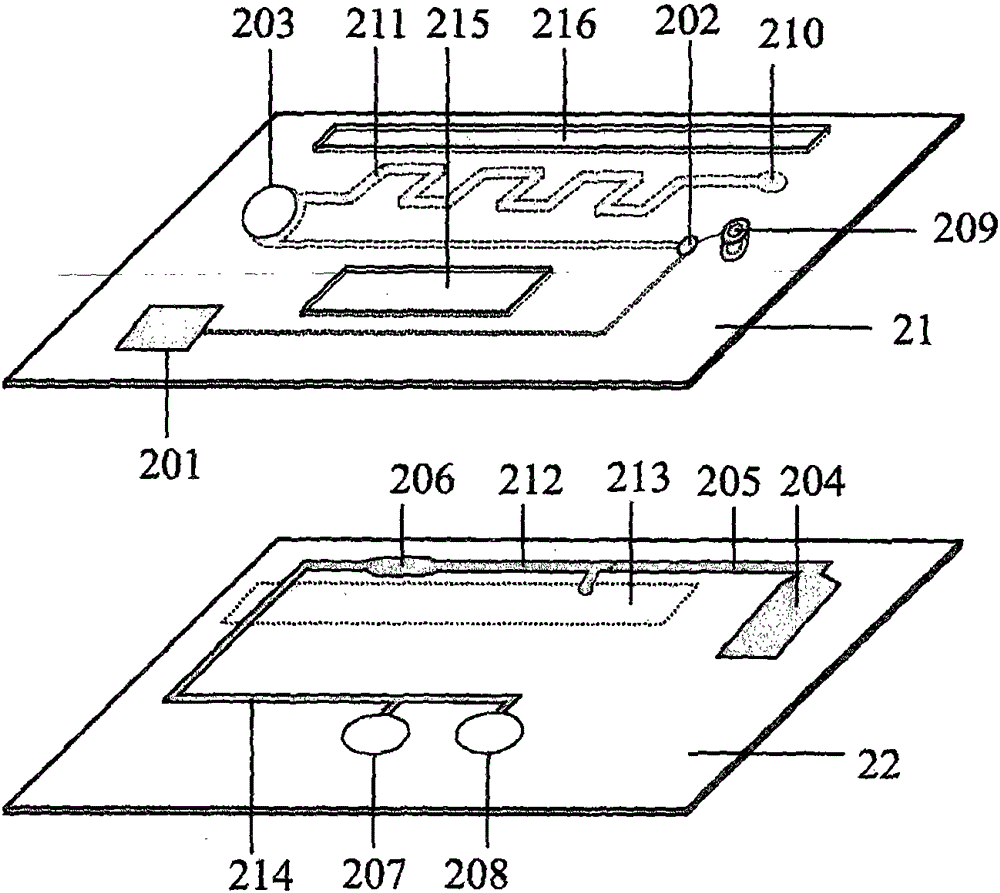
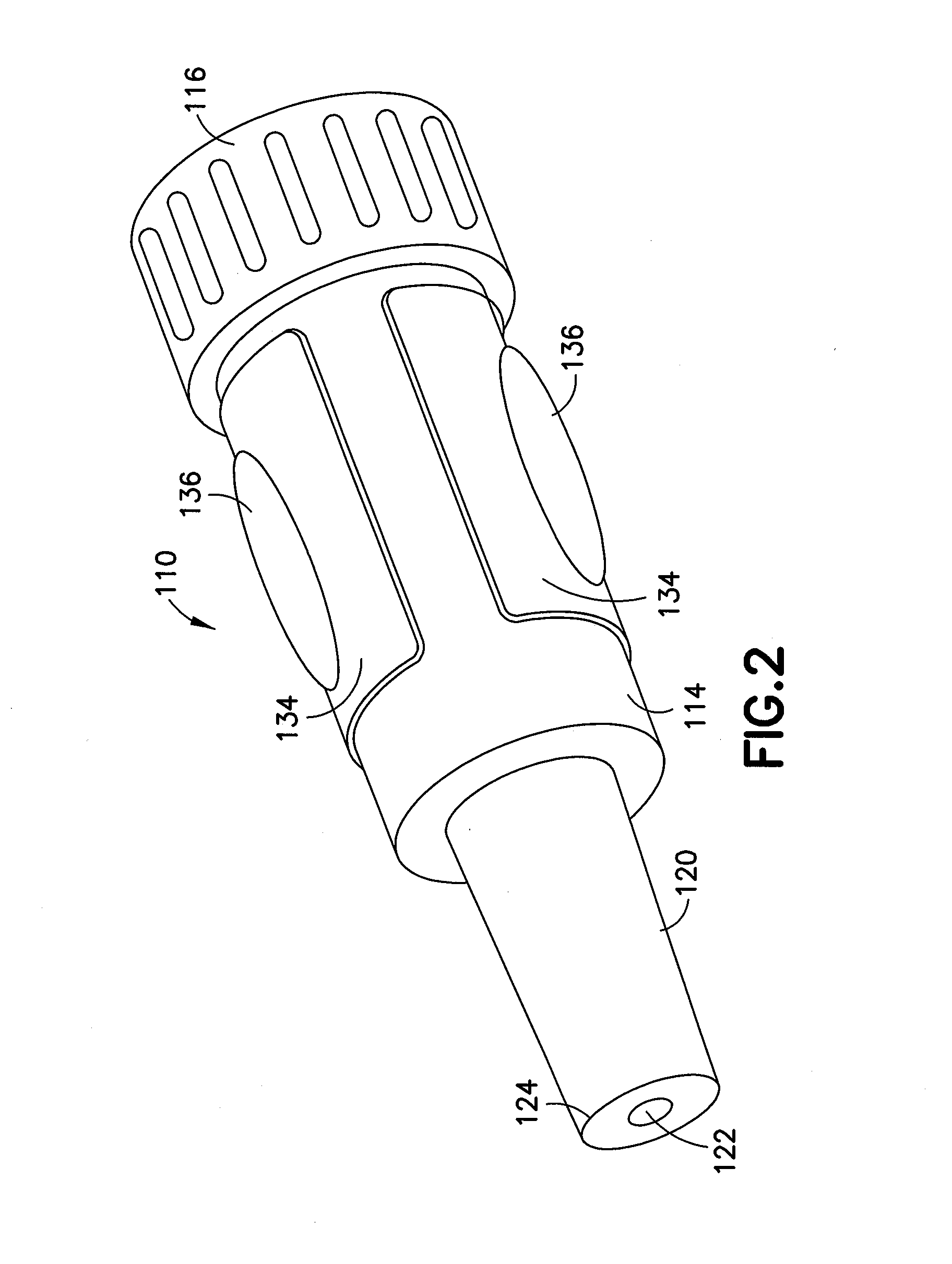
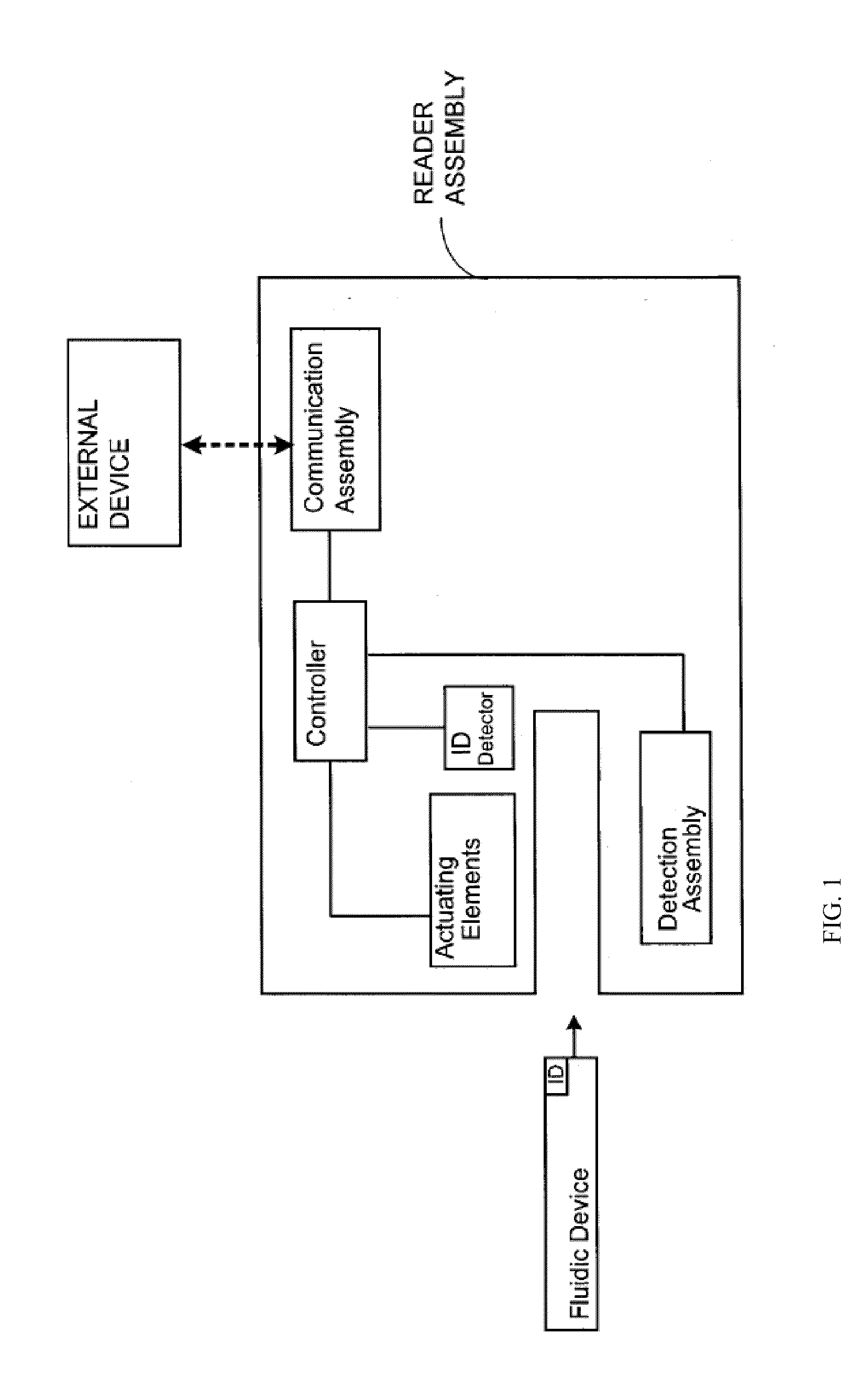
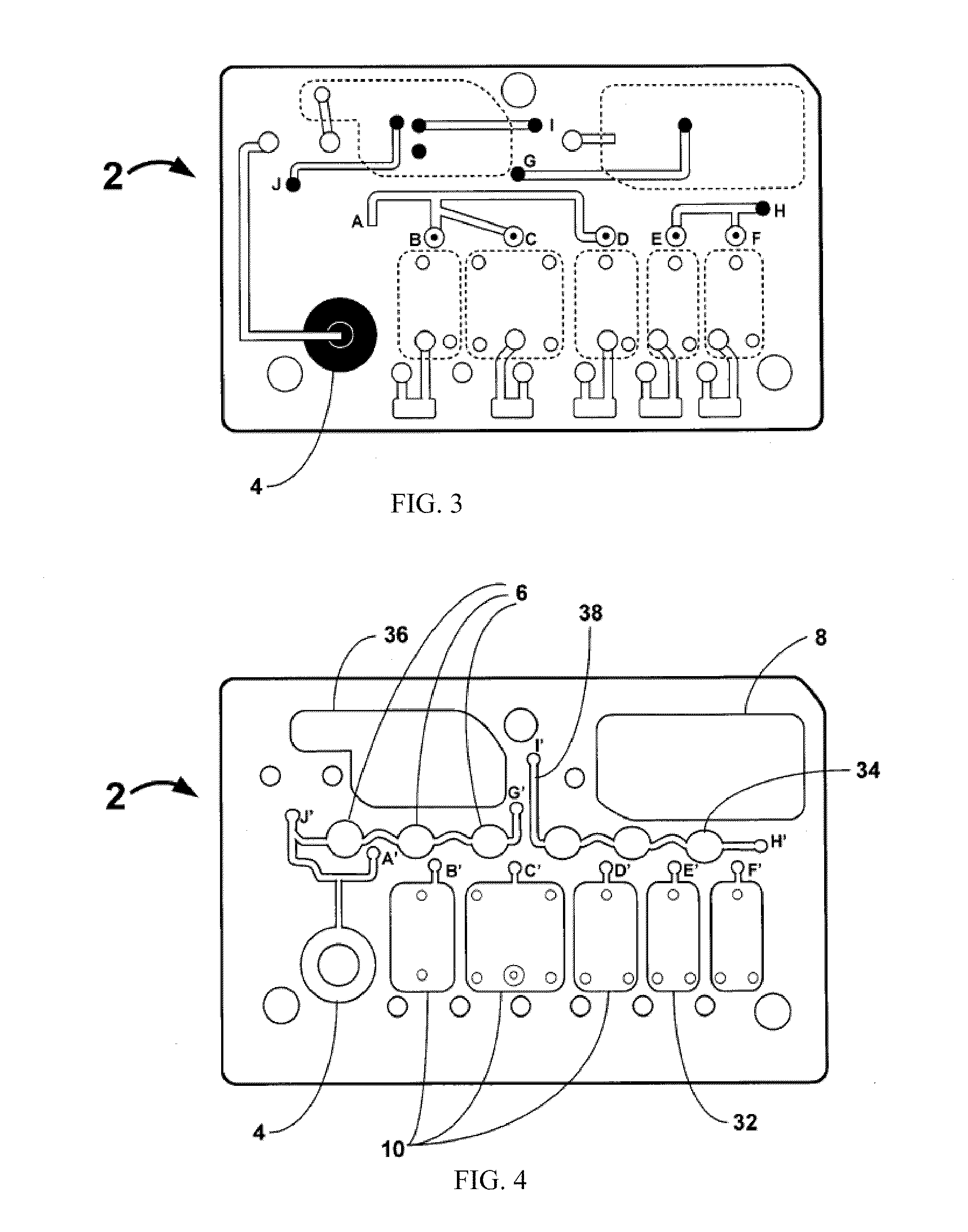
Smart disposable plastic lab-on-a-chip for point-of-care testing
InactiveUS20050130292A1None of measures has been particularly successfulRelieve painBioreactor/fermenter combinationsCombination devicesVenous bloodLab-on-a-chip
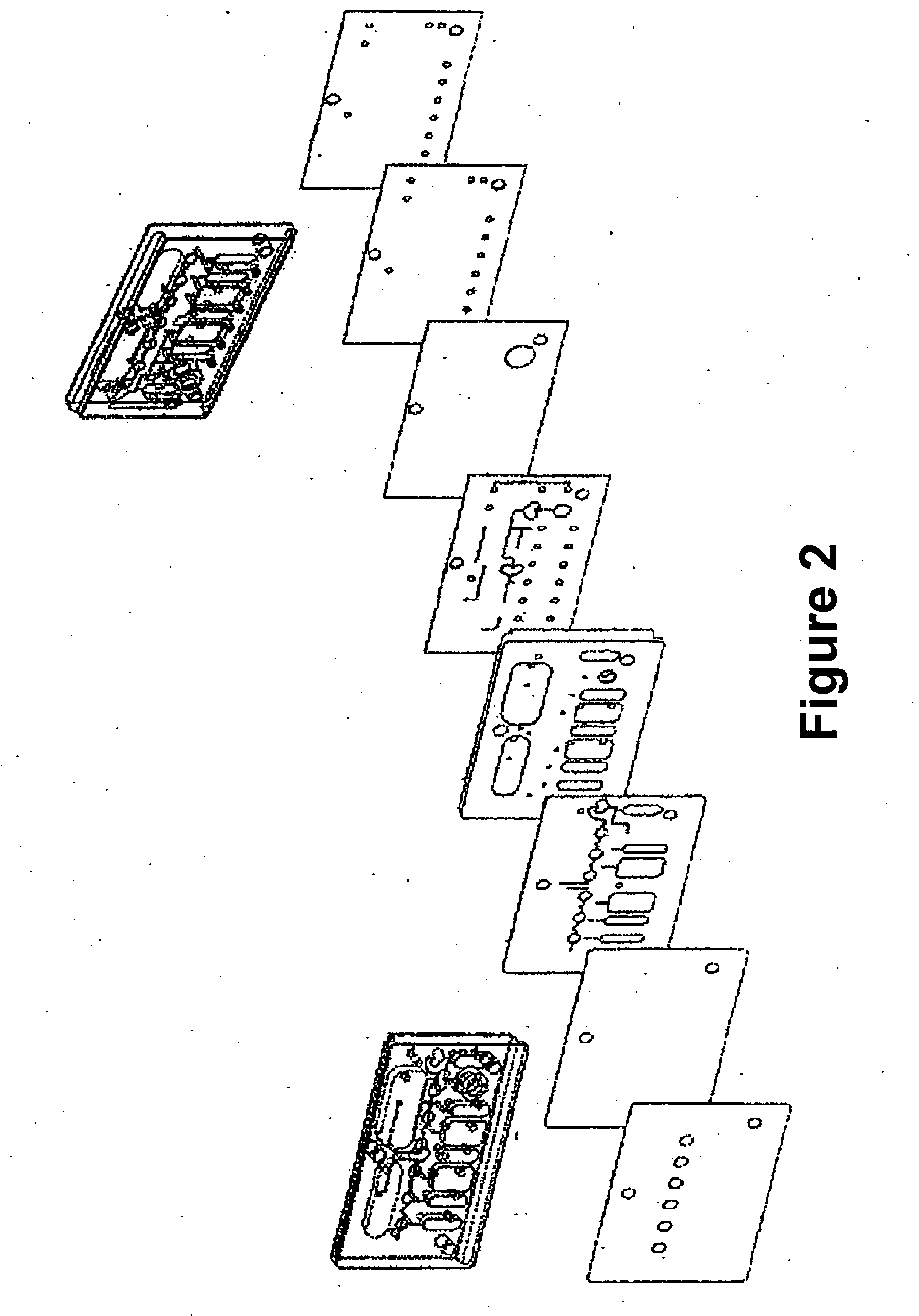
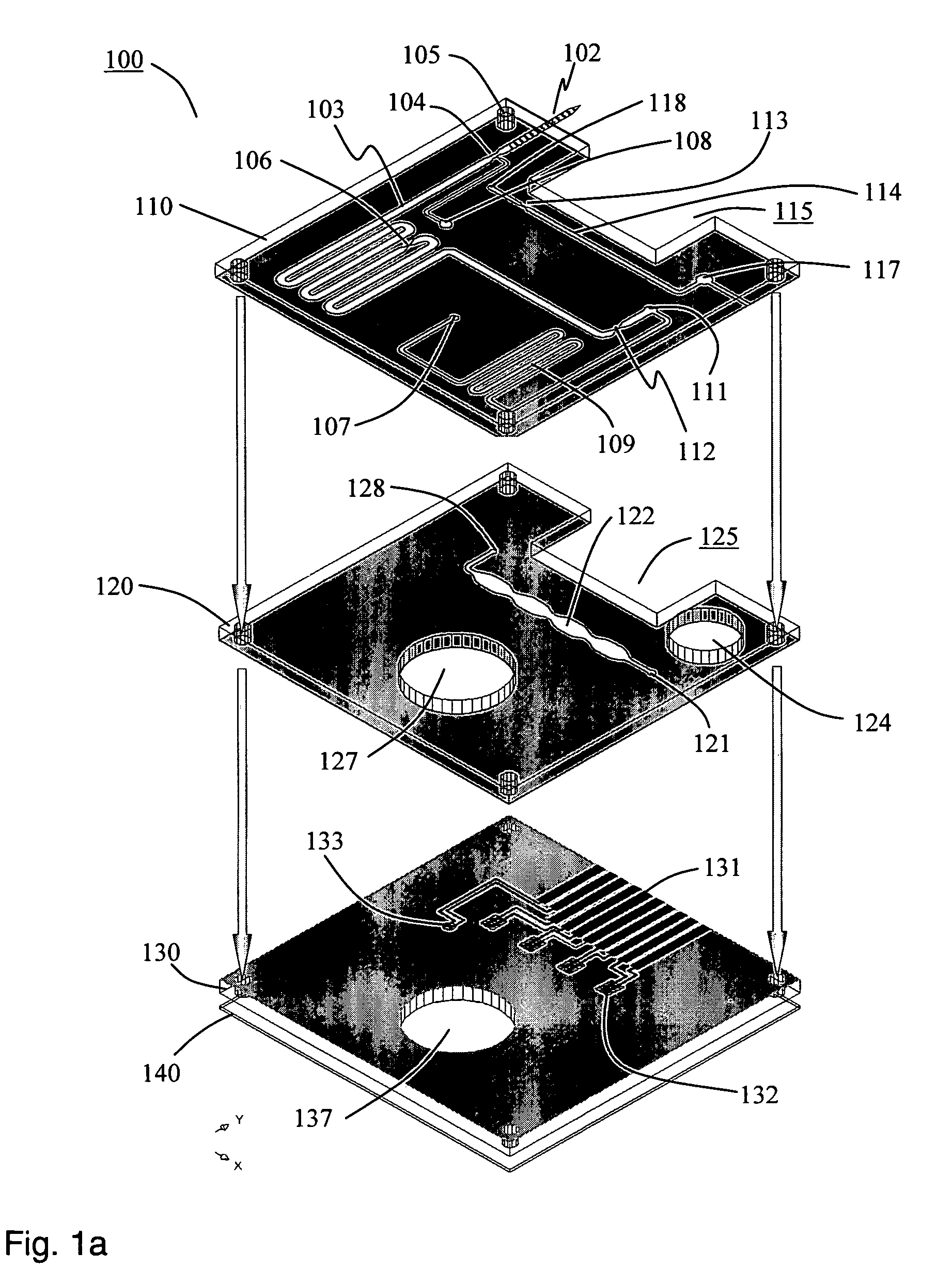
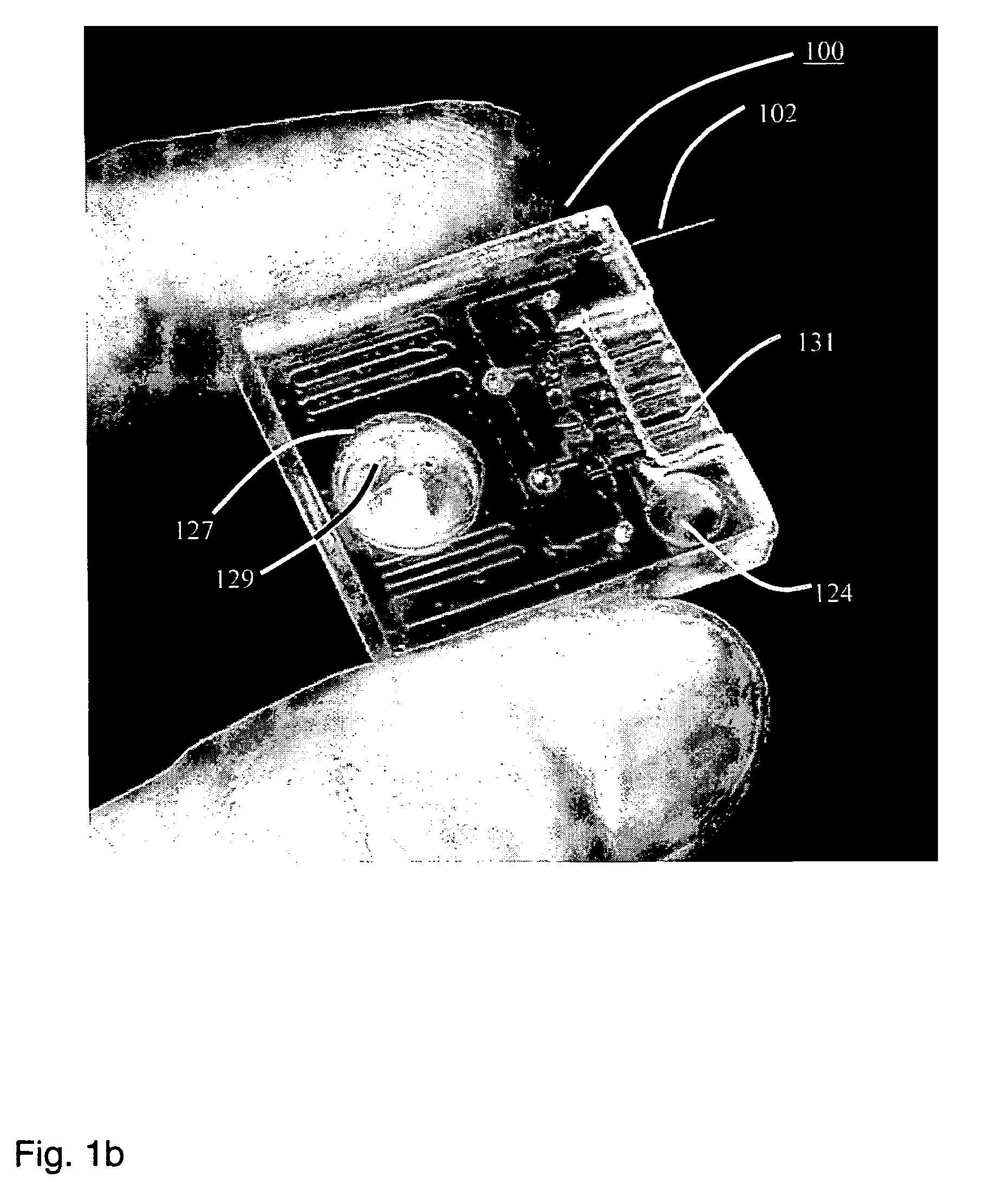
Disclosed herein is a fully-integrated, disposable biochip for point-of-care testing of clinically relevant parameters. Specifically, in accordance with an embodiment of the present invention, the biochip is designed for POCT (point-of-care-testing) of an array of metabolic parameters including partial pressure of oxygen, Glucose, and Lactate concentration from venous blood samples. The biochip is fabricated on a low-cost plastic substrate using mass manufacturing compatible fabrication processes. Furthermore, the biochip contains a fully-integrated metallic micro-needle for blood sampling. The biochip also uses smart passive microfluidics in conjunction with low-power functional on-chip pressure generators for microfluidic sequencing. The design, configuration, assembly and operation of the biochip are ideally suited for a disposable biochip specifically targeted towards POCT applications.
Owner:UNIVERSITY OF CINCINNATI
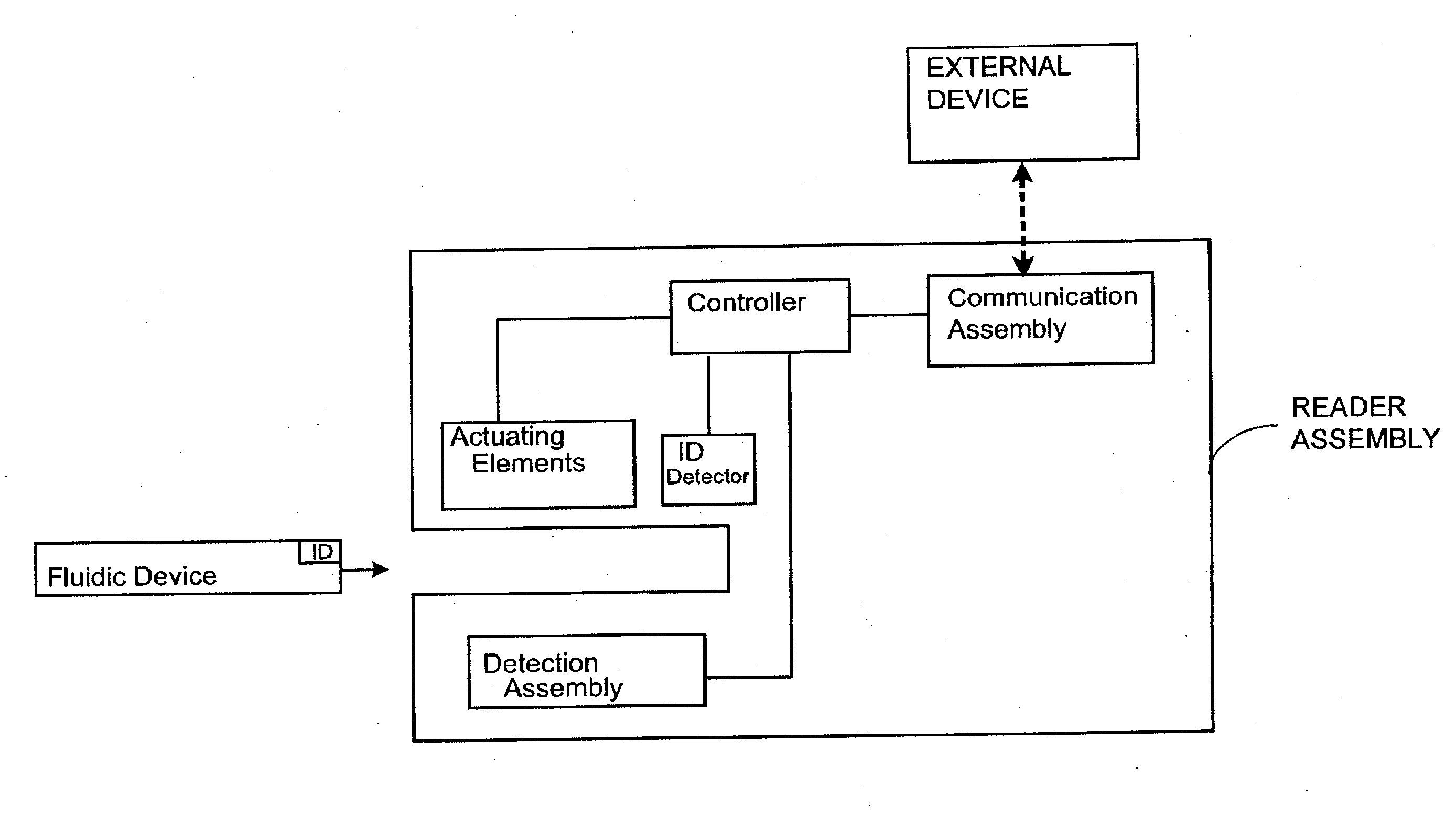
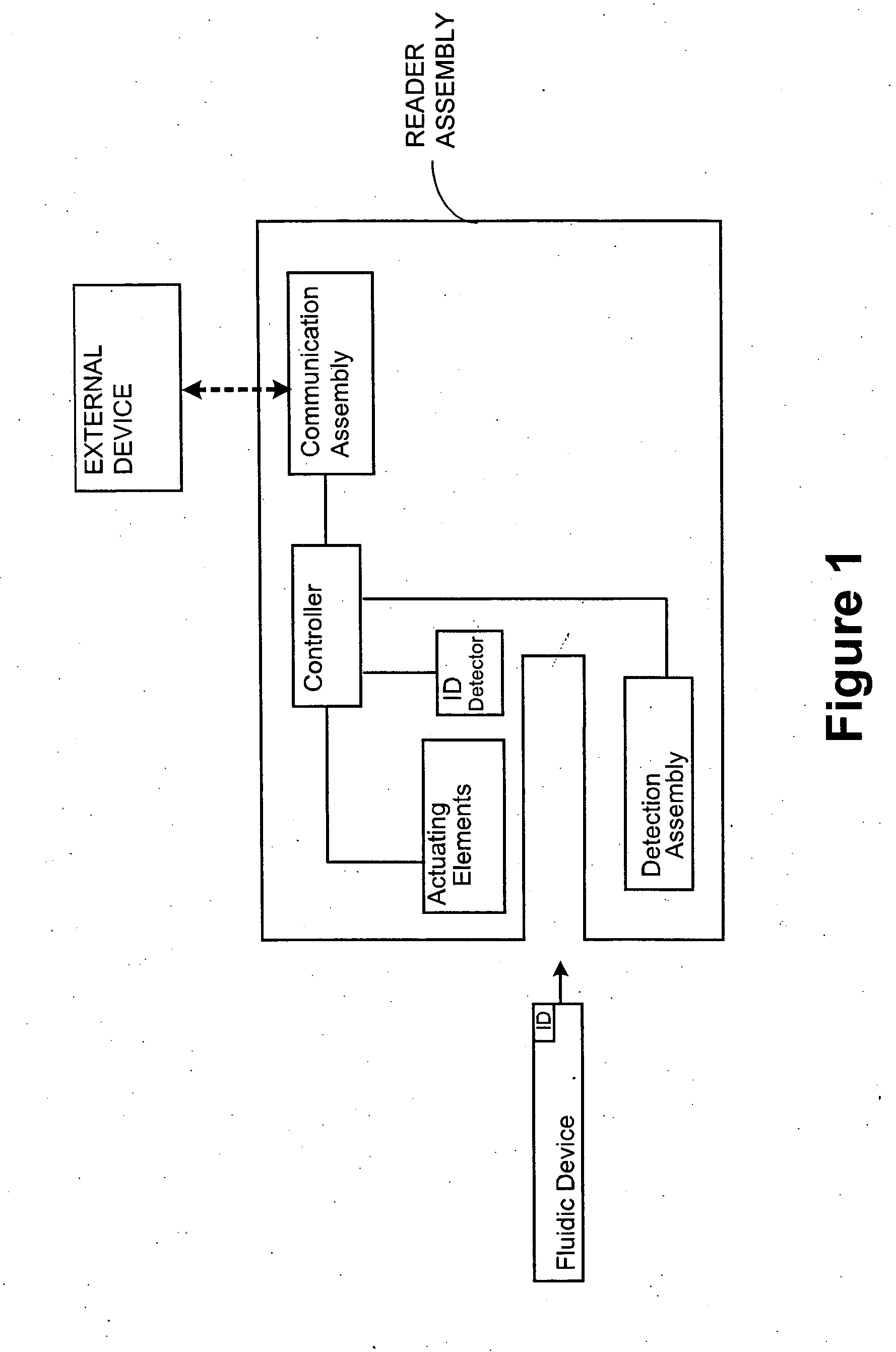
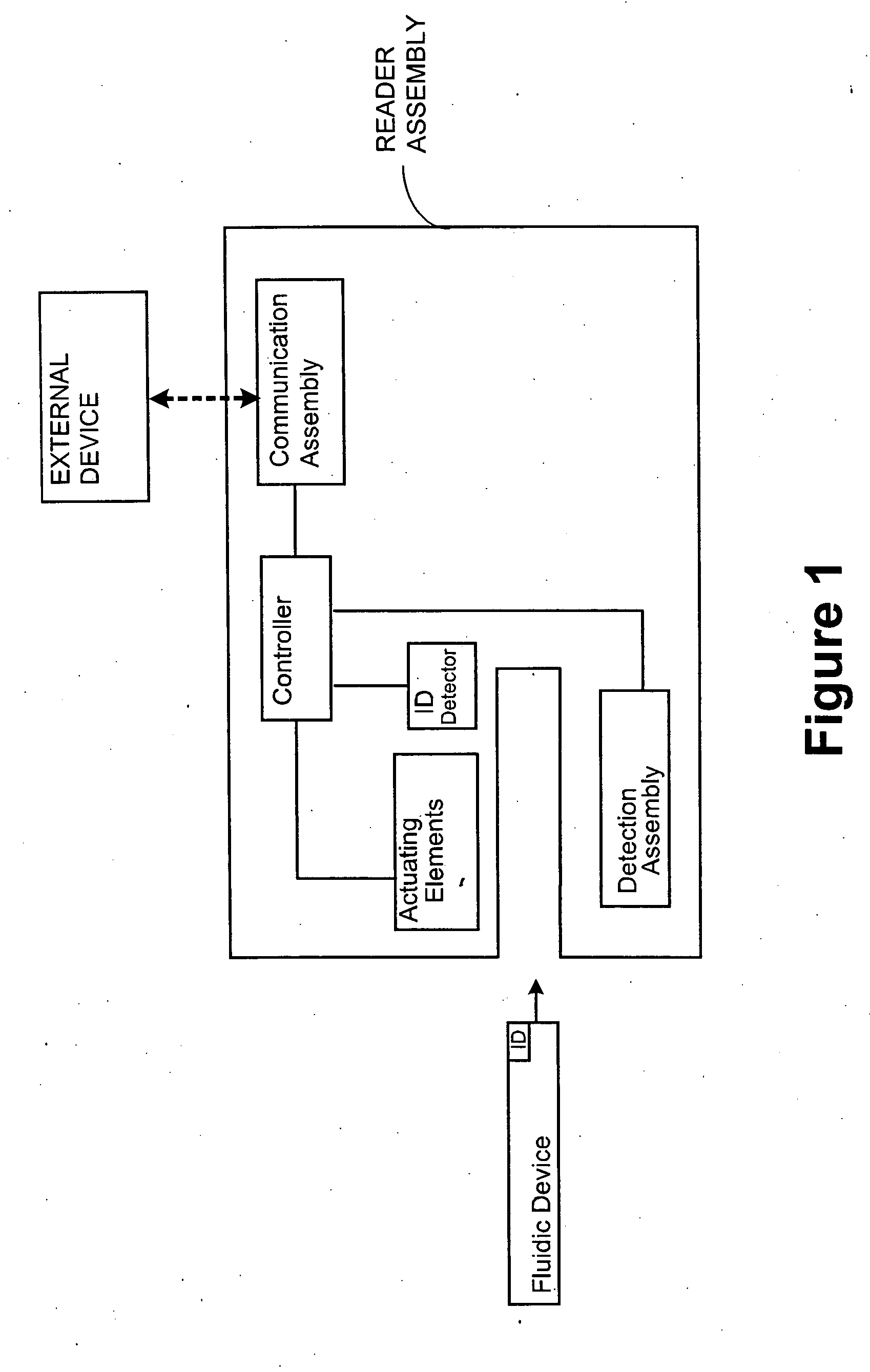
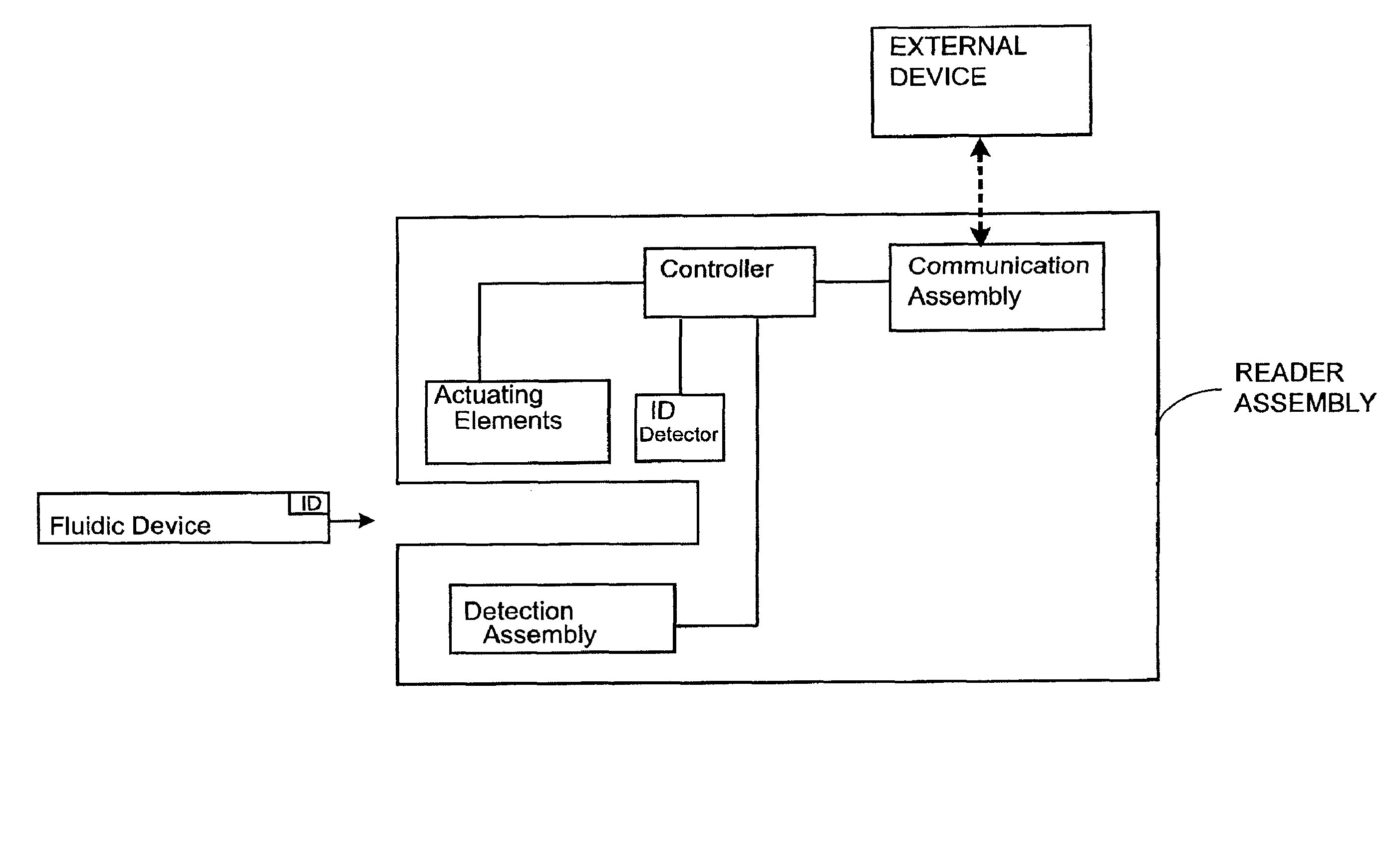
Systems and Methods of Sample Processing and Fluid Control in a Fluidic System
ActiveUS20070224084A1Reduce Interfering SignalsMicrobiological testing/measurementCatheterAnalyteFluid control
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:GOLDEN DIAGNOSTICS CORP
Systems and methods for monitoring pharmacological parameters
InactiveUS20060264783A1Simplify laborUseful in detectionCompound screeningApoptosis detectionAnalyteMedicine
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:THERANOS +2
Systems and methods for conducting animal studies
ActiveUS20060264780A1Simplify laborFacilitate high throughput point-of-care testingCompound screeningApoptosis detectionAnalytePoint-of-care testing
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:GOLDEN DIAGNOSTICS CORP
Systems and methods of fluidic sample processing
ActiveUS8158430B1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalytePoint-of-care testing
The present invention provides fluidic devices and systems that allow detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
Detection and quantification of analytes in bodily fluids
InactiveUS20080113391A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalytePoint-of-care testing
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:THERANOS
In situ lysis of cells in lateral flow immunoassays
ActiveUS20100015634A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteLysis
Devices and methods incorporate lysis agents into a point-of-care testing device. The sample is loaded, and then the sample travels until it encounters a lysis agent. The lysis agent is preferably pre-loaded onto the collection device. In a preferred embodiment, the initially lysis agent is localized between the sample application zone and the conjugate zone. The lysis agent is preferably soluble or miscible in the sample transport liquid, and the lysis agent is solubilized and activated upon contact with the sample transport liquid. The sample transport liquid then contains both lysis agent in solution or suspension and sample components in suspension. Any lysis-susceptible components in a sample, then being exposed in suspension to the lysis agent, are themselves lysed in situ. The running buffer then carries the analyte, including any lysis-freed components, to the detection zone.
Owner:RAPID PATHOGEN SCREENING INC
Detection and quantification of analytes in bodily fluids
ActiveUS8778665B2Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalytePoint-of-care testing
Owner:LABRADOR DIAGNOSTICS LLC
Systems and methods of fluidic sample processing
The present invention provides fluidic devices and systems that allow detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
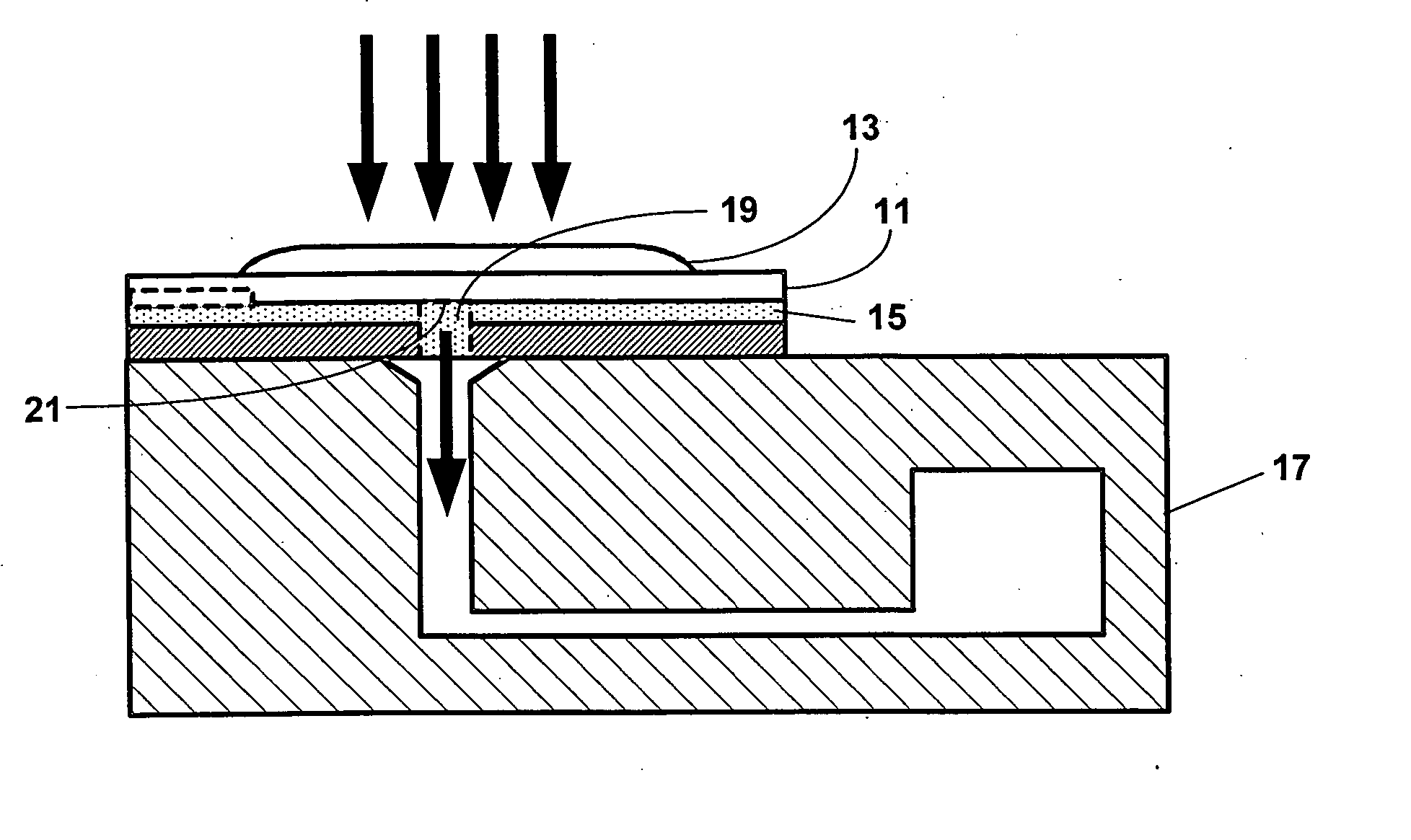
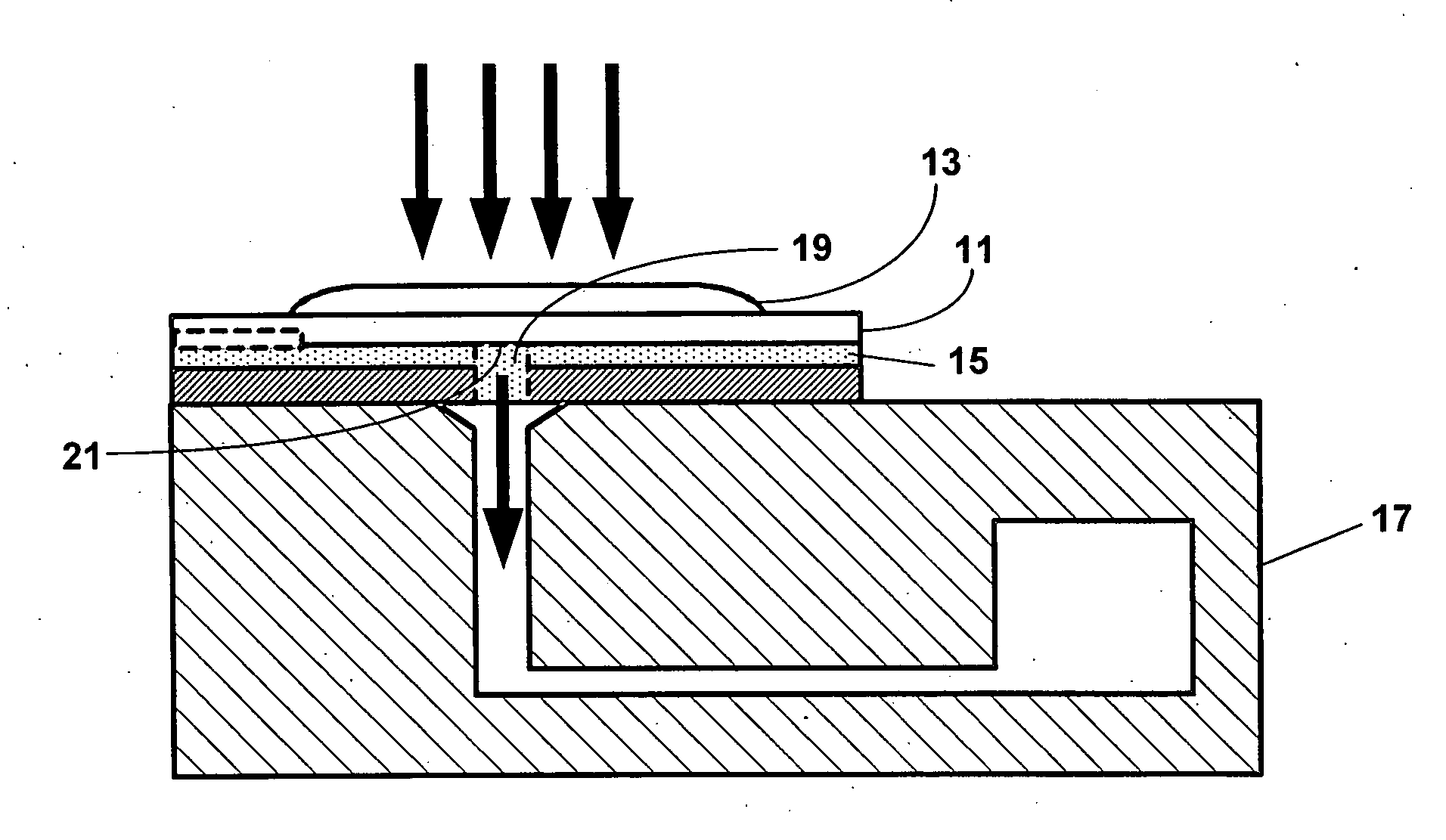
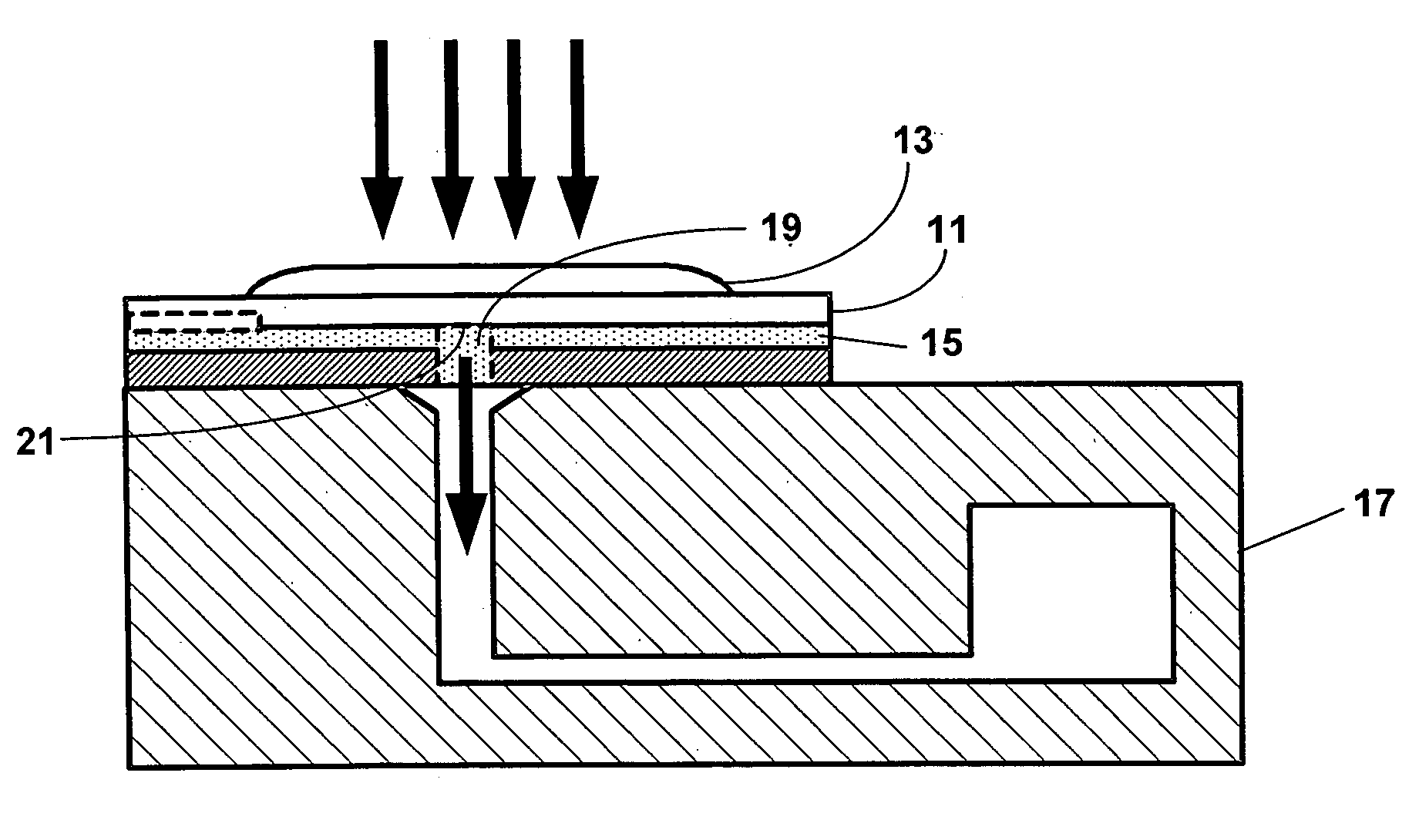
Reducing optical interference in a fluidic device
ActiveUS8012744B2Reduce distractionsBioreactor/fermenter combinationsBiological substance pretreatmentsAnalytePoint-of-care testing
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications. In particular, the medical device reduces interference with an optical signal which is indicative of the presence of an analyte in a bodily sample.
Owner:LABRADOR DIAGNOSTICS LLC
Systems and methods for improving medical treatments
ActiveUS20080009766A1Simplify laborFacilitate high throughput point-of-care testingCompound screeningApoptosis detectionAnalytePoint-of-care testing
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:GOLDEN DIAGNOSTICS CORP
In situ lysis of cells in lateral flow immunoassays
Devices and methods incorporate lysis agents into a point-of-care testing device. The sample is loaded, and then the sample travels until it encounters a lysis agent. The lysis agent is preferably pre-loaded onto the collection device. In a preferred embodiment, the initially lysis agent is localized between the sample application zone and the conjugate zone. The lysis agent is preferably soluble or miscible in the sample transport liquid, and the lysis agent is solubilized and activated upon contact with the sample transport liquid. The sample transport liquid then contains both lysis agent in solution or suspension and sample components in suspension. Any lysis-susceptible components in a sample, then being exposed in suspension to the lysis agent, are themselves lysed in situ. The running buffer then carries the analyte, including any lysis-freed components, to the detection zone.
Owner:RAPID PATHOGEN SCREENING INC
Reducing optical interference in a fluidic device
ActiveUS8008034B2Reduce distractionsBioreactor/fermenter combinationsBiological substance pretreatmentsAnalytePoint-of-care testing
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications. In particular, the medical device reduces interference with an optical signal which is indicative of the presence of an analyte in a bodily sample.
Owner:LABRADOR DIAGNOSTICS LLC
Systems and methods of sample processing and fluid control in a fluidic system
ActiveUS8741230B2Reduce Interfering SignalsMicrobiological testing/measurementLaboratory glasswaresFluid controlAnalyte
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:GOLDEN DIAGNOSTICS CORP
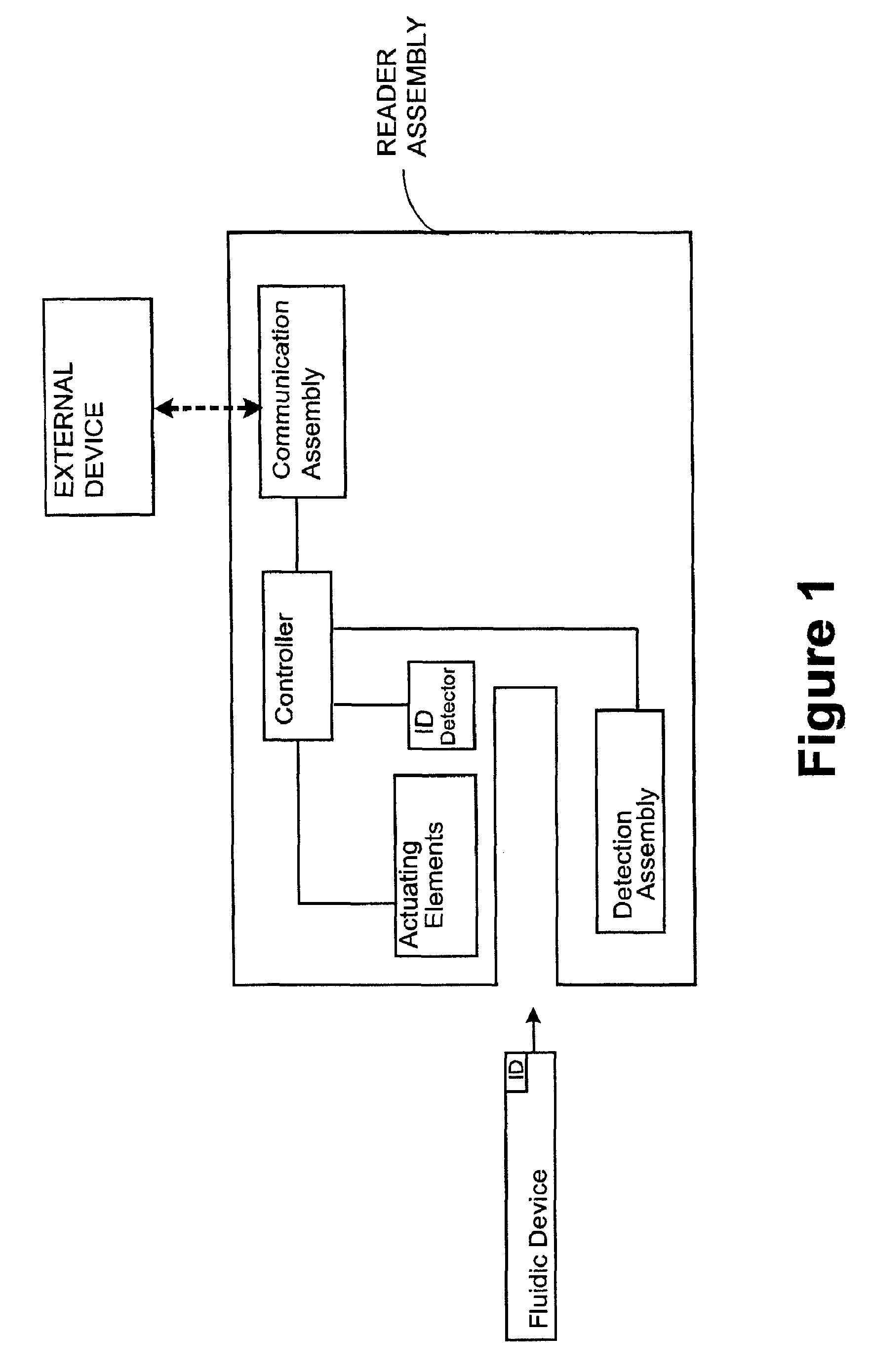
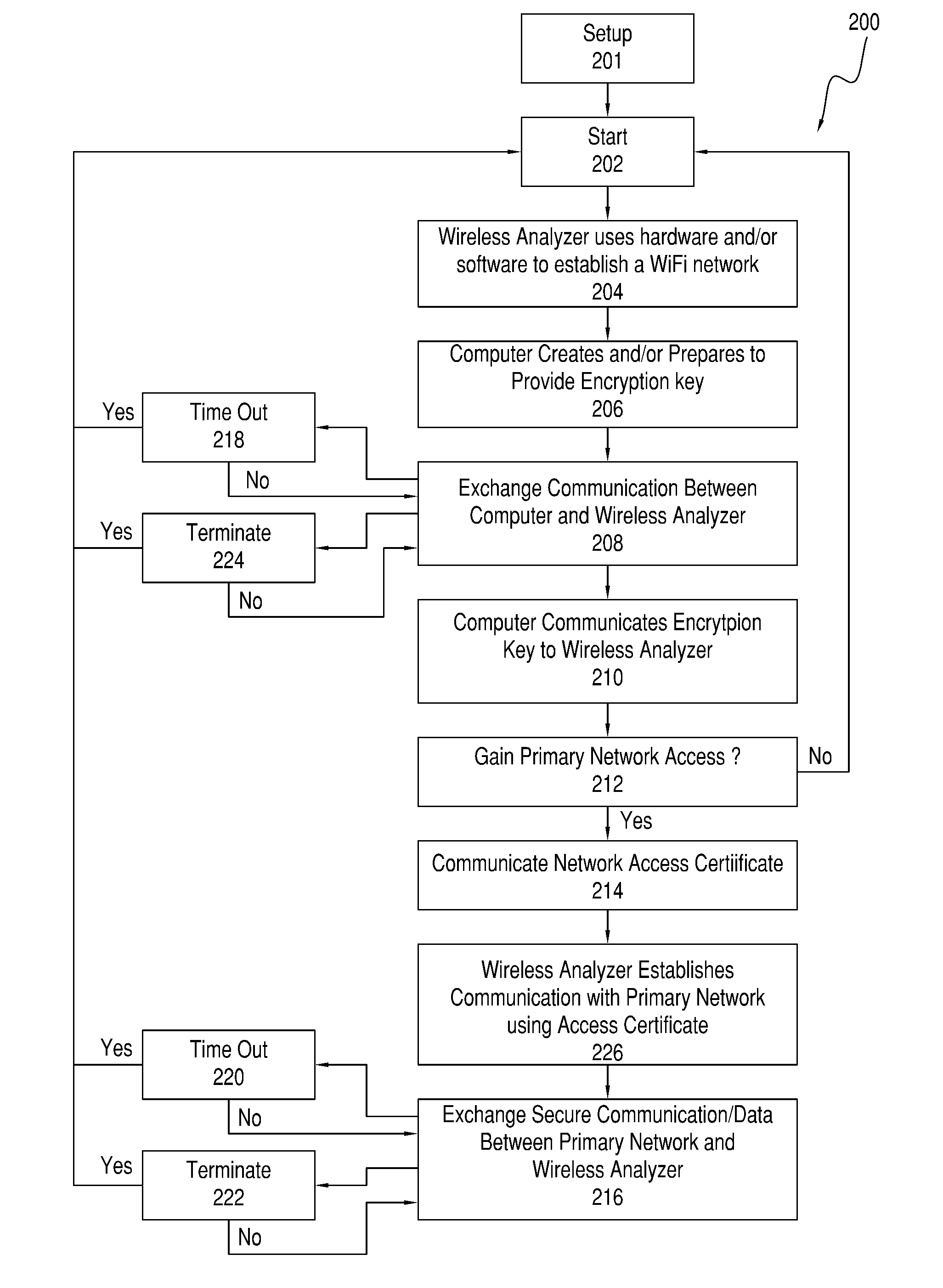
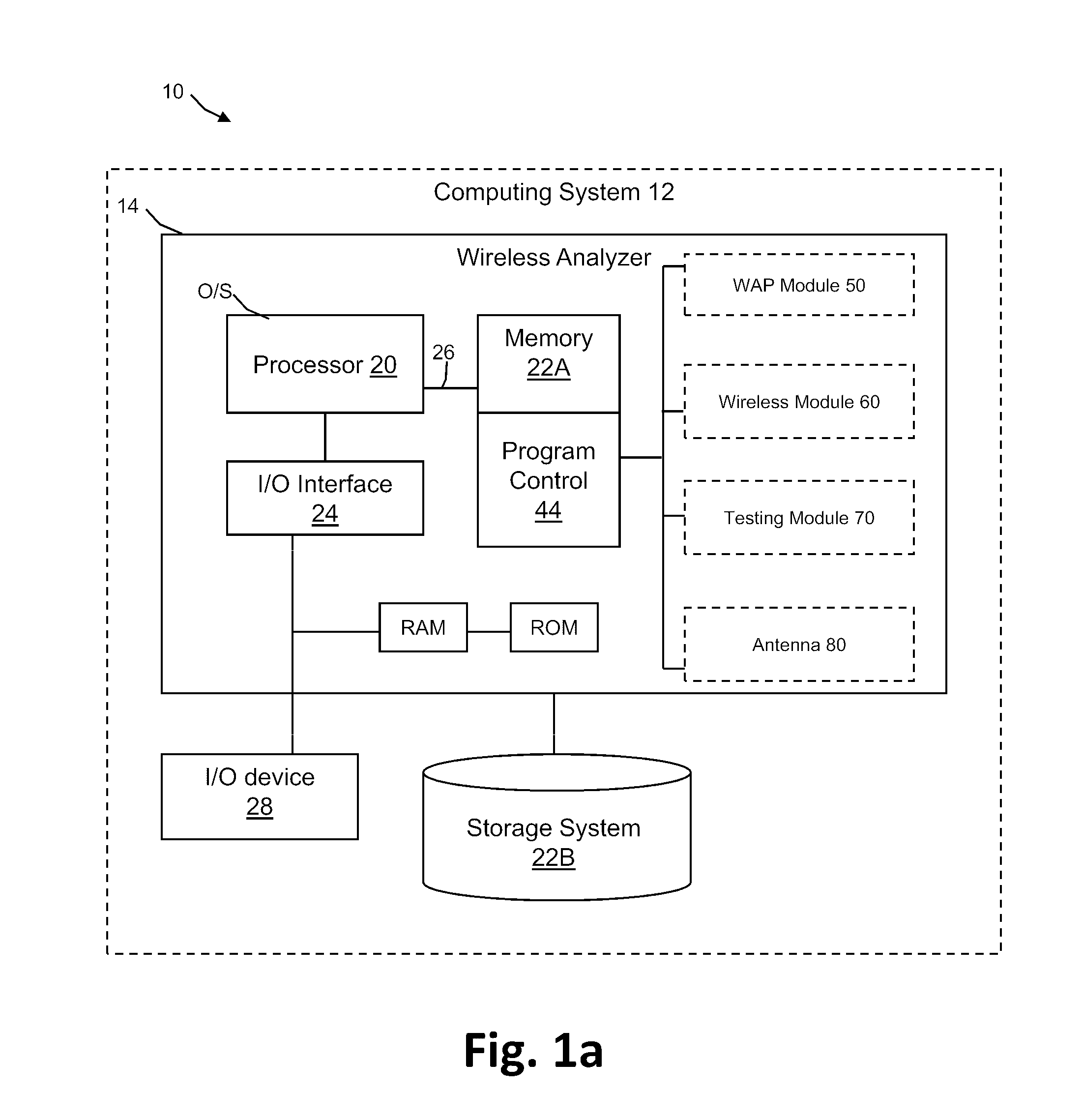
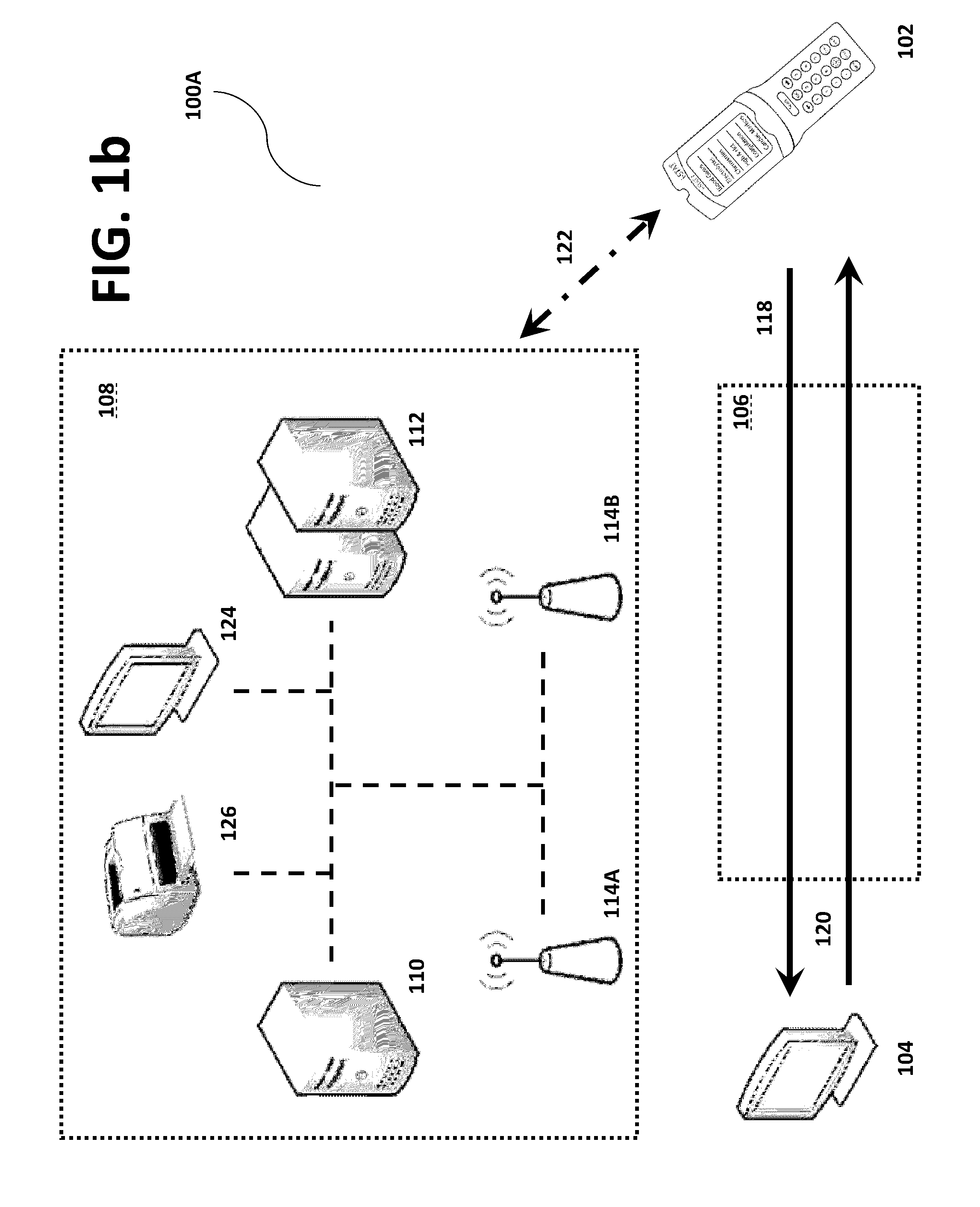
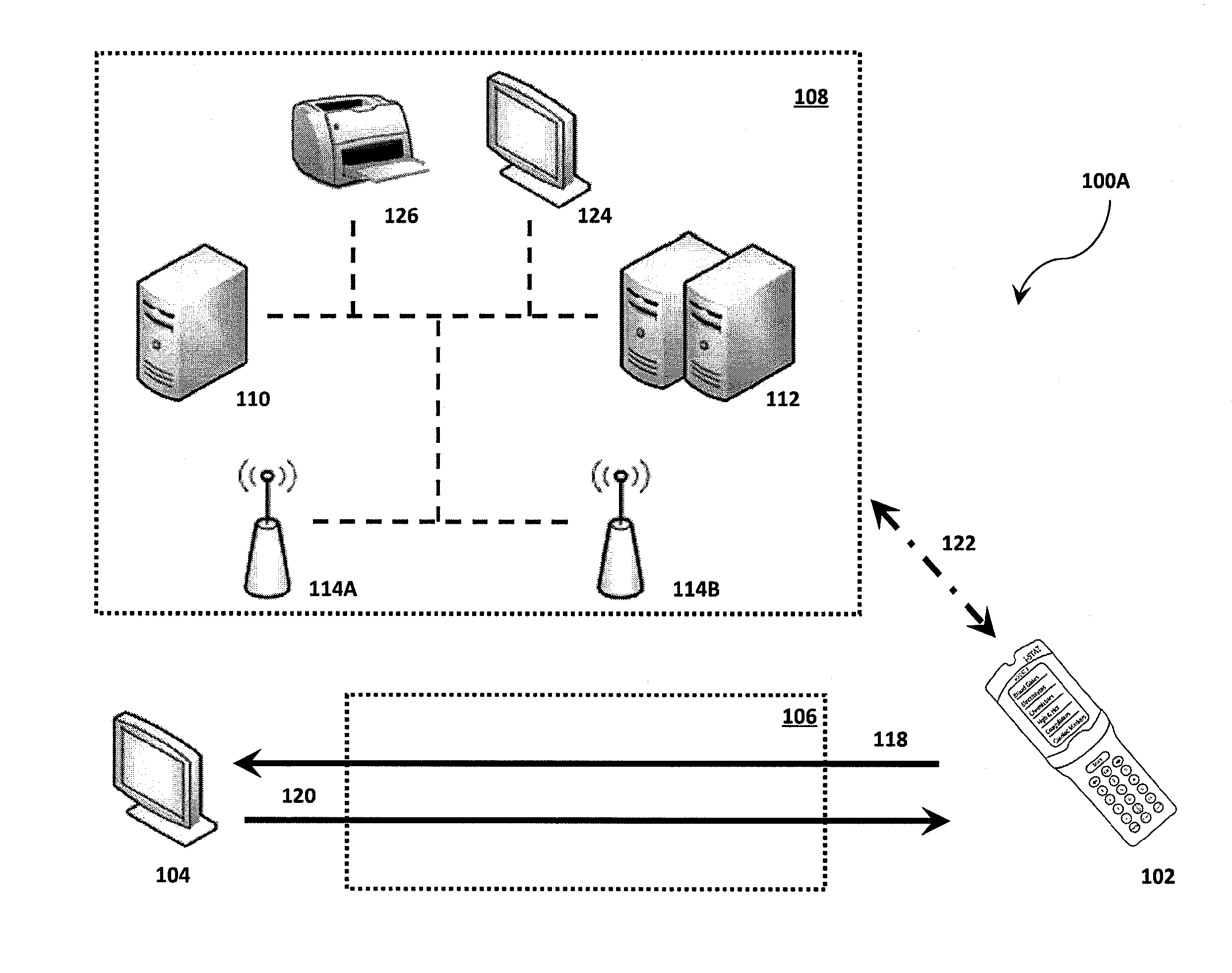
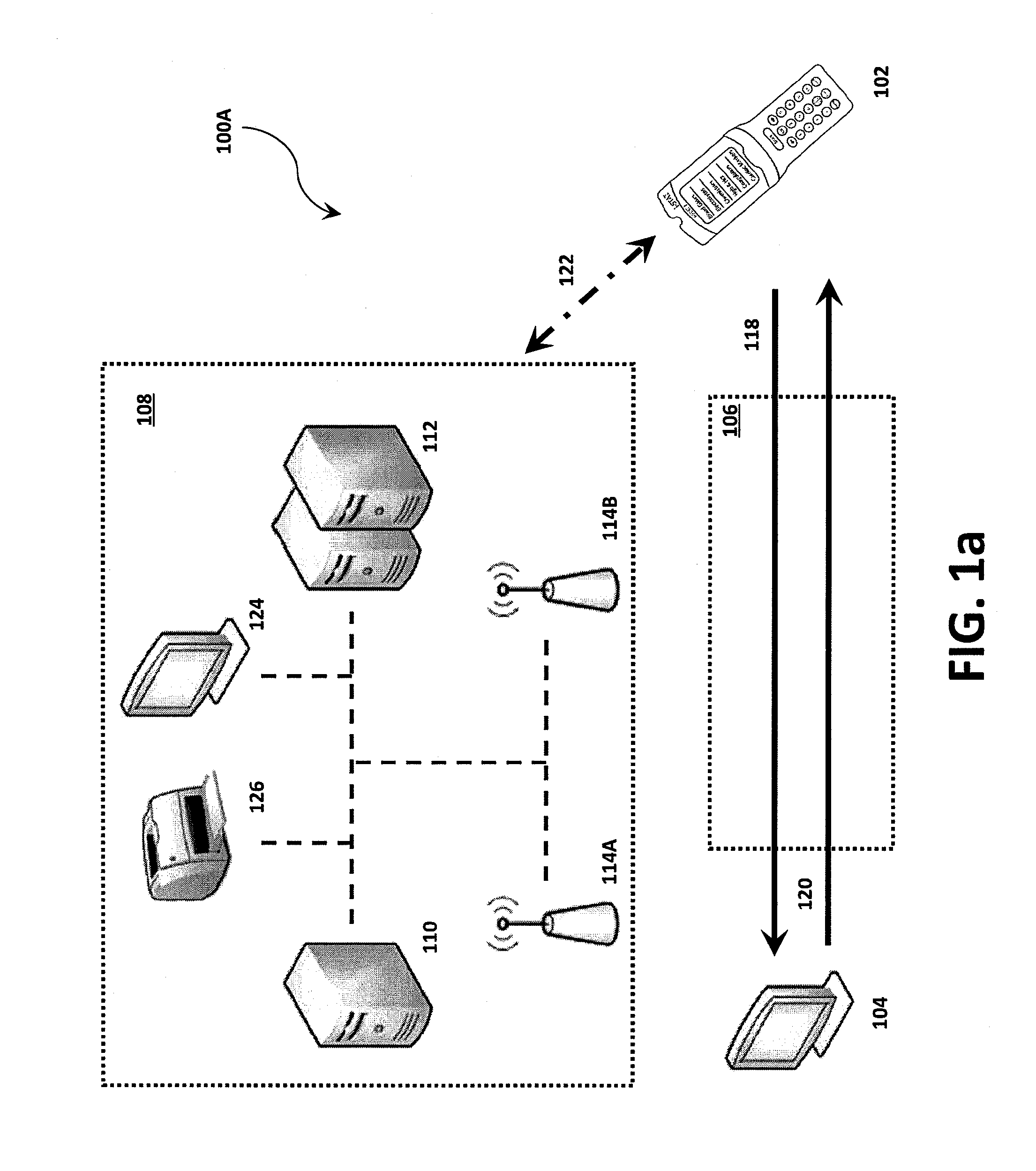
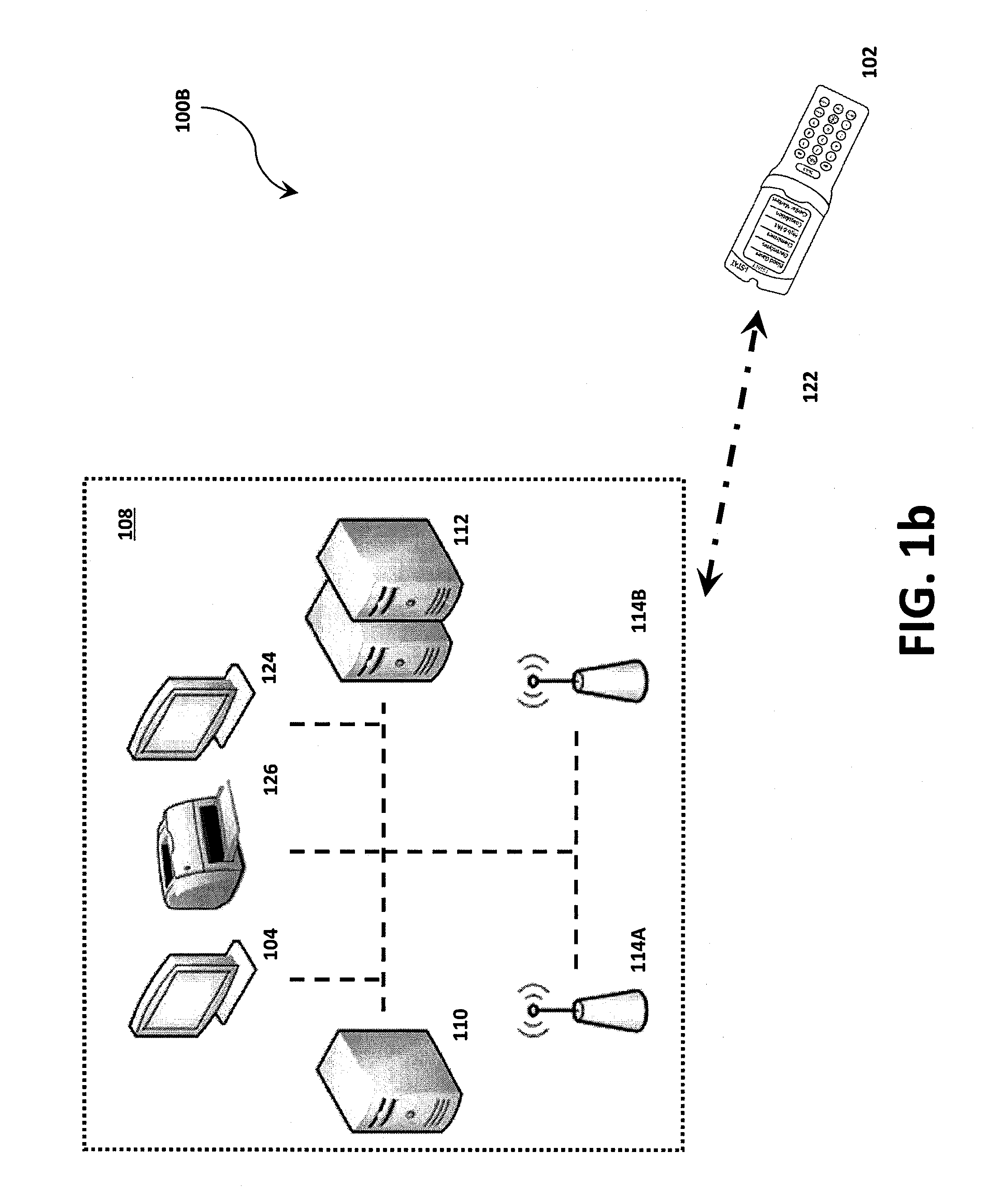
Systems, methods and analyzers for establishing a secure wireless network in point of care testing
ActiveUS8549600B2Secure CommunicationsDigital data processing detailsUser identity/authority verificationPoint of careWi-Fi
A system and method for initiating and maintaining a secure wireless communication between a wireless analyzer and a target network (e.g., a hospital network connected to a LIS and / or HIS). The present disclosure provides novel processes and systems for securely networking a wireless analyzer with a Wi-Fi network without the need for an operator or user to engage in manual initiation steps on, or through, the wireless analyzer.
Owner:ABBOTT POINT CARE
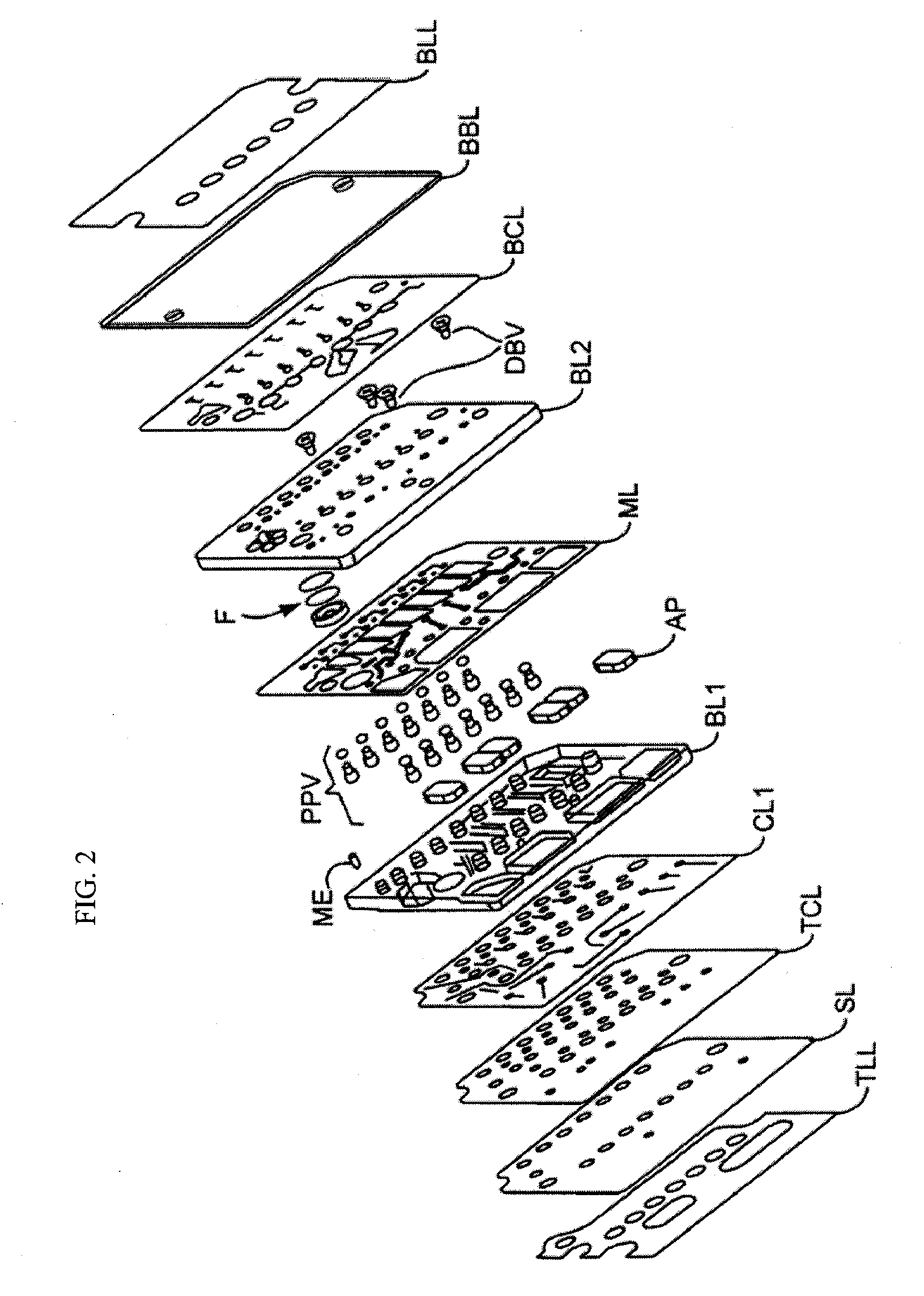
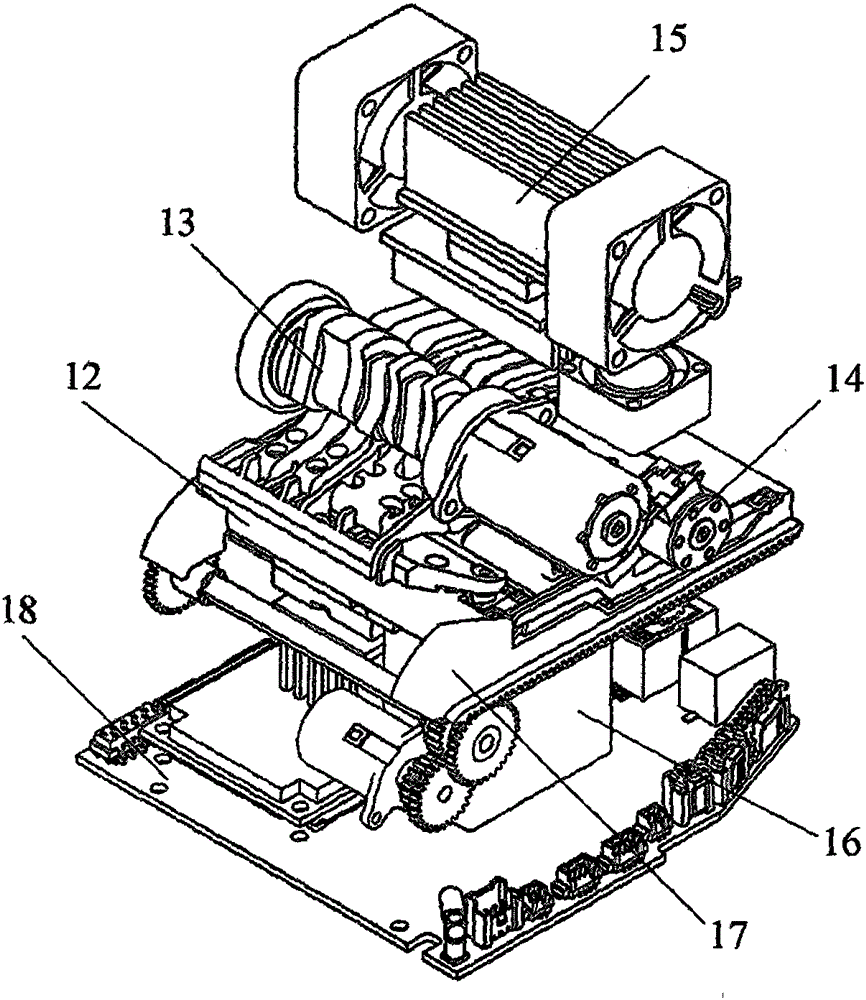
POCT (Point of Care Testing) chemiluminescence immunoassay system and method
ActiveCN105203746ASimple structureReduce volumeBiological testingTemperature controlPoint-of-care testing
The invention relates to a POCT (Point of Care Testing) chemiluminescence immunoassay system and a POCT chemiluminescence immunoassay method. The POCT chemiluminescence immunoassay system comprises a testing card, an integrated fluid driving module, a magnet control module, a temperature control module, an optical signal detection module, an automatic testing card pushing device and a circuit analysis control module. Whole blood can be adopted as a detection sample of the system, and can be directly used without centrifugal treatment after being sampled, POCT is achieved beside a patient, and a diagnosis result is quickly obtained.
Owner:SHENZHEN HUAMAIXINGWEI MEDICAL TECH CO LTD
Integrated instrumentation for the analysis of biofluids at the point-of-care
ActiveUS20150004717A1Improve performanceMinimize intra-assay CVMaterial analysis by optical meansChemical methods analysisPoint of careLab-on-a-chip
Owner:RICE UNIV
Systems and methods of fluidic sample processing
ActiveUS20120258472A1Component separationChemiluminescene/bioluminescenceAnalytePoint-of-care testing
The present invention provides fluidic devices and systems that allow detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
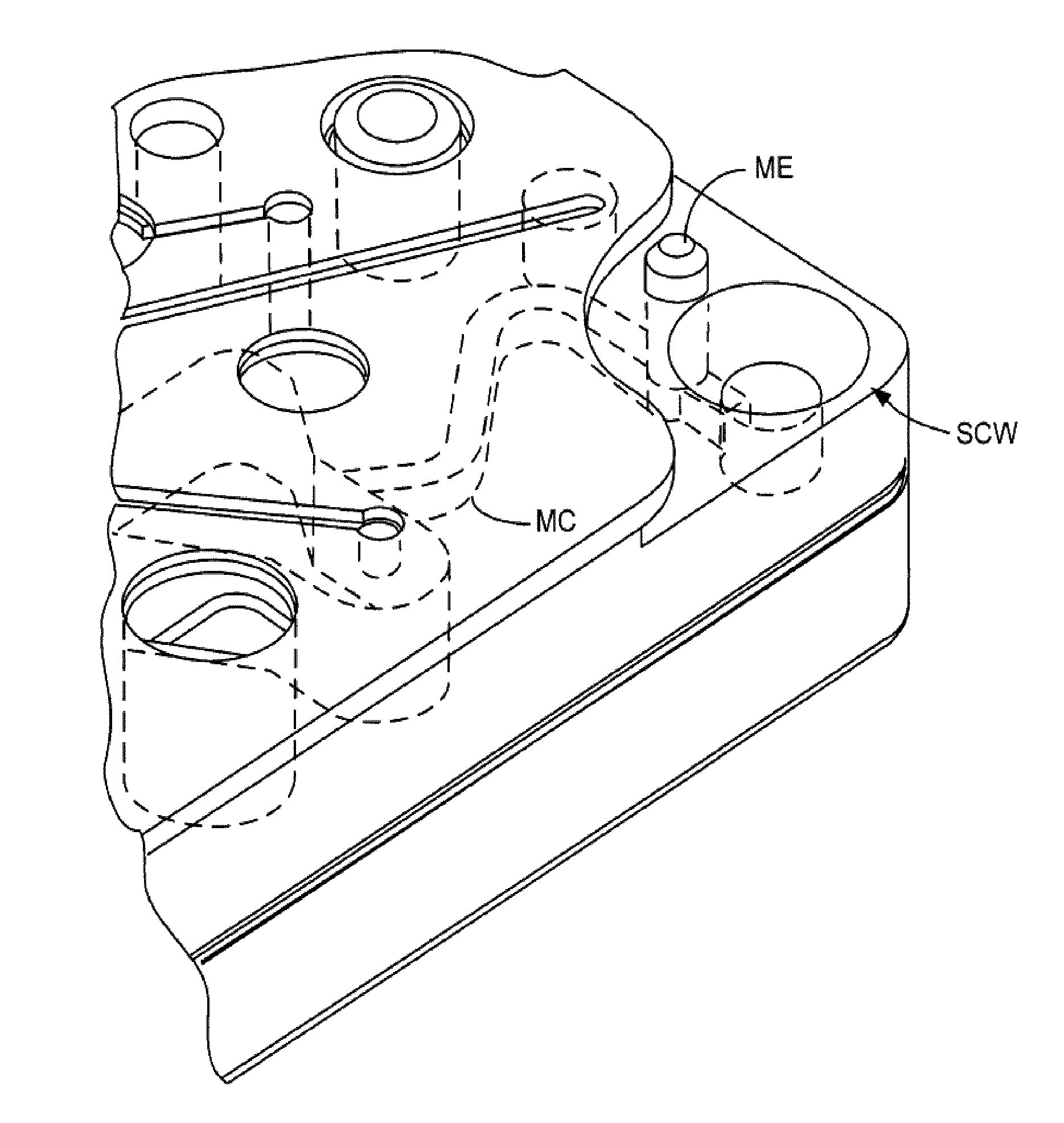
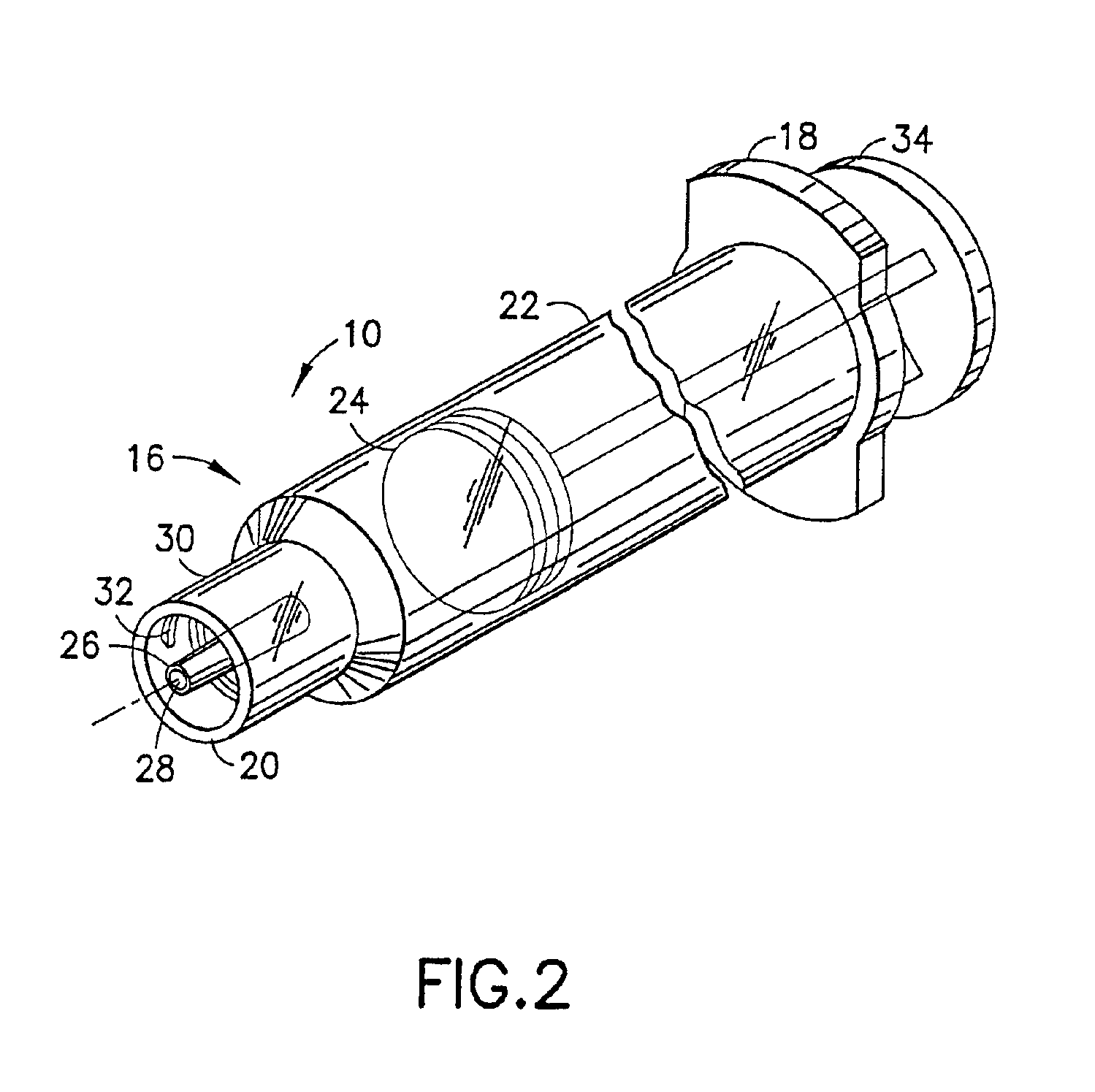
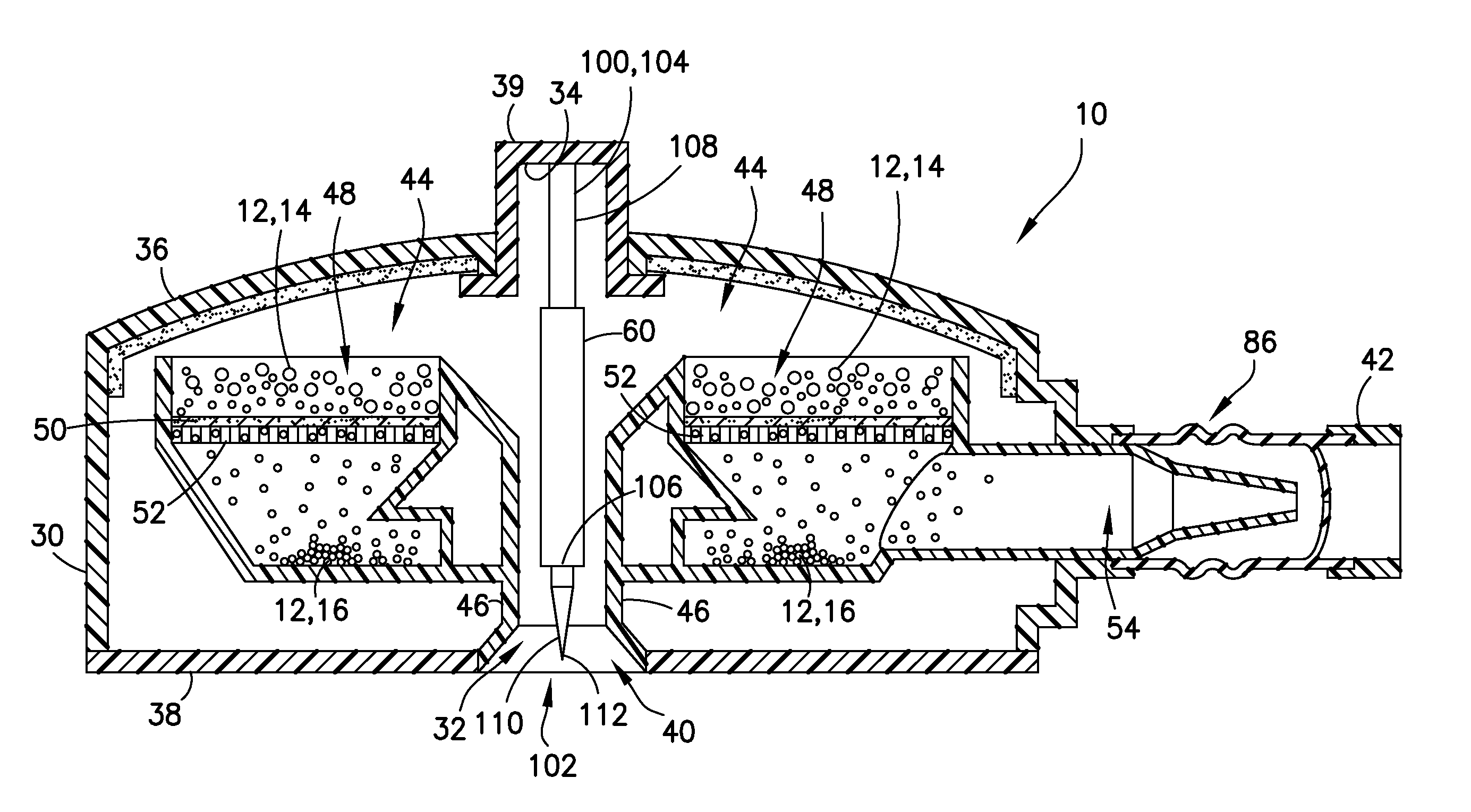
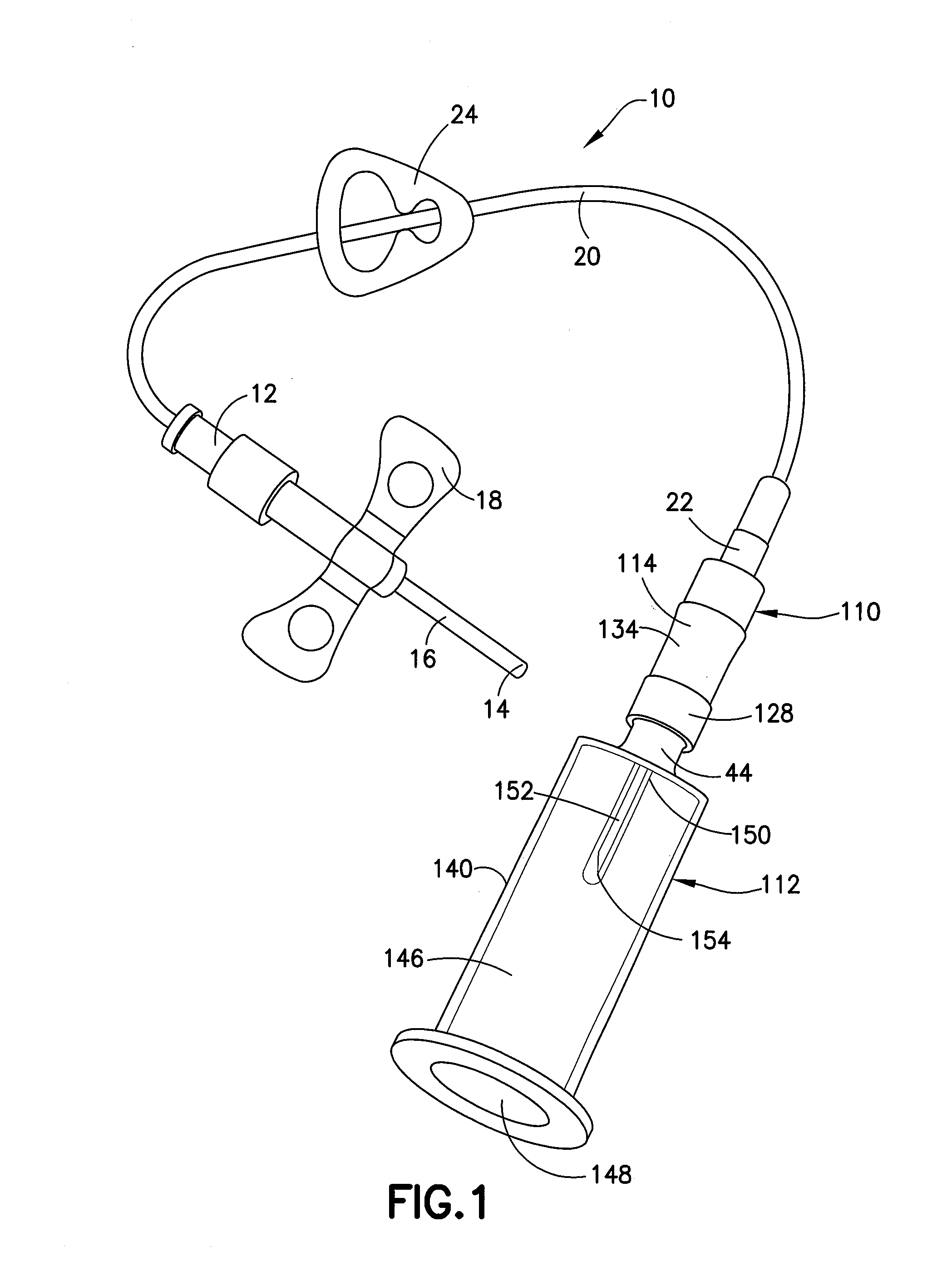
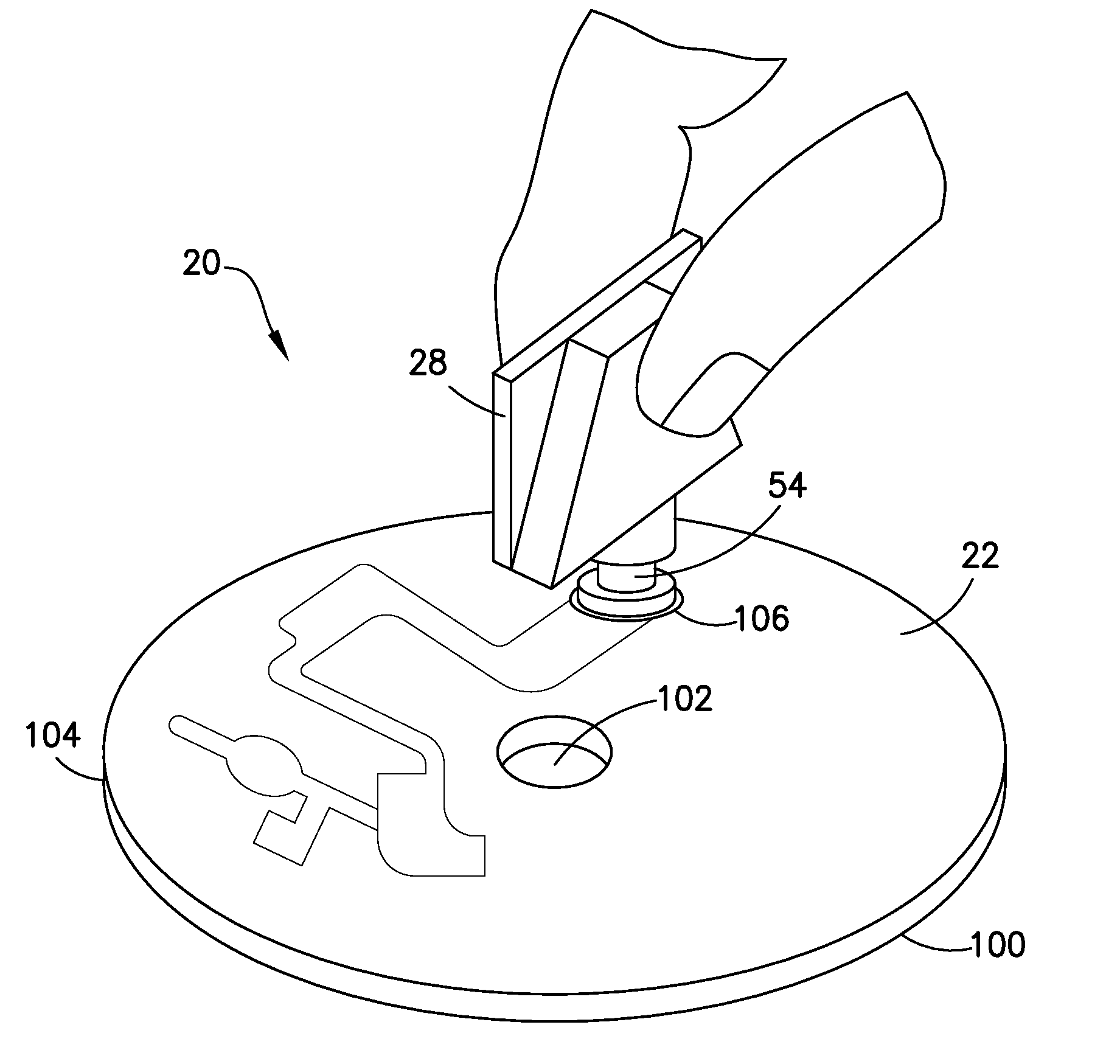
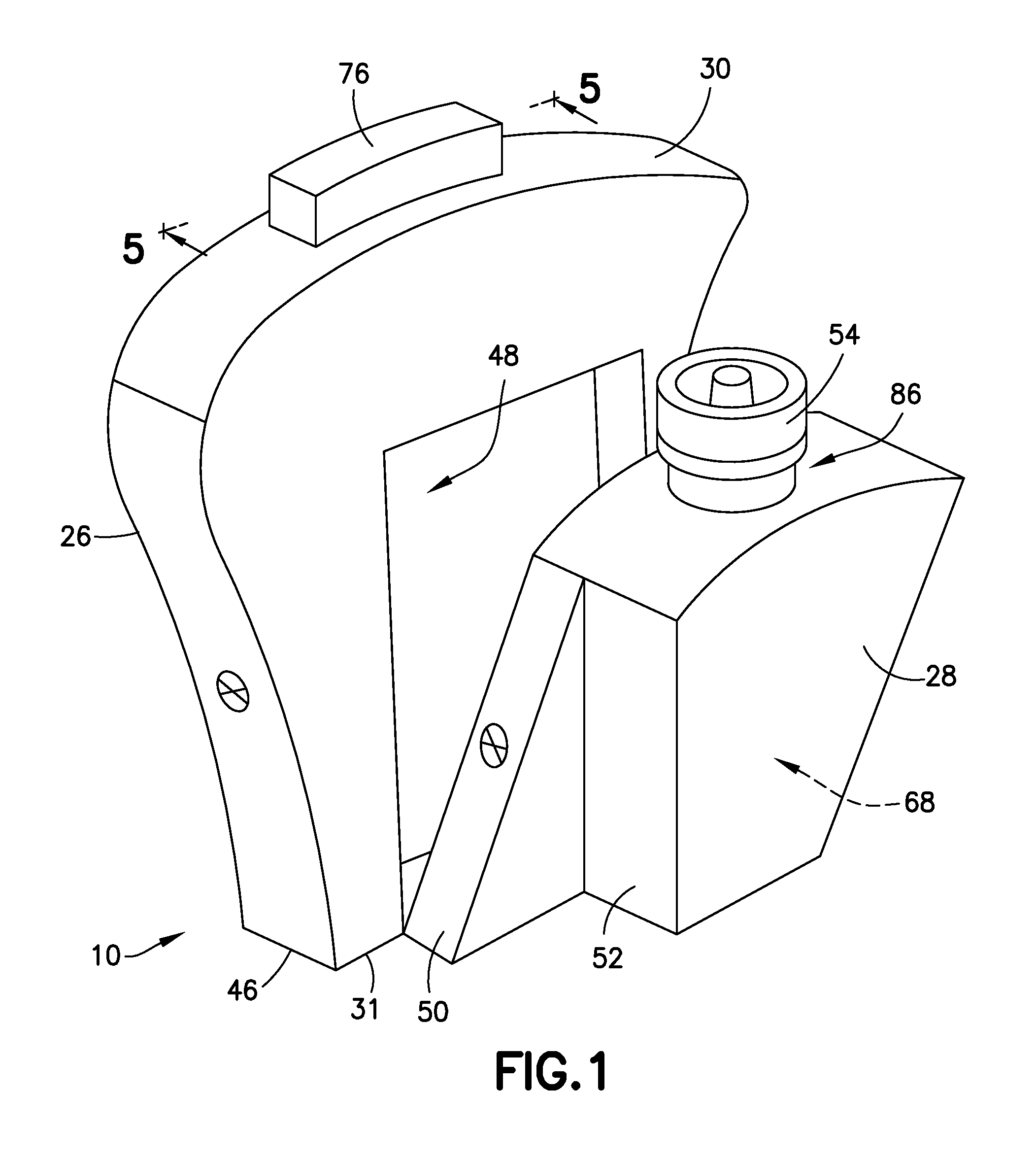
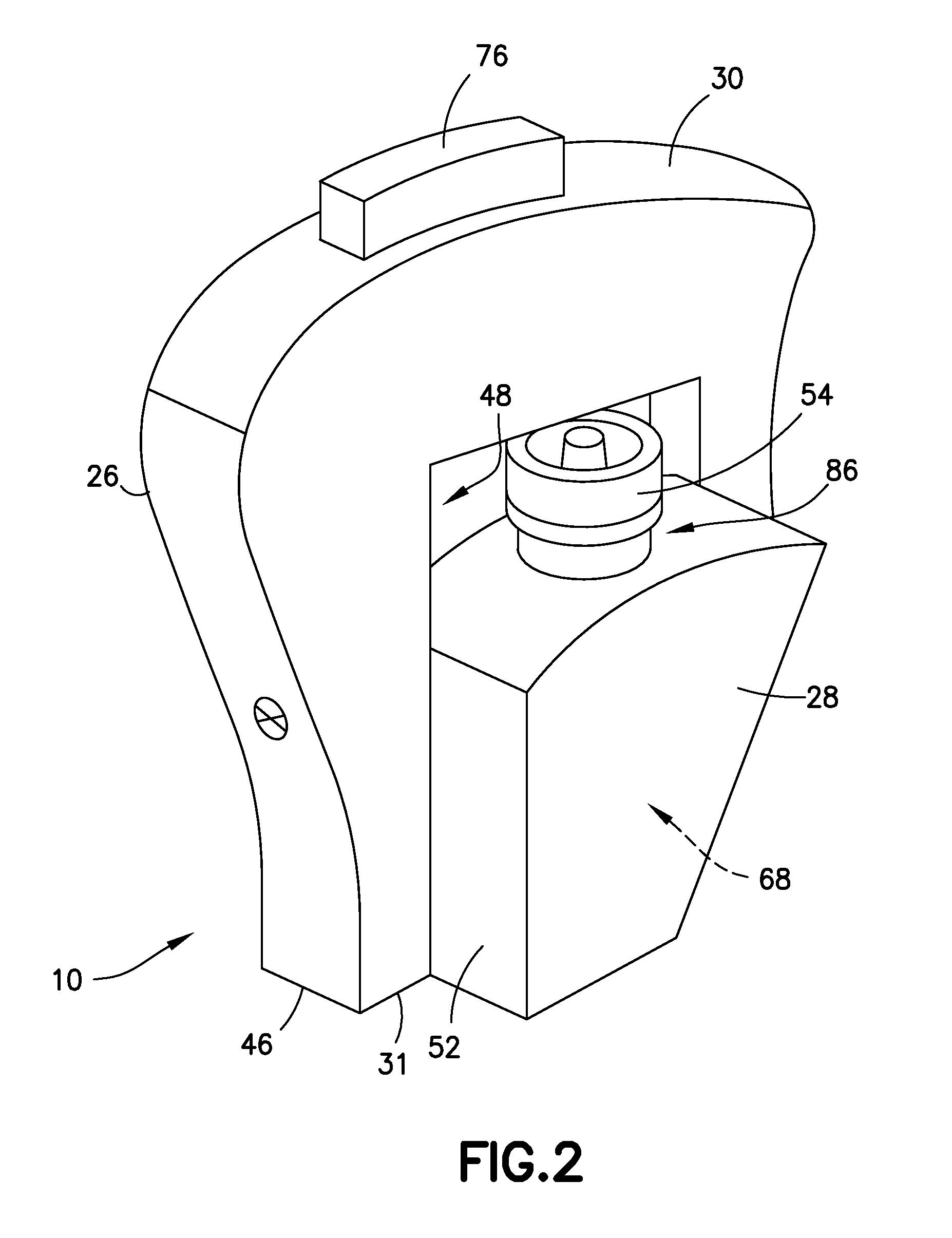
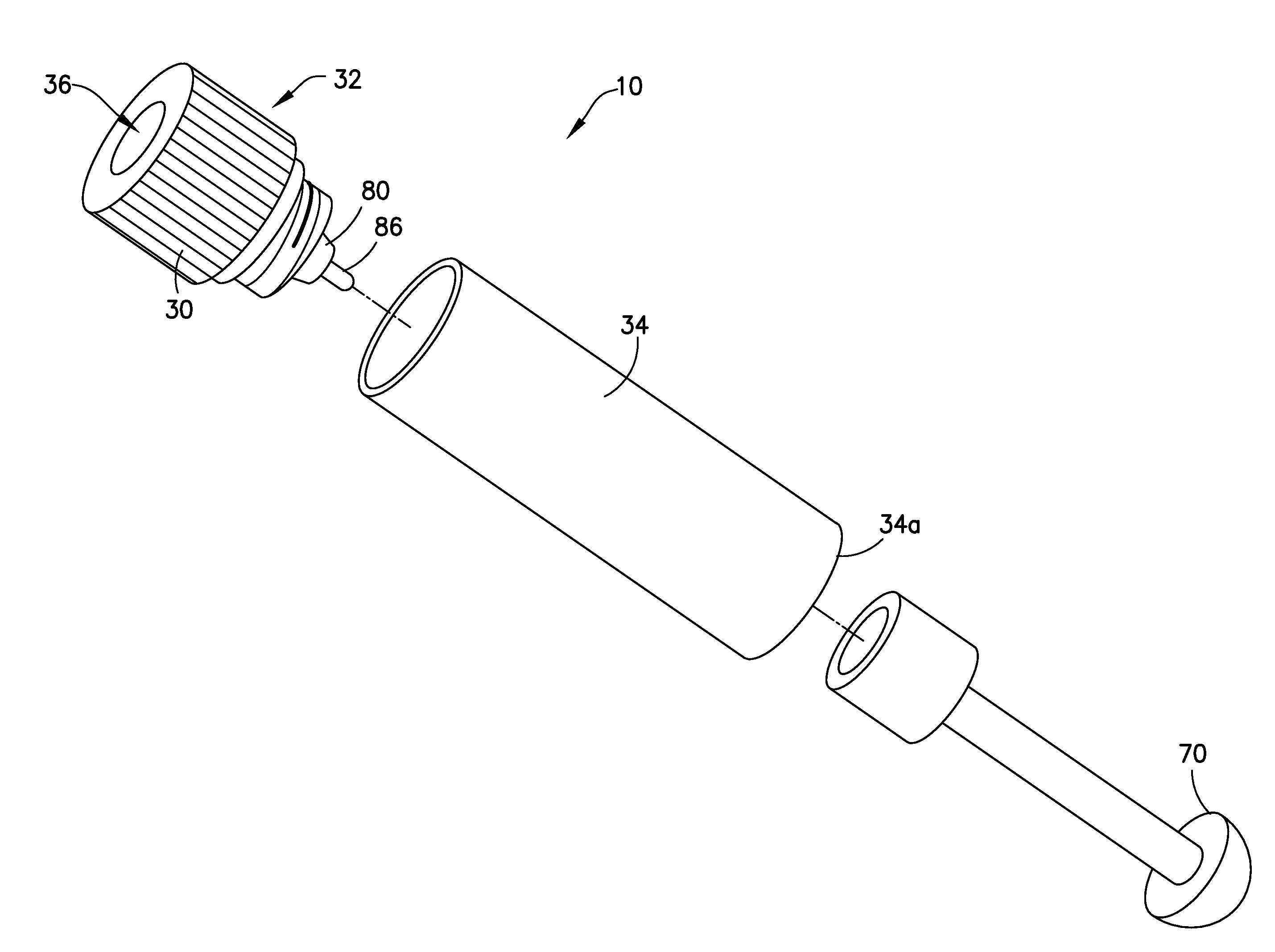
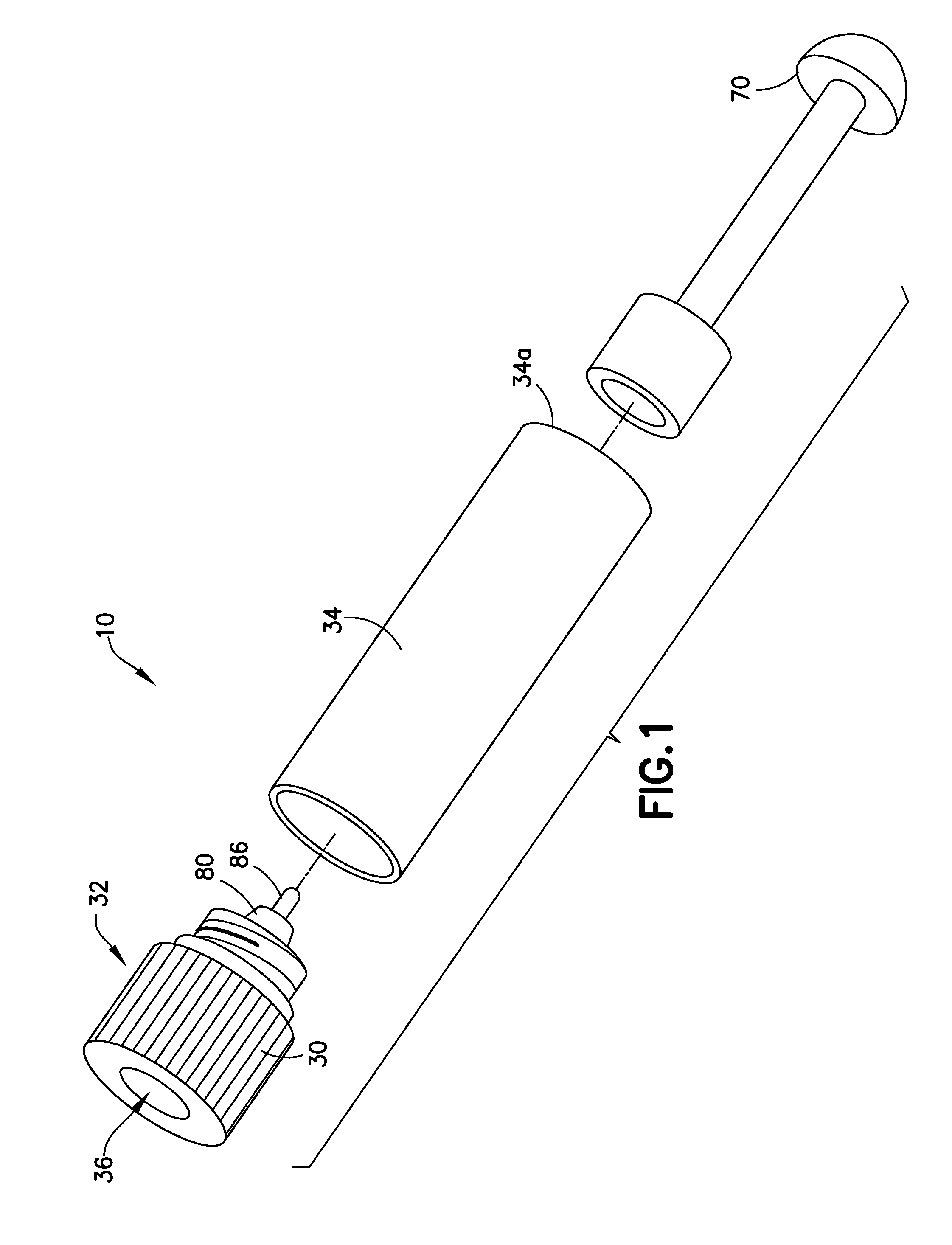
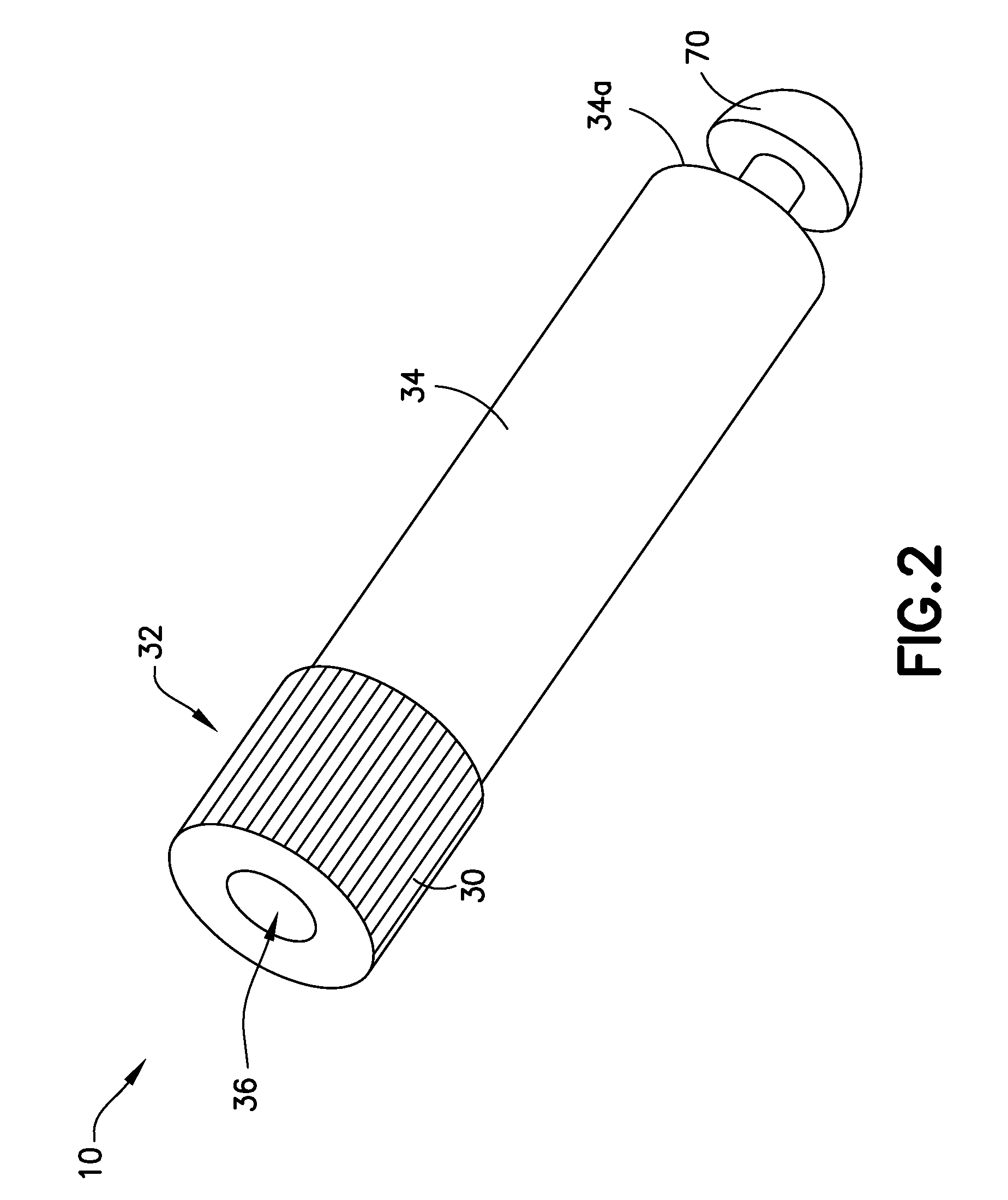
Blunt cannula and filter assembly and method of use with point-of-care testing cartridge
InactiveUS6869405B2Infusion syringesWithdrawing sample devicesCellular componentPoint-of-care testing
Owner:BECTON DICKINSON & CO
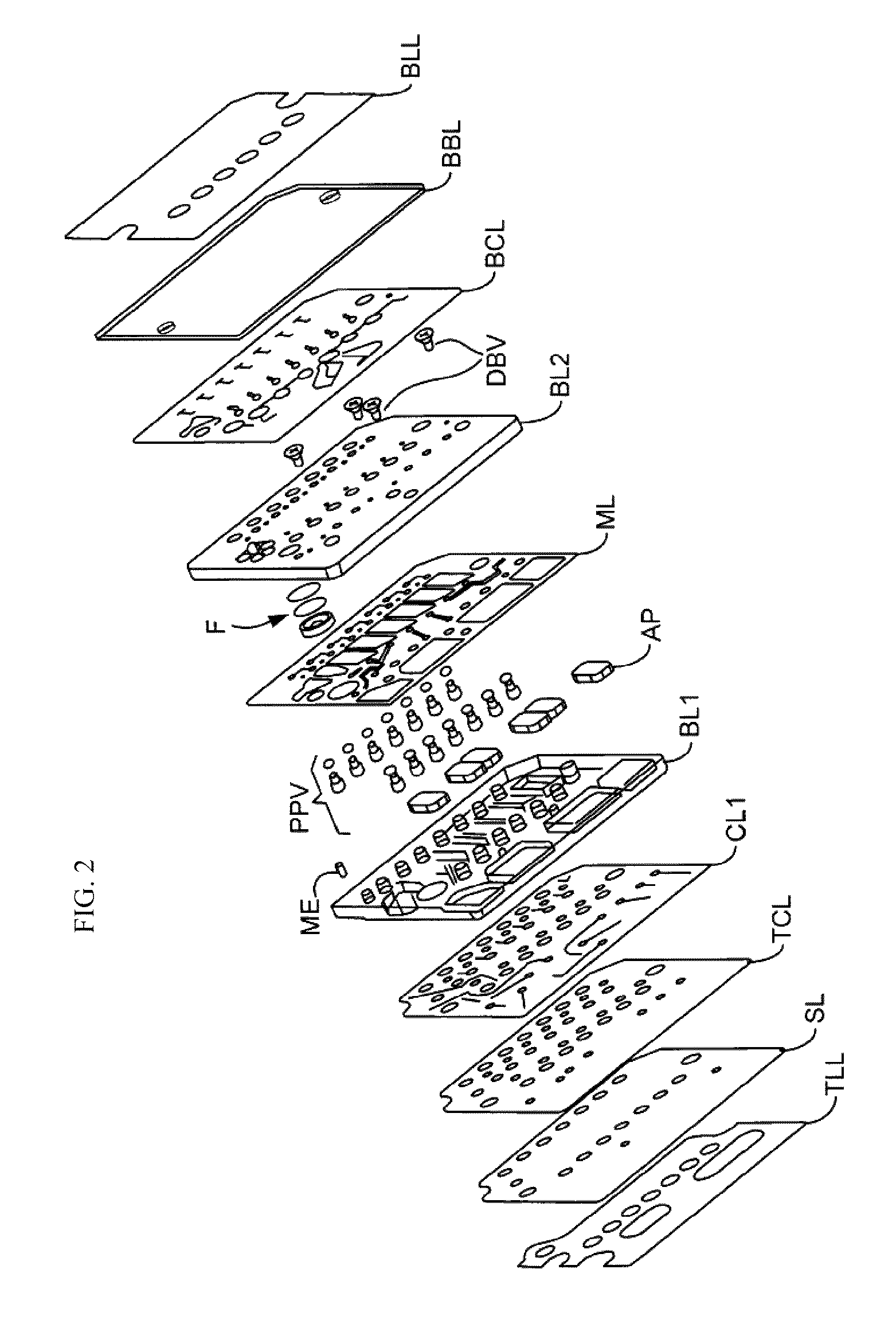
Biological Fluid Collection Device and Biological Fluid Collection and Testing System
ActiveUS20140309556A1Reduce blood exposureQuick mixAnalysis material containersOther blood circulation devicesBlood Collection TubeBlood test
A blood collection device adapted to receive a multi-component blood sample is disclosed. After collecting the blood sample, the blood collection device separates a plasma portion from a cellular portion. After separation, the blood collection device is able to transfer the plasma portion of the blood sample to a point-of-care testing device. The blood collection device of the present disclosure also provides a closed collection and transfer system that reduces the exposure of a blood sample and provides fast mixing of a blood sample with a sample stabilizer. The blood collection device is engageable with a blood testing device for closed transfer of a portion of the plasma portion from the blood collection device to the blood testing device. The blood testing device is adapted to receive the plasma portion to analyze the blood sample and obtain test results.
Owner:BECTON DICKINSON & CO
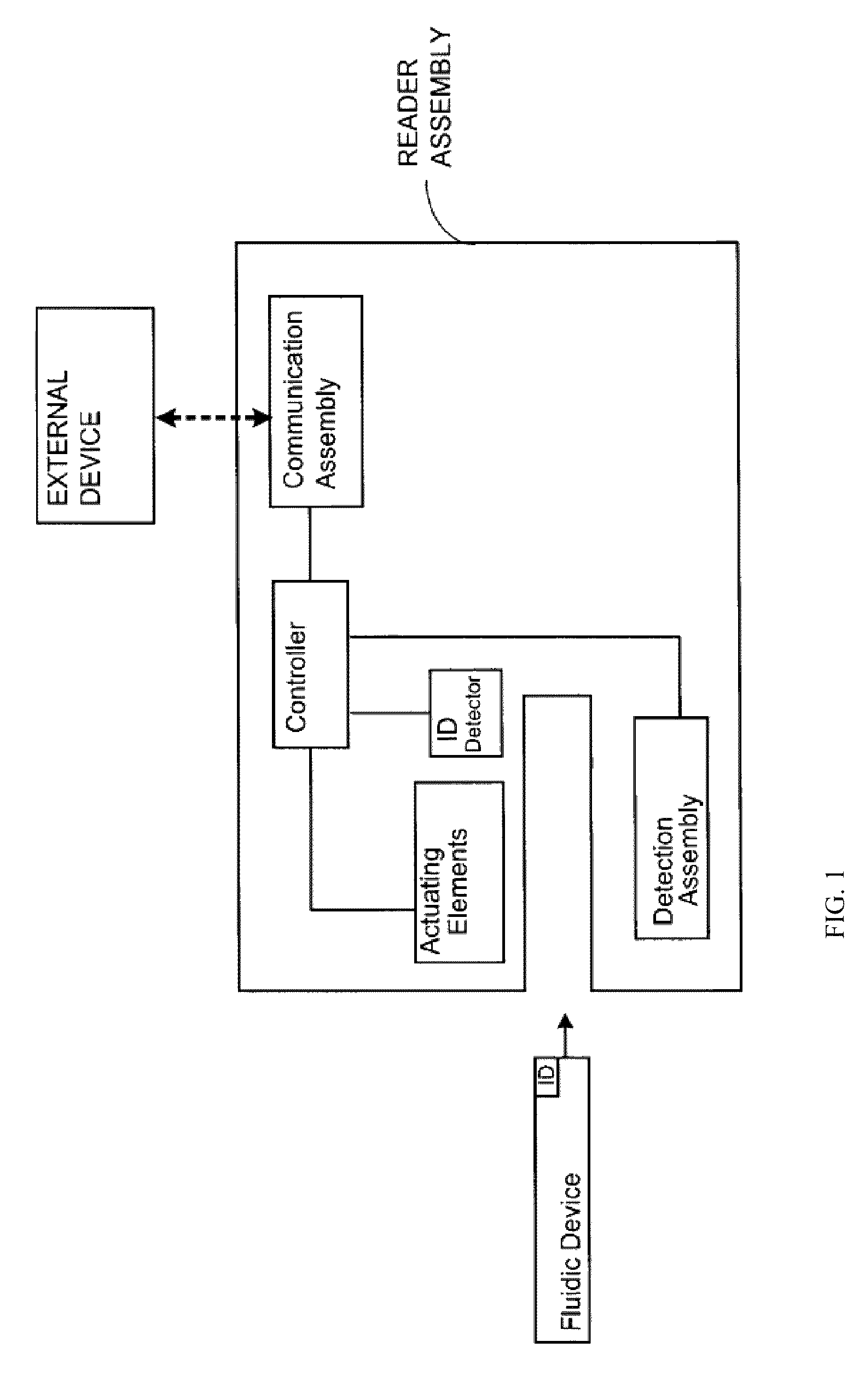
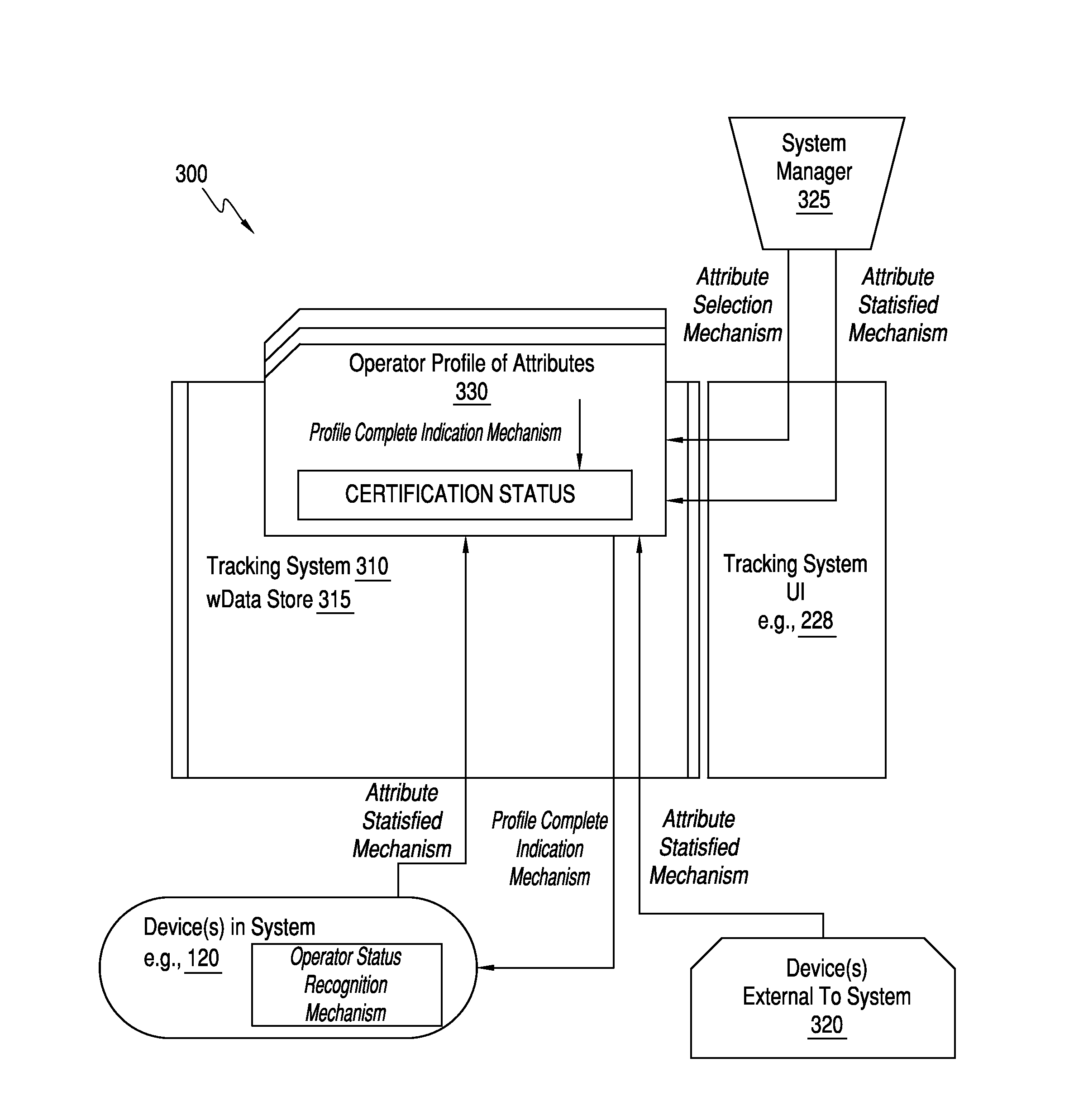
Management system for point of care testing
The present invention relates to a method and system for quality compliance, system and operator verification, and process management for point of care biological sample testing systems used in hospitals and other medical delivery environments. Specifically, the present invention may be directed to a computing device configured to generate a plurality of attributes configured to assess a competency level of an operator to operate at least one sample testing instrument, obtain operator derived data pertaining to the operator's ability to operate the at least one sample testing instrument, and determine a competency level of the operator for the at least one sample testing instrument based the plurality of attributes and the operator derived data.
Owner:ABBOTT POINT CARE
Medical Device for Collection of a Biological Sample
ActiveUS20140309551A1Analysis material containersOther blood circulation devicesPoint of careVascular Access Devices
A biological fluid sampling device for collecting a blood sample from a separate vascular access device and for ejecting a portion of the collected sample to a point-of-care testing device for analysis is provided. The biological fluid sampling device includes a body enclosing a reservoir. The reservoir has an internal volume sufficient to contain enough blood for use in a diagnostic test. The sampling device further includes: an access lumen extending from a distal end of the body for establishing fluid communication between a separate vascular access device and the reservoir; an outflow lumen also in fluid communication with the reservoir; and a removable vented cap attached to the outflow lumen including a gas permeable vent in gaseous communication between the reservoir and ambient air. In addition, several sample and transfer devices are provided for obtaining a sample from a subject and transferring the sample to a point-of-care testing device.
Owner:BECTON DICKINSON & CO
Method for detection of foot-and-mouth disease virus with chromatographic strip test
InactiveUS20080280296A1Improve detection efficiencyQuick stepsMicrobiological testing/measurementReverse transcriptasePoint-of-care testing
The present invention discloses a method for detection of foot-and-mouth disease virus with chromatographic strip test. Firstly, the nucleic acid sequence of FMDV NSPs is set up, the nucleic acid sequence is amplified by the reverse transcriptase polymerase chain reaction (RT-PCR) method, the recombinant vector is constructed and performed through a prokaryotic system to transform and express the recombinant protein, and the purified recombinant protein is mass produced. Design principles of the method are based on immunoassay and chromatographic analysis. The advantages are easy and simple to handle, no need of elaborate equipment, only one drop of body fluid is required to quickly complete the qualitative test in 10-20 minutes, and operating with a portable POCT (Point of care testing) instrument to complete the quantitative detection within 40-50 minutes.
Owner:NAT INST FOR ANIMAL HEALTH COUNCIL AGRI EXECUTIVE YUAN
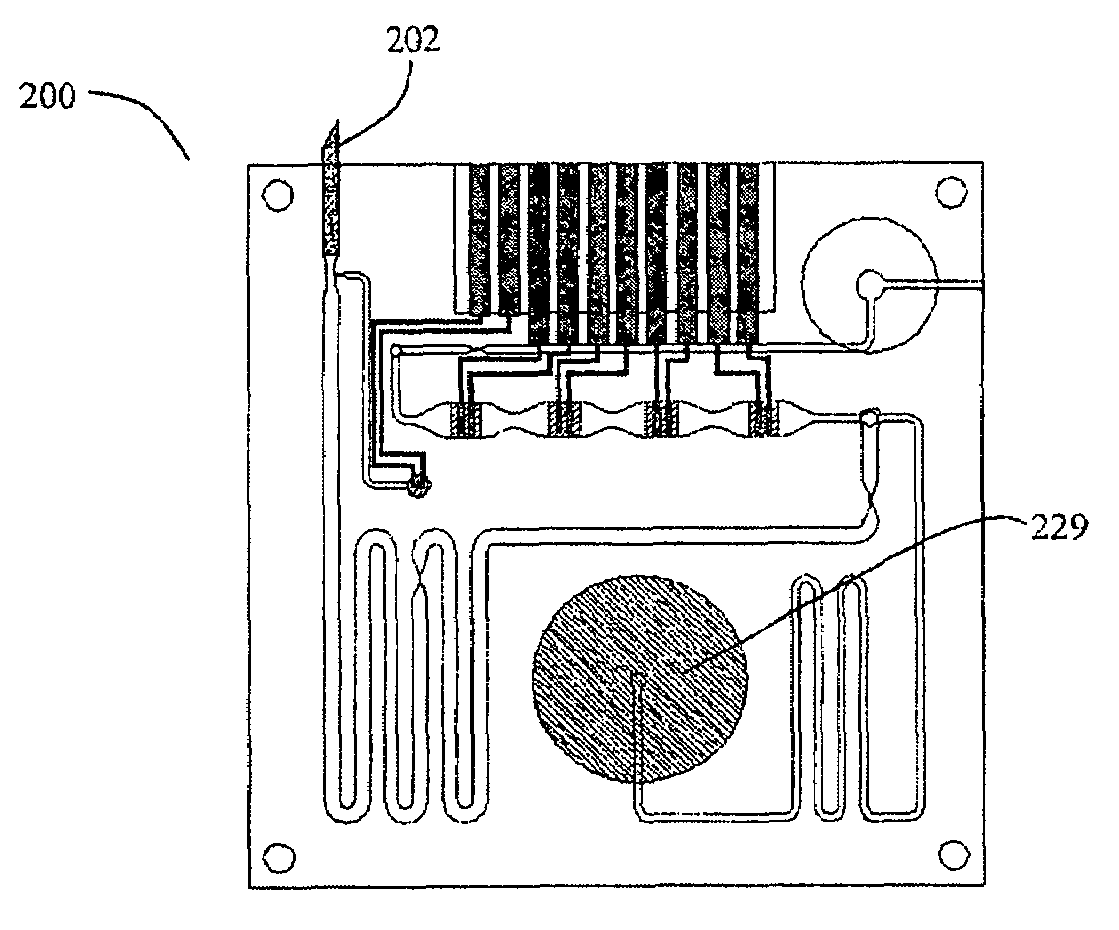
Smart disposable plastic lab-on-a-chip for point-of-care testing
InactiveUS7524464B2None of measures has been particularly successfulRelieve painBioreactor/fermenter combinationsCombination devicesVenous bloodLab-on-a-chip
Disclosed herein is a fully-integrated, disposable biochip for point-of-care testing of clinically relevant parameters. Specifically, in accordance with an embodiment of the present invention, the biochip is designed for POCT (point-of-care-testing) of an array of metabolic parameters including partial pressure of oxygen, Glucose, and Lactate concentration from venous blood samples. The biochip is fabricated on a low-cost plastic substrate using mass manufacturing compatible fabrication processes. Furthermore, the biochip contains a fully-integrated metallic micro-needle for blood sampling. The biochip also uses smart passive microfluidics in conjunction with low-power functional on-chip pressure generators for microfluidic sequencing. The design, configuration, assembly and operation of the biochip are ideally suited for a disposable biochip specifically targeted towards POCT applications.
Owner:UNIVERSITY OF CINCINNATI
Systems, methods and analyzers for establishing a secure wireless network in point of care testing
ActiveUS8776246B2Digital data processing detailsMeasurement arrangements for variablePoint of careWi-Fi
A system and method for initiating and maintaining a secure wireless communication between a wireless analyzer and a target network (e.g., a hospital network connected to a LIS and / or HIS). The present disclosure provides novel processes and systems for securely networking a wireless analyzer with a Wi-Fi network without the need for an operator or user to engage in manual initiation steps on, or through, the wireless analyzer.
Owner:ABBOTT POINT CARE
Detection and quantification of analytes in bodily fluids
ActiveUS20100248277A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalytePoint-of-care testing
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
Biological Fluid Collection Device and Biological Fluid Separation and Testing System
ActiveUS20140309555A1Reduce blood exposureQuick mixAnalysis material containersOther blood circulation devicesPoint of careBlood specimen
A biological fluid collection device that is adapted to receive a blood sample having a cellular portion and a plasma portion is disclosed. After collecting the blood sample, the biological fluid collection device is able to transfer the blood sample to a point-of-care testing device or a biological fluid separation and testing device. After transferring the blood sample, the biological fluid separation and testing device is able to separate the plasma portion from the cellular portion and analyze the blood sample and obtain test results.
Owner:BECTON DICKINSON & CO
Biological Fluid Collection Device and Biological Fluid Separation and Testing System
ActiveUS20140305196A1Reduce blood exposureQuick mixAnalysis material containersSemi-permeable membranesMedicineTransfer system
A biological fluid collection device that is adapted to receive a blood sample having a cellular portion and a plasma portion is disclosed. After collection of the blood sample, the plasma portion is separated from the cellular portion. After separation, the biological fluid collection device is able to transfer the plasma portion of the blood sample to a point-of-care testing device. The biological fluid collection device also provides a closed sampling and transfer system that reduces the exposure of a blood sample and provides fast mixing of a blood sample with a sample stabilizer. The biological fluid collection device is engageable with a biological fluid testing device for closed transfer of a portion of the plasma portion from the biological fluid collection device to the biological fluid testing device. The biological fluid testing device is adapted to receive the plasma portion to analyze the blood sample.
Owner:BECTON DICKINSON & CO
Urine creatine quantitative test card
InactiveCN101865911AReal-time measurementSimple structureBiological testingPipettePoint-of-care testing
The invention relates to a urine creatine quantitative test card, which comprises an upper shell, a lower shell, reaction test paper and developing test paper arranged in shell cavity, a pipette and a quantitative absorbing rod. The upper shell is provided with a sample loading hole; a solution tank is arranged in the shell; and the tail end of the reaction test paper is positioned in the solution tank. The urine creatine quantitative test card can realize instantaneous measurement of creatine in urine by combining simple and convenient urine quantitative dilution, enzyme reaction and chromatography developing technology, and has the advantages of simple structure, no need of mating instruments, convenient use, low cost, and accordance with application trend of point-of-care testing (POCT).
Owner:顾瑜
Rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on amino acid spacer arm
InactiveCN104198723AReduce binding steric hindranceHigh sensitivityBiological testingGelatinasesMicrosphere
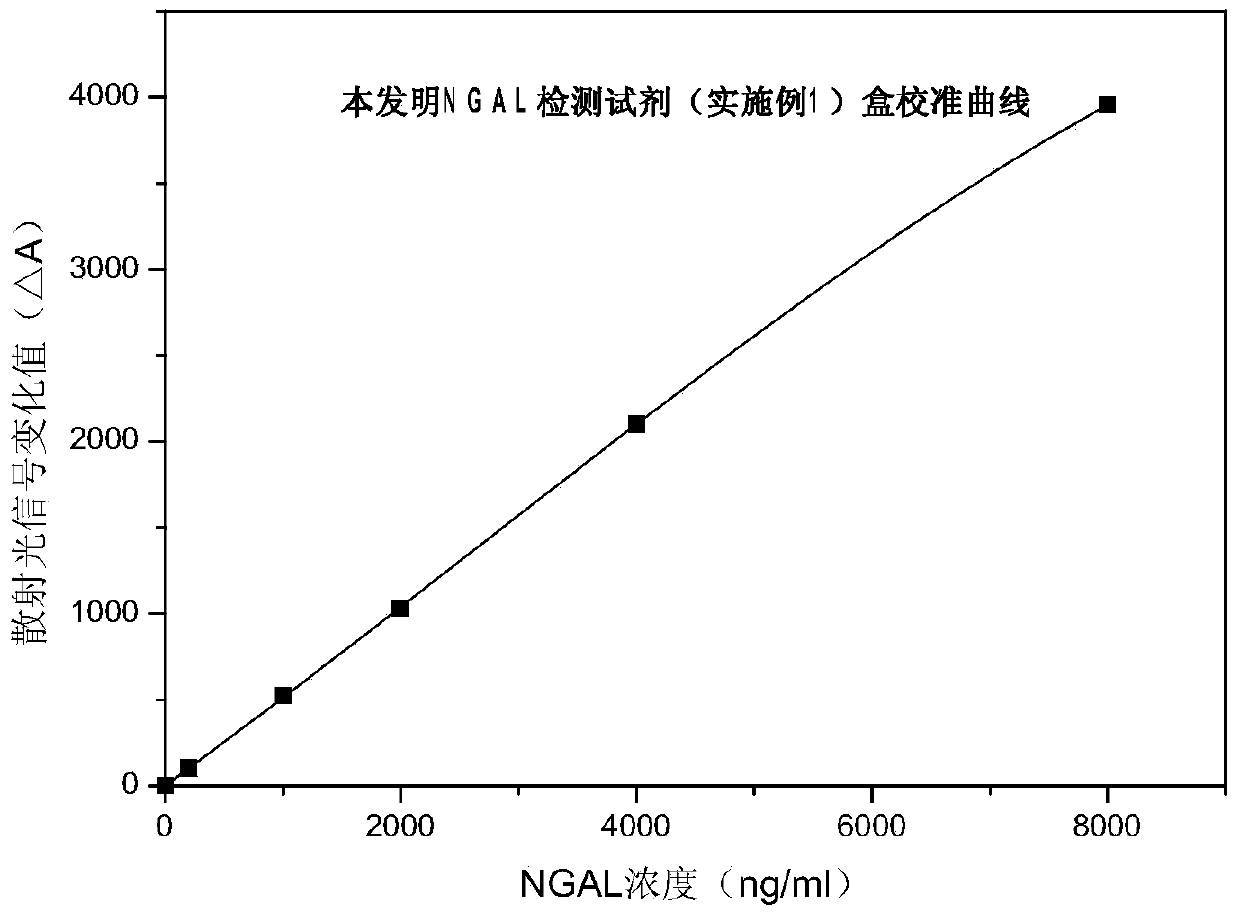
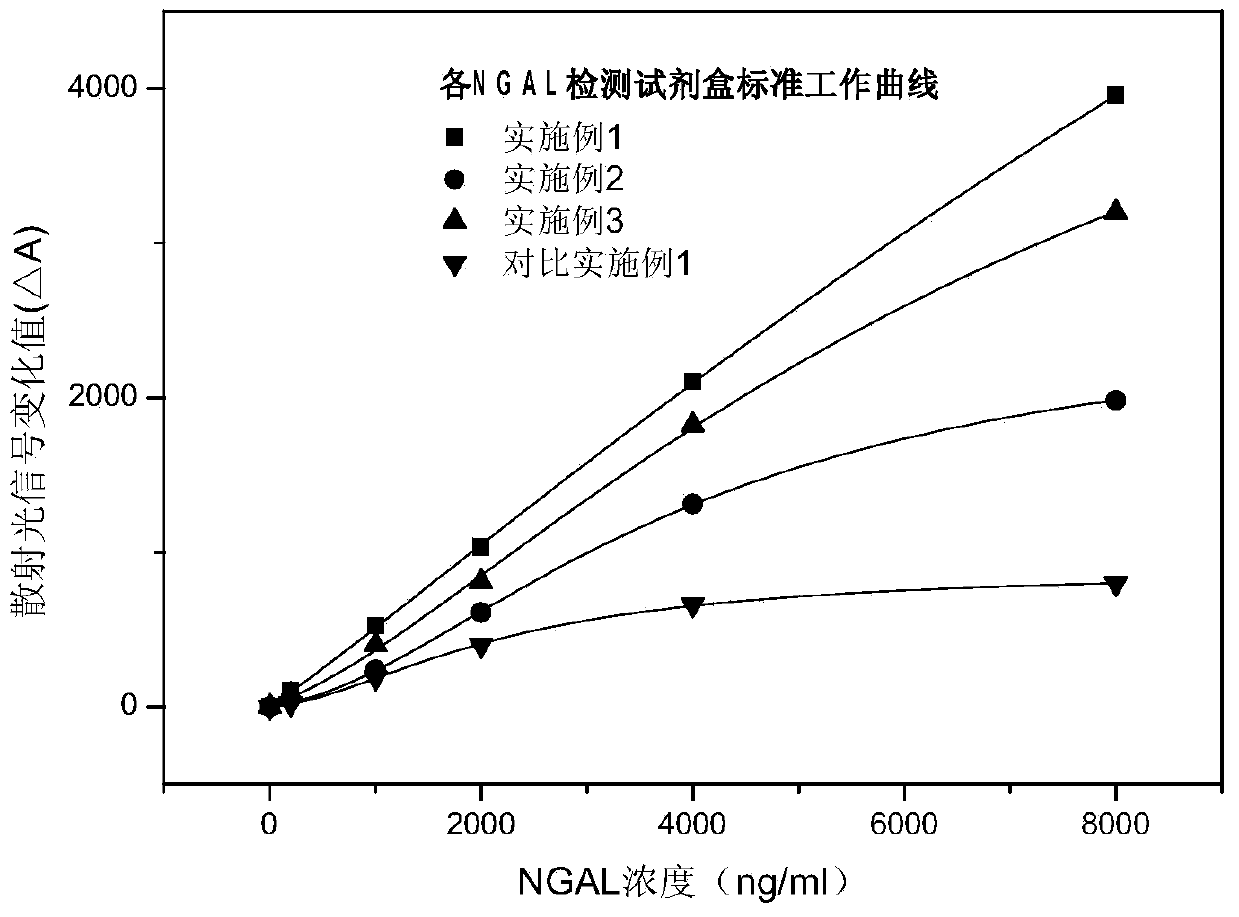
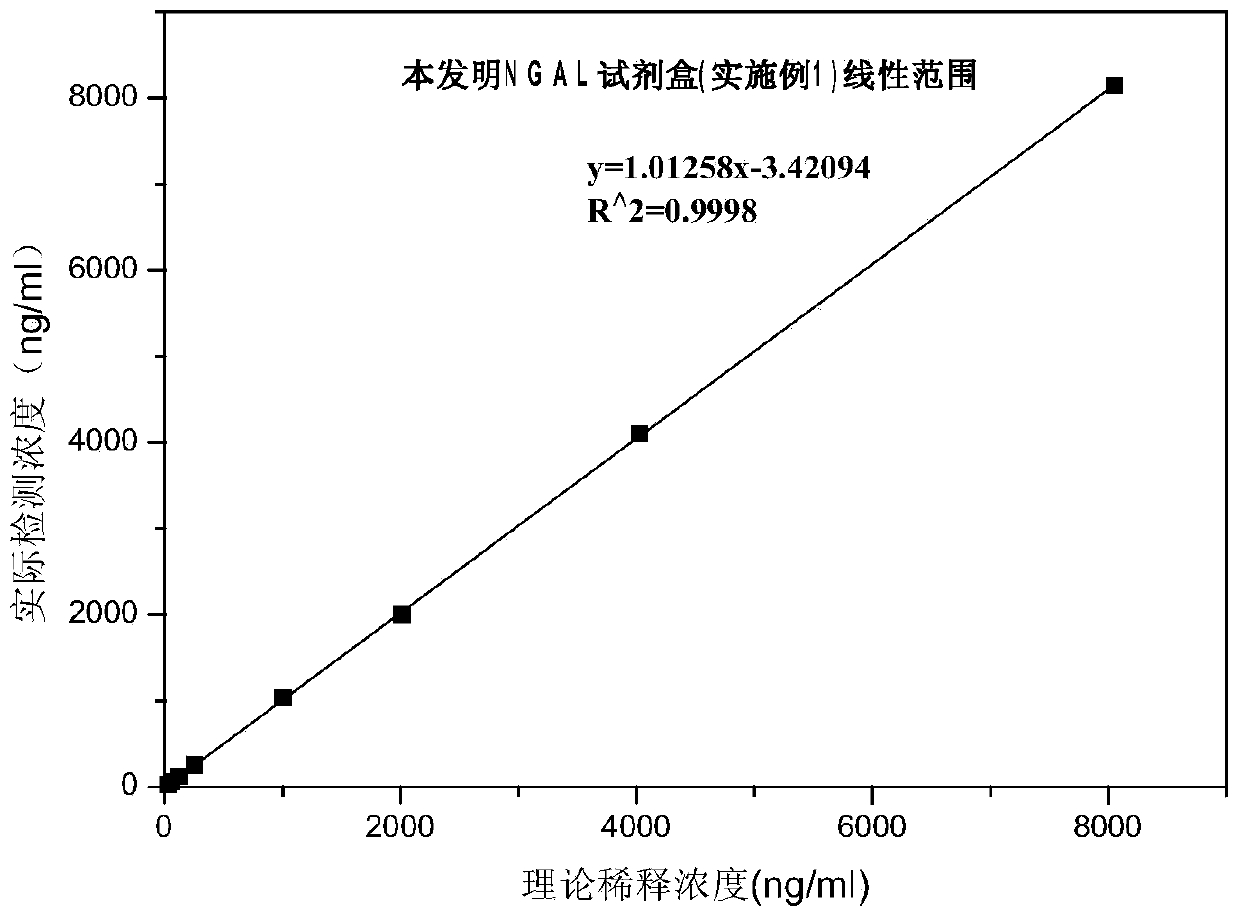
The invention discloses a rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on an amino acid spacer arm and used for quantitatively and rapidly determining NGAL in human serum (plasma) or urine. The kit comprises a reagent 1 (R1) and a reagent 2 (R2), wherein the R1 comprises a biological buffer solution, a surfactant, a stabilizer, a coagulation accelerator and a preservative; the R2 comprises latex particles for enveloping an NGAL antibody, a biological buffer solution, a protective agent, a surfactant and a preservative; in R2, an antibody against human NGAL is connected with a polystyrene latex microsphere through the amino acid spacer arm. The invention also discloses a preparation method of the latex particles for enveloping the antibody against the human NGAL. The kit has high sensitivity and wide linear range; when being used in cooperation with a POCT (Point Of Care Testing) scattering nephelometry analyzer, the kit can achieve the aim of accurately and rapidly determining the NGAL in human serum (plasma) or urine.
Owner:NANJING PERLONG MEDICAL EQUIP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com