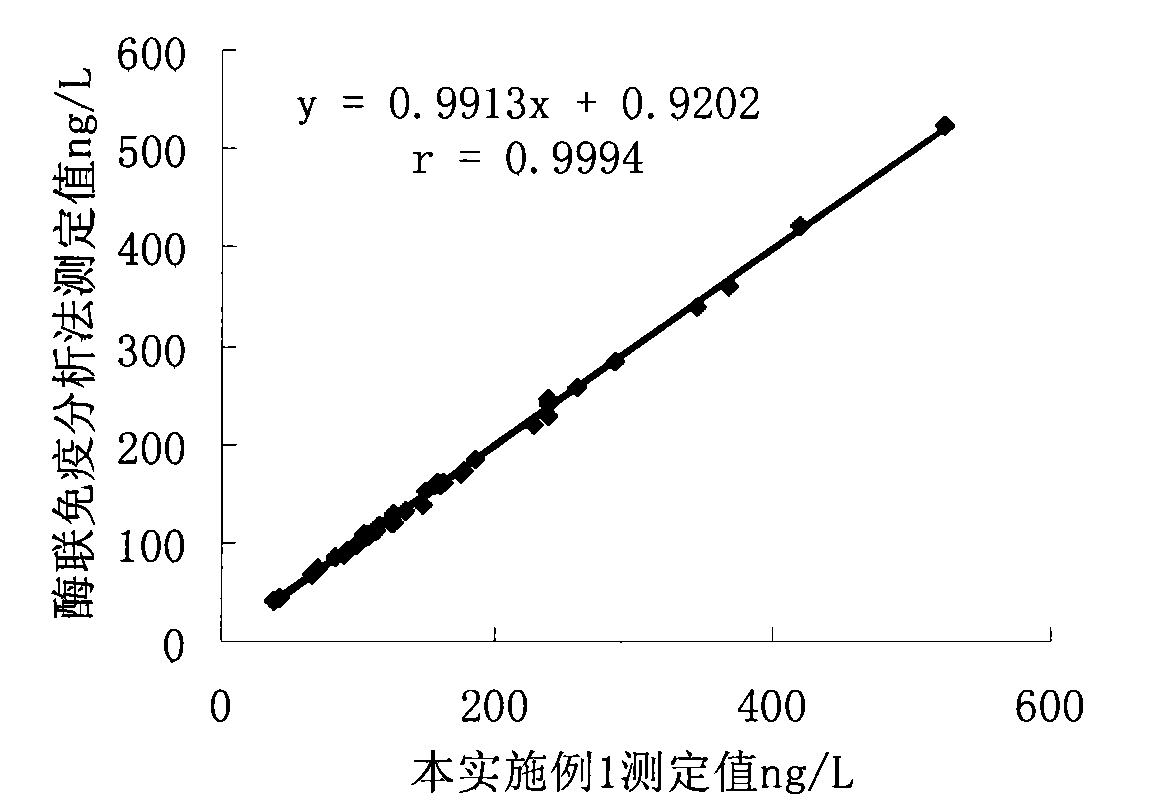
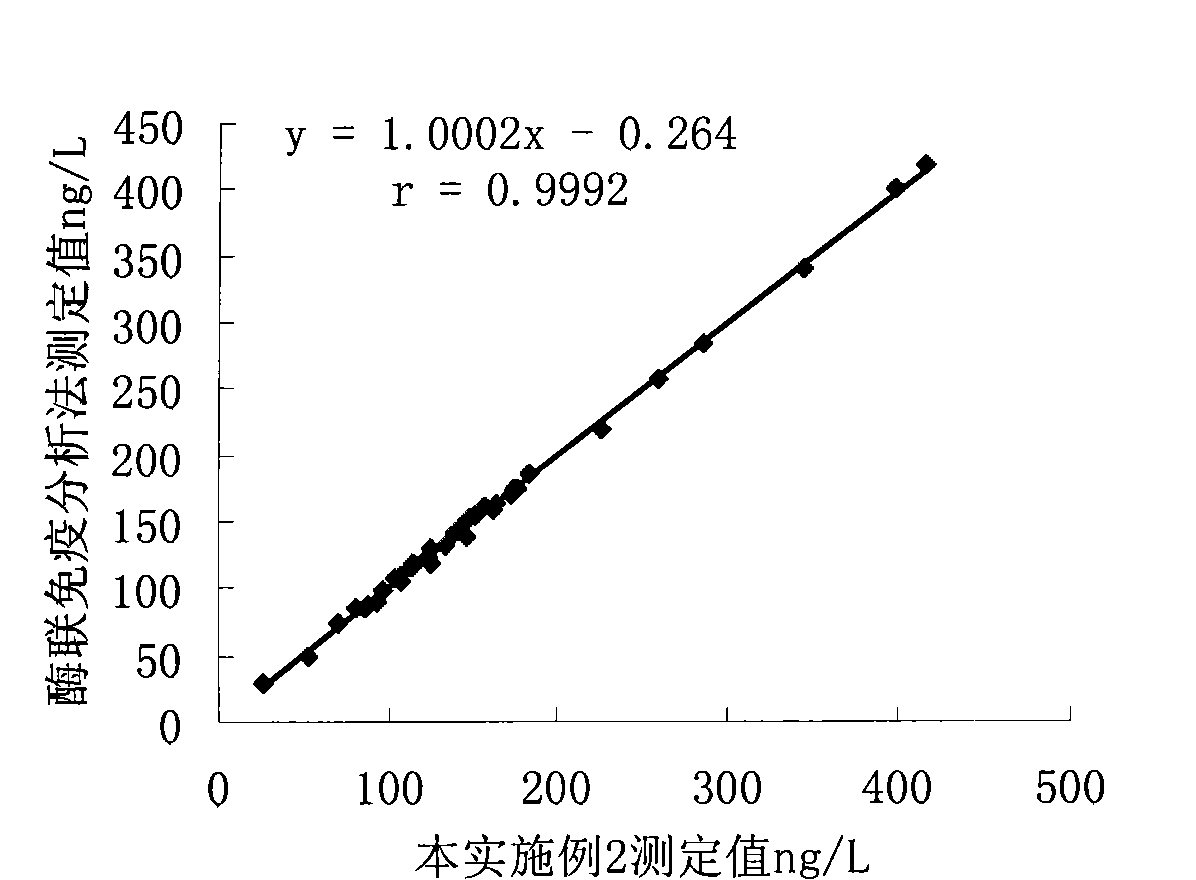
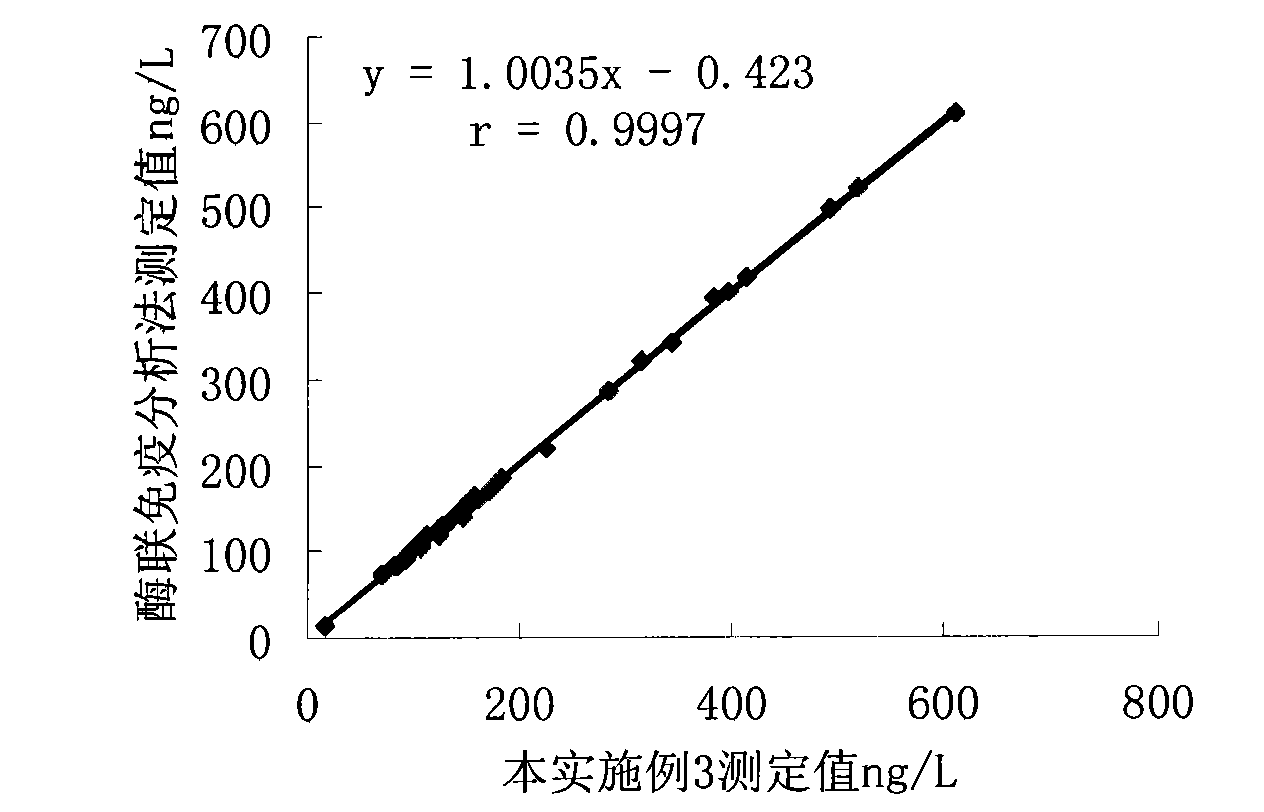
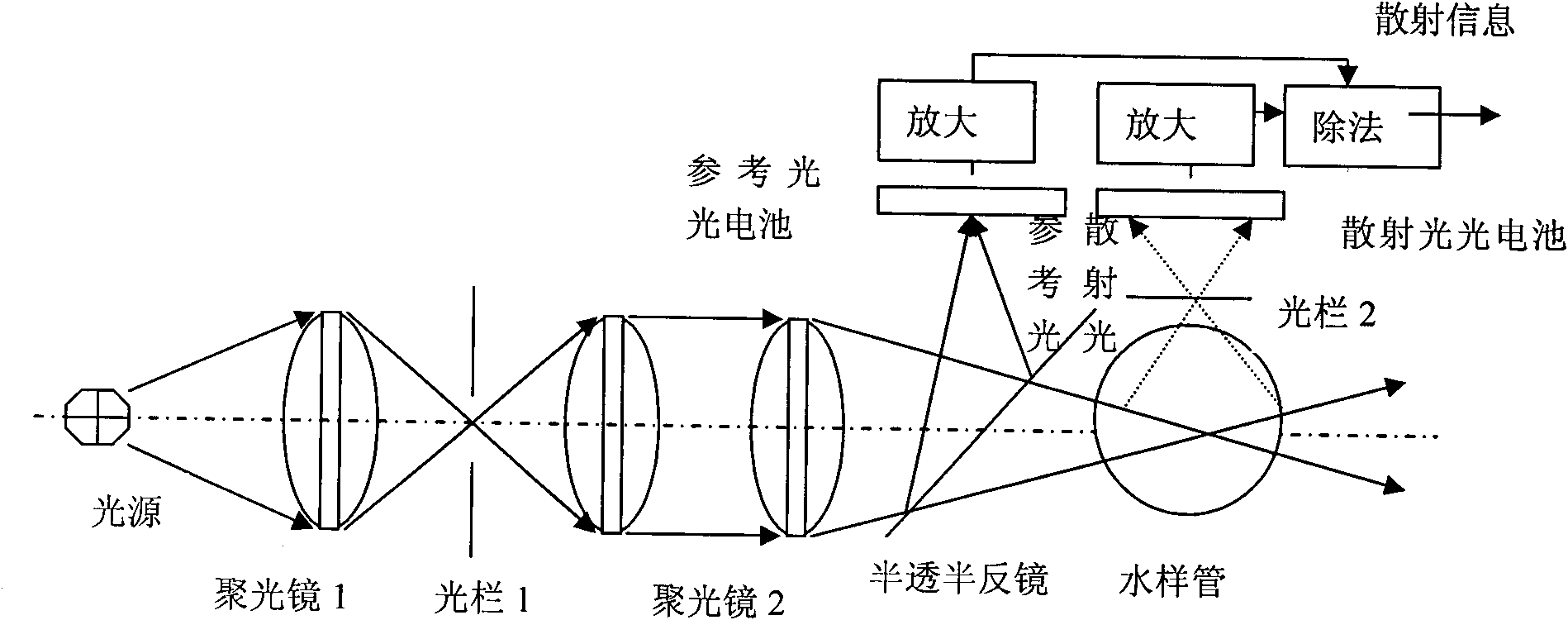
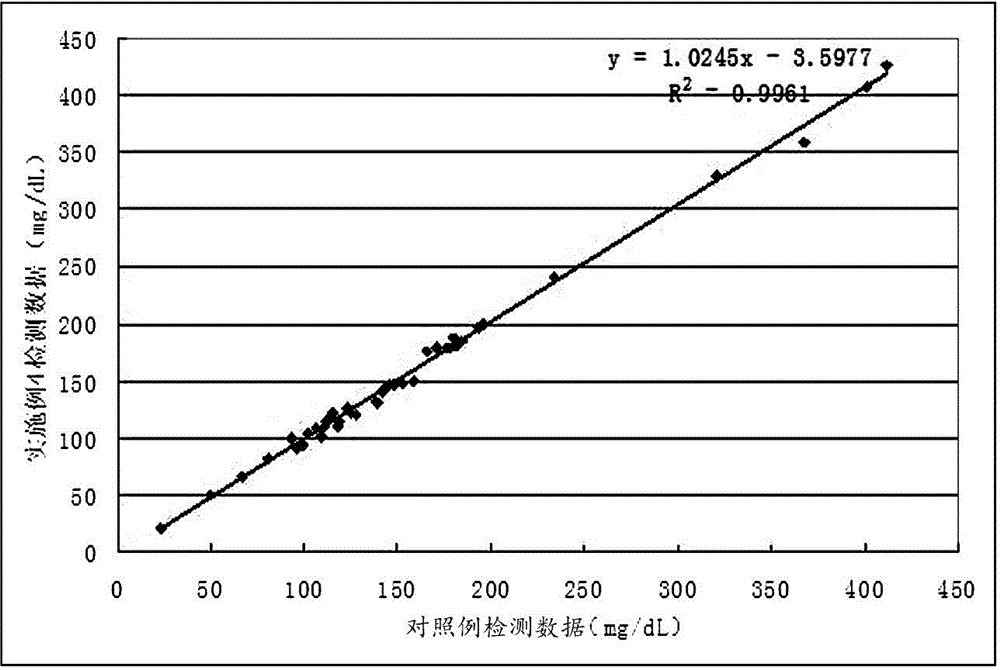
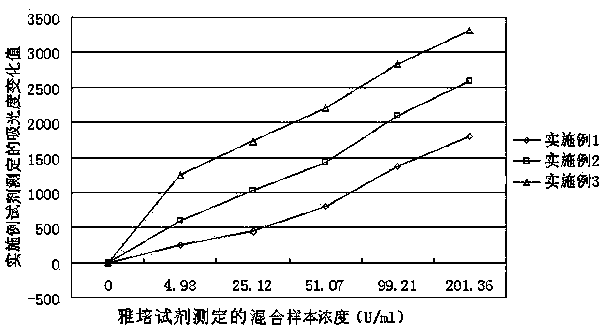
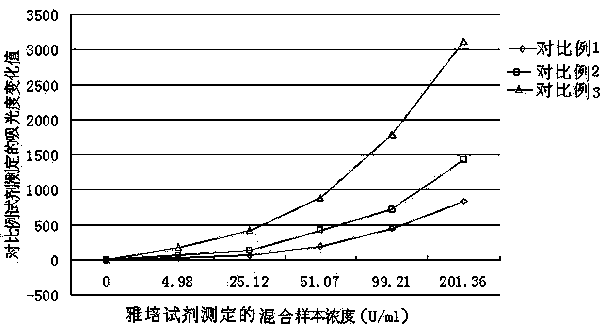
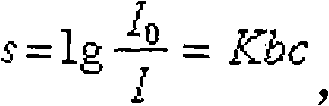Patents
Literature
97 results about "Nephelometry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kit for detecting brain natriuretic peptide by nano microsphere immunonephelometry
The invention relates to a kit for detecting brain natriuretic peptide in blood serum and provides the kit for detecting brain natriuretic peptide by nano microsphere immunonephelometry, which has the characteristics of no need of dilution of a sample, simple operation, high precision, good repeatability and suitability for a clinically common used automatic biochemical analyzer, in order to solve technical problems. The invention adopts a technical scheme that the kit for detecting the brain natriuretic peptide by nano microsphere immunonephelometry comprises the following components: a, a reagent R1 which comprises buffer solution, a preservative, a stabilizing agent, an electrolyte, a surfactant, and the balance of water; b, a reagent R2 which comprises buffer solution, nano microspheres combined with brain natriuretic peptide antibodies and a preservative, wherein the diameter of the microspheres is 50 to 150 nm; and c, a reference calibration material which comprises buffer solution, a stabilizing agent, a preservative, a recombinant brain natriuretic peptide pure product which is added in a corresponding amount according to the required concentration of the BNP (brain natriuretic peptide) reference calibration material, and the balance of water.
Owner:浙江伊利康生物技术有限公司
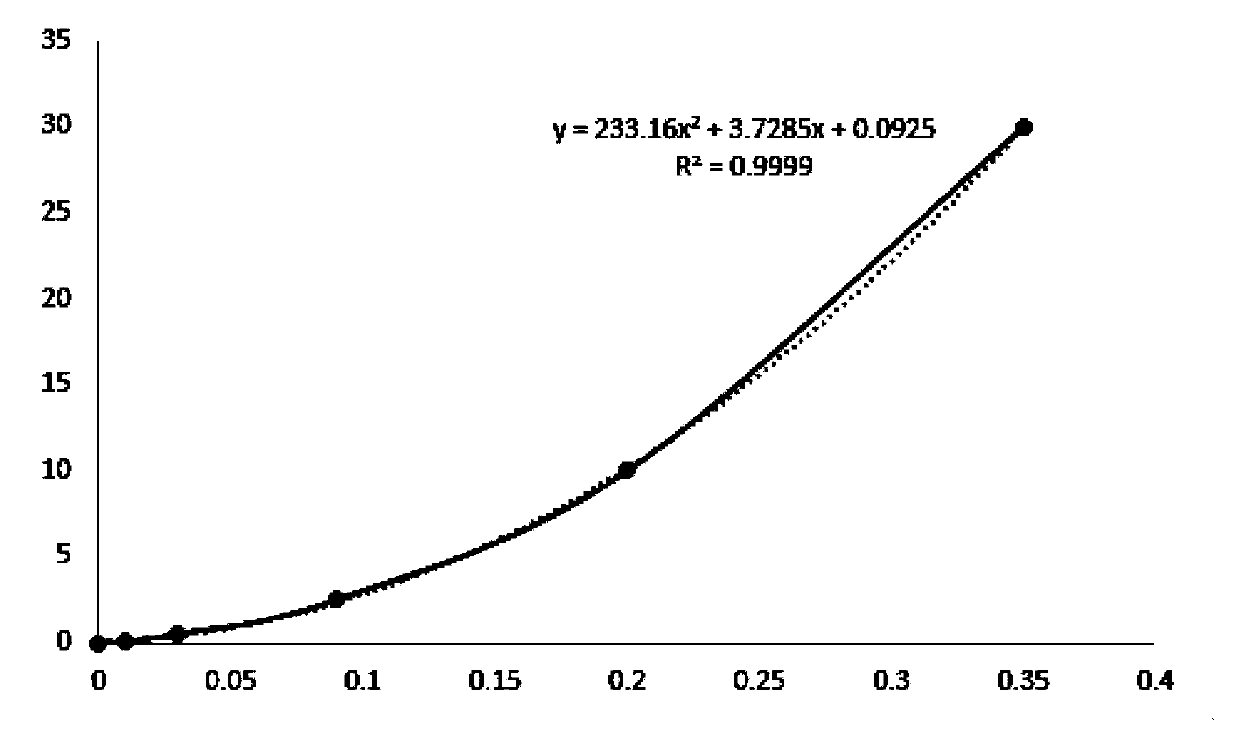
Method for detecting concentration of nano particles in solution by nephelometry
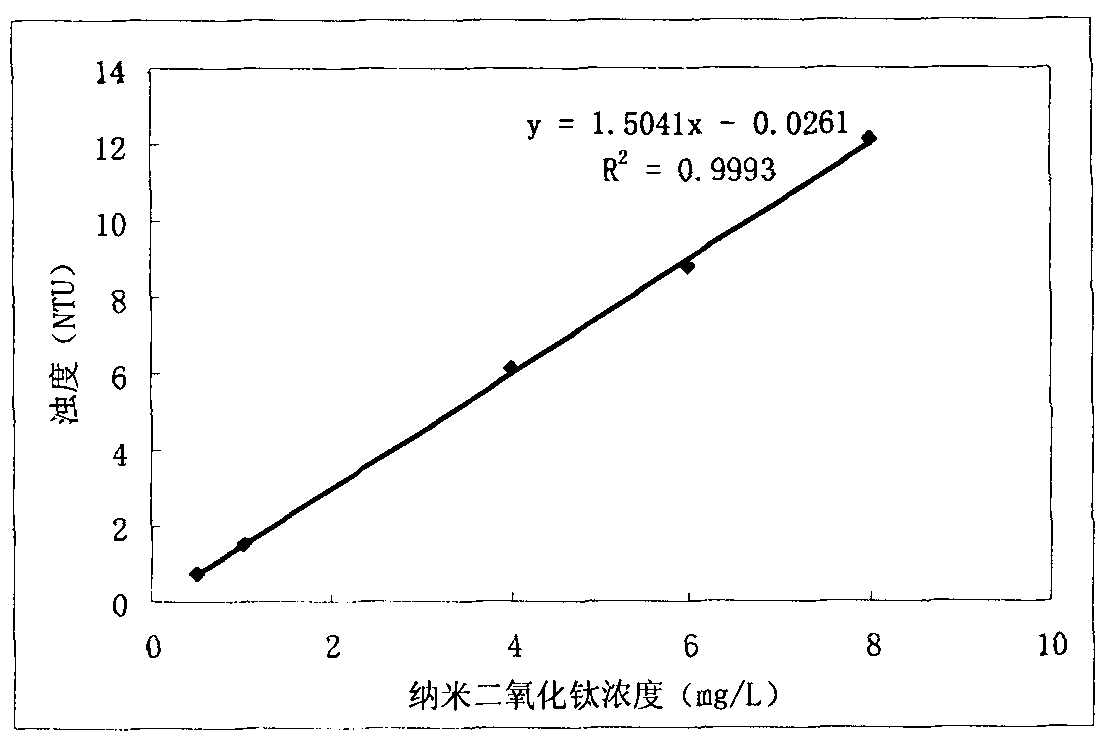
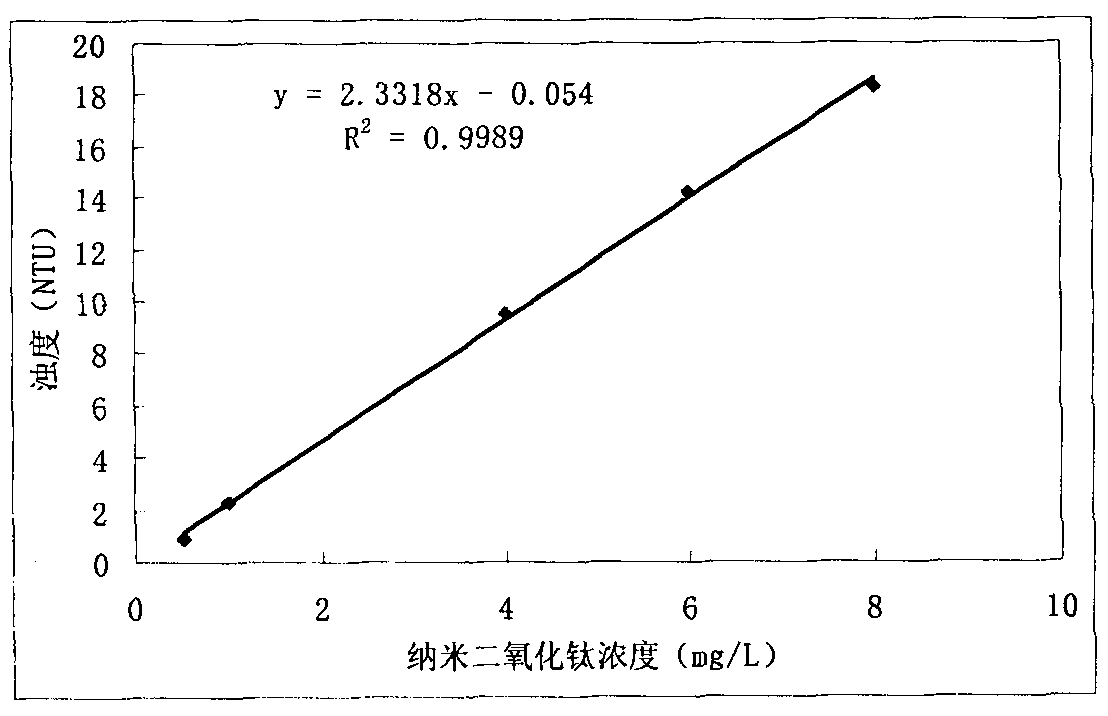
InactiveCN102338733AReduce analysis costsHigh precisionMaterial analysis by observing effect on chemical indicatorPermeability/surface area analysisWater bathsTurbidity
The invention discloses a method for detecting a concentration of nano particles in solution by a nephelometry, which comprises the following steps of: a first step of preparing standard dispersed solution and carrying out ultrasonic dispersion to form uniform standard dispersed solution for later use; a second step of drawing a calibration curve between the concentration of the nano particles and the turbidity, calculating to obtain a standard curvilinear equation, and returning to the first step to restart if the linearity r of the calibration curve is lower than 0.999; and a third step of measuring the concentration of the nano particles in a sample to be measured, placing the sample to be measured into an ultrasonic water bath to carry out ultrasonic dispersion, taking and moving uniformly dispersed solution to be measured into a cuvette cell of a turbidity meter, measuring to obtain a turbidity value of the solution to be measured on a nephelometer by taking a liquid solvent difficult to volatilize as a blank reference, and obtaining the corresponding concentration value of the nano particles according to the calibration curvilinear equation obtained in the second step. The method has the characteristics of strong pertinence, simple, visual and rapid analysis method, capability of field analysis, high precision and accuracy and the like and also serves for enterprises which produce and apply nano materials.
Owner:NANJING UNIV OF SCI & TECH
Kit for detecting serum amyloid protein and application thereof
InactiveCN106353507AEnhanced detection signalHigh detection sensitivityBiological testingChemistryAntibody
The invention provides a kit for detecting serum amyloid protein and application thereof. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and interference elimination protein; the reagent R2 is prepared from the buffer solution, the inorganic salt, the surfactant, the preservative, the stabilizer and a polystyrene latex particle mixture; the polystyrene latex particle mixture is cross-linked with an SAA (Serum Amyloid A) antibody; the polystyrene latex particle mixture is a mixture of large-diameter polystyrene latex particles and small-diameter polystyrene latex particles. The kit disclosed by the invention is based on PETIA (Particle-enhanced Turbidimetric Immunoassay) and can be generally used for analysis of all kinds of full-automatic biochemical analyzer; during use, the required determining time is short, the specificity is high, the precision degree is high, and the accuracy degree is high.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
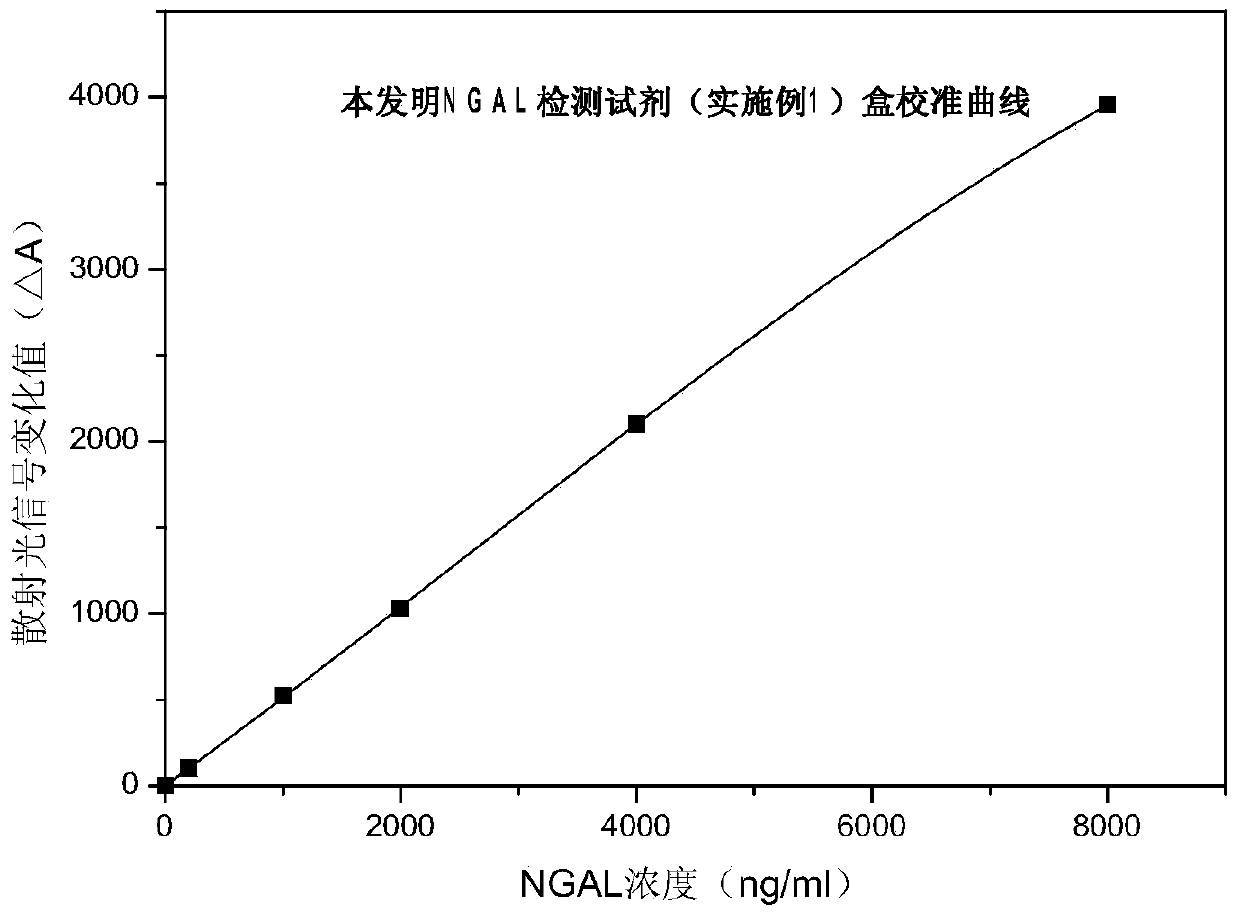
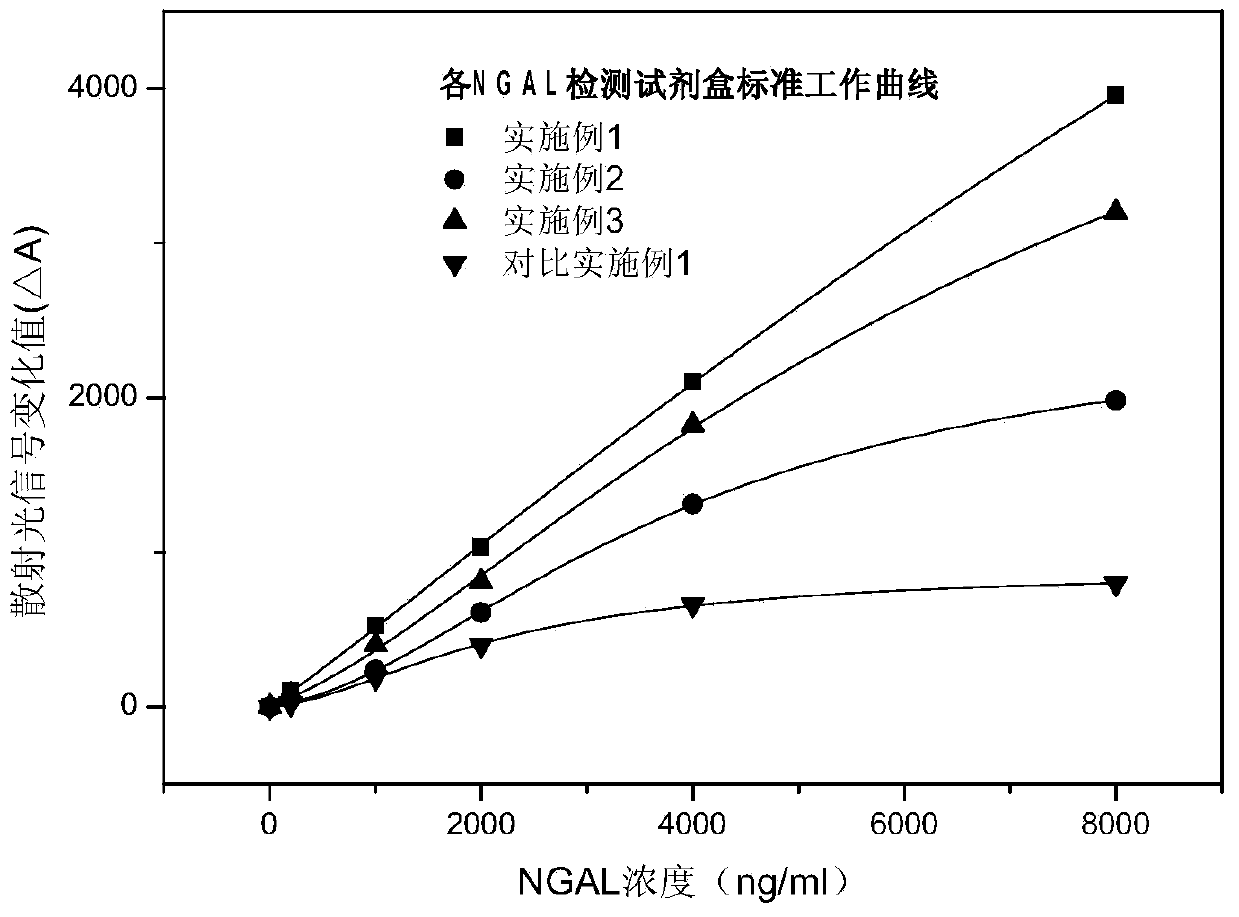
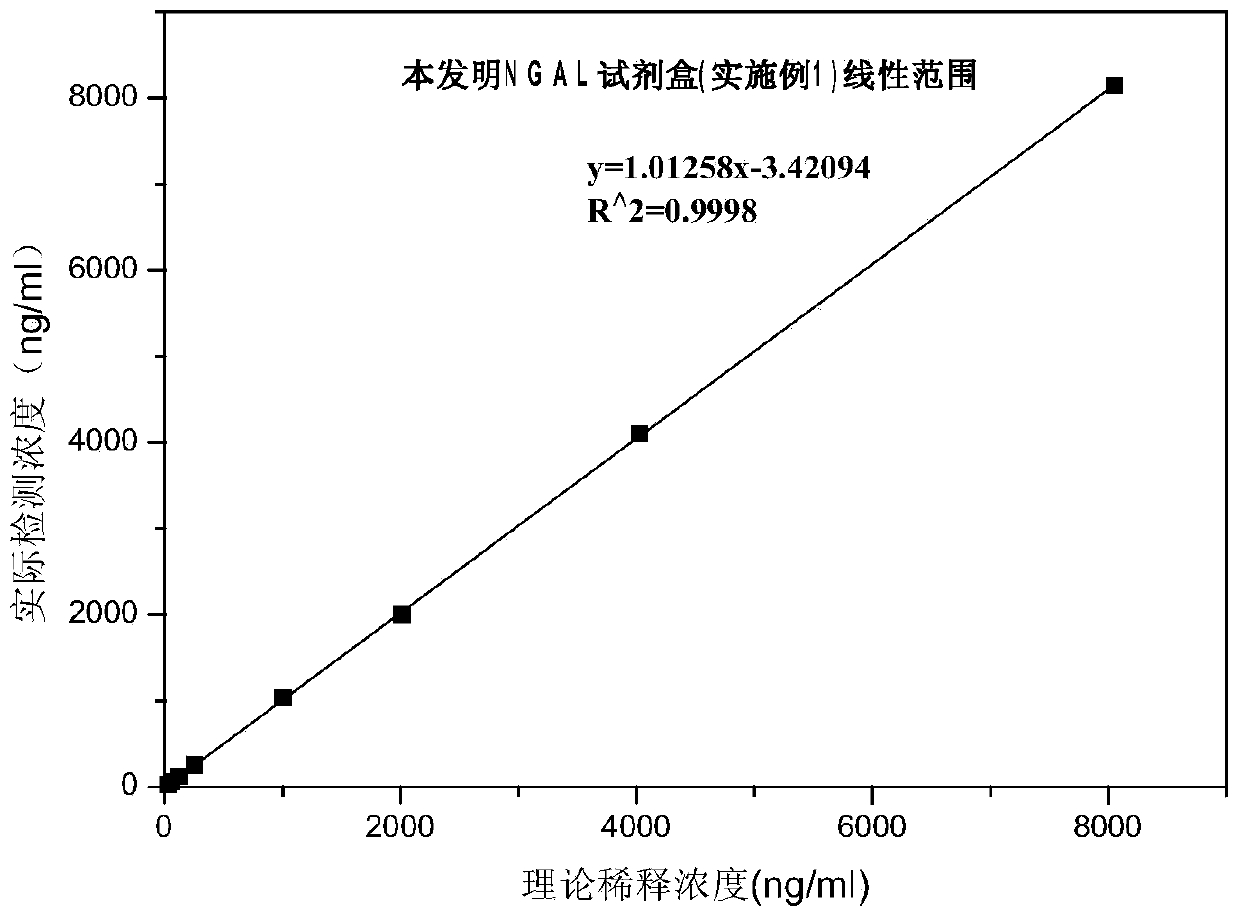
Rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on amino acid spacer arm
InactiveCN104198723AReduce binding steric hindranceHigh sensitivityBiological testingGelatinasesMicrosphere
The invention discloses a rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on an amino acid spacer arm and used for quantitatively and rapidly determining NGAL in human serum (plasma) or urine. The kit comprises a reagent 1 (R1) and a reagent 2 (R2), wherein the R1 comprises a biological buffer solution, a surfactant, a stabilizer, a coagulation accelerator and a preservative; the R2 comprises latex particles for enveloping an NGAL antibody, a biological buffer solution, a protective agent, a surfactant and a preservative; in R2, an antibody against human NGAL is connected with a polystyrene latex microsphere through the amino acid spacer arm. The invention also discloses a preparation method of the latex particles for enveloping the antibody against the human NGAL. The kit has high sensitivity and wide linear range; when being used in cooperation with a POCT (Point Of Care Testing) scattering nephelometry analyzer, the kit can achieve the aim of accurately and rapidly determining the NGAL in human serum (plasma) or urine.
Owner:NANJING PERLONG MEDICAL EQUIP
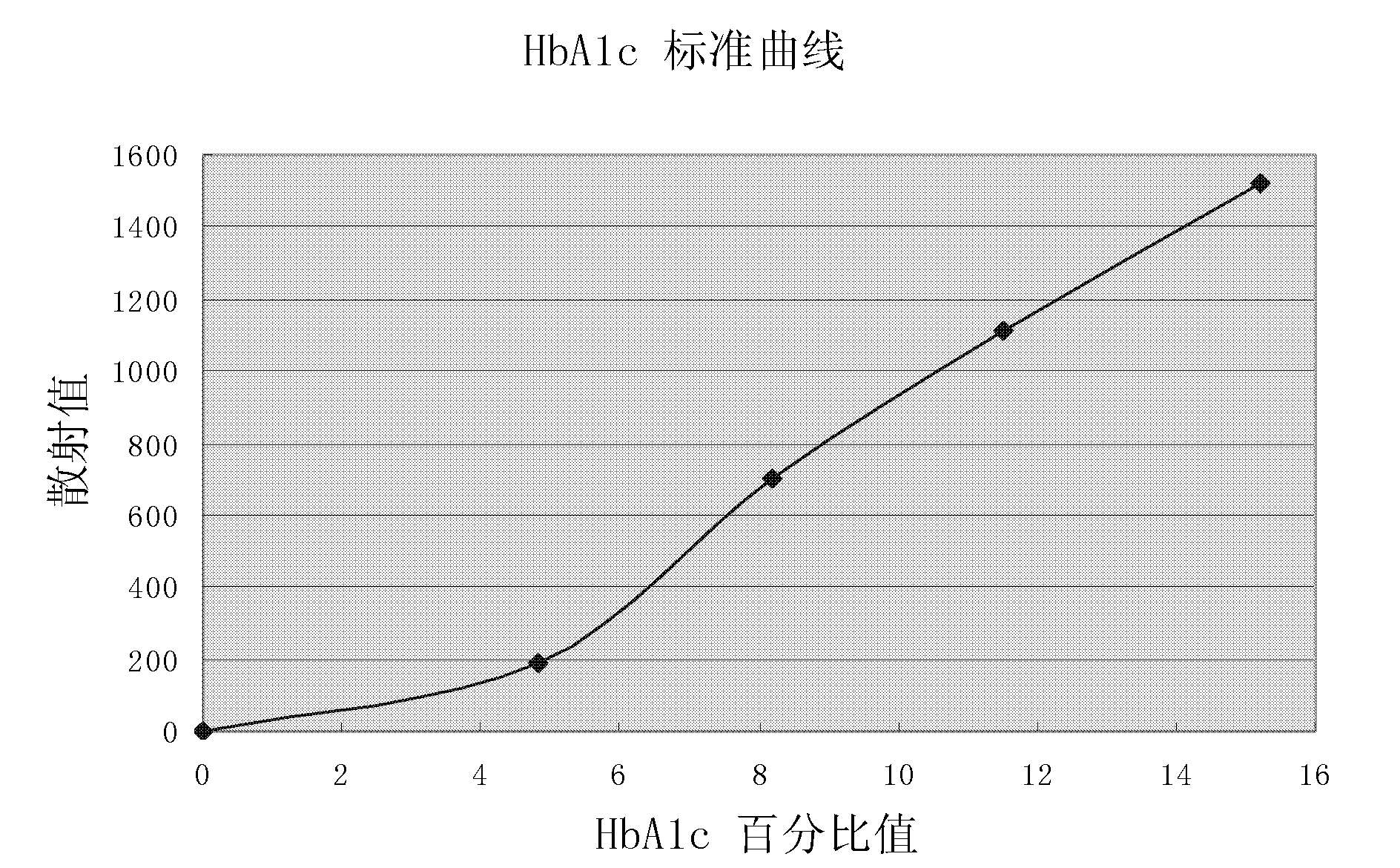
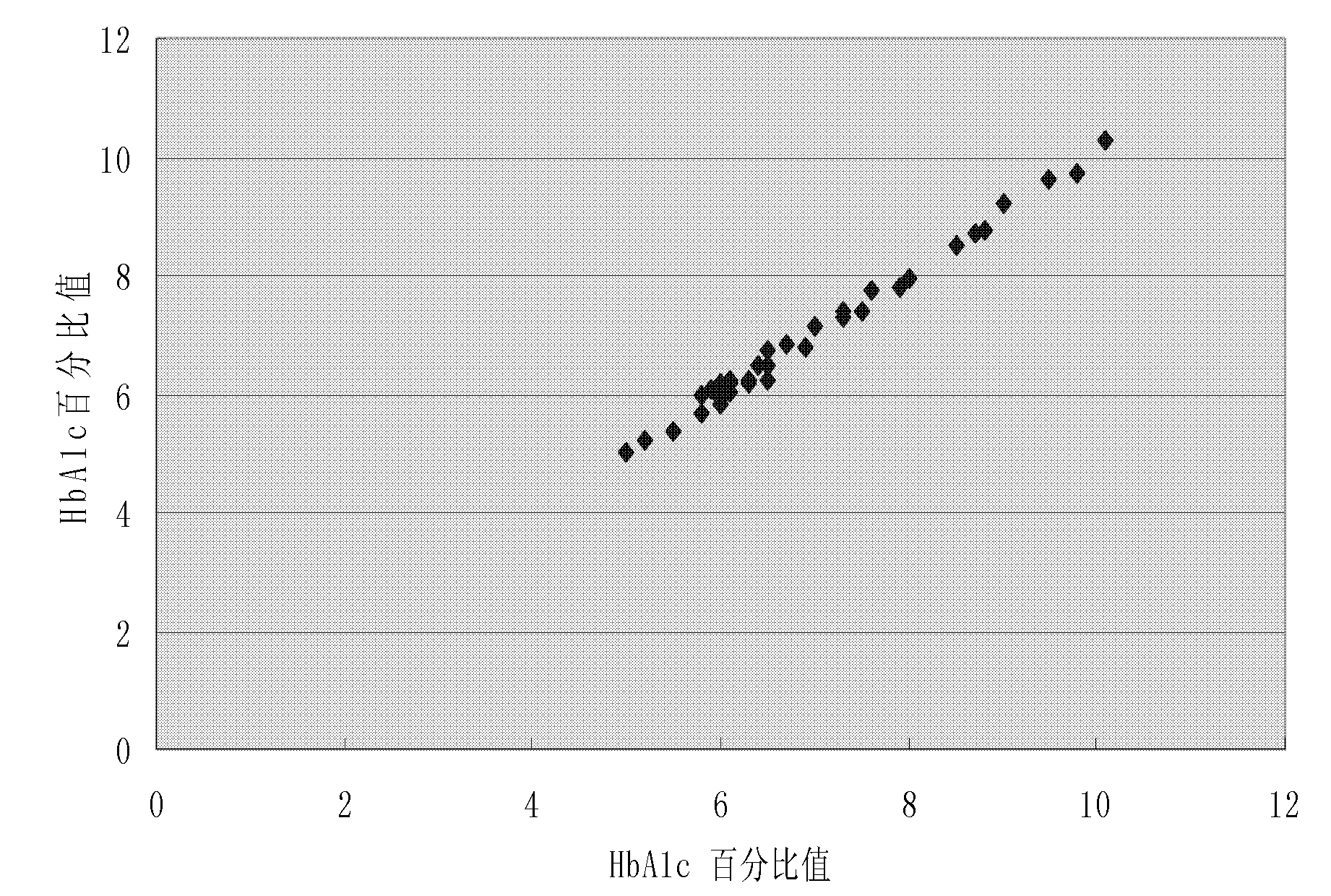
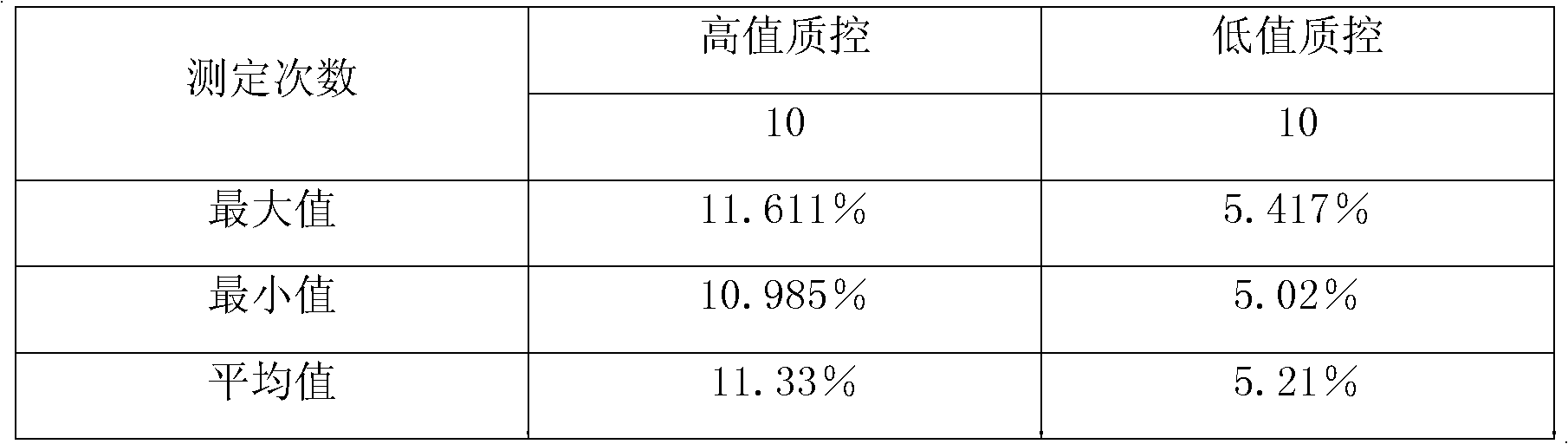
Method for measuring glycosylated hemoglobin content
ActiveCN101915849AExtend effective use timeEasy to operateMaterial analysis by observing effect on chemical indicatorBiological testingLight irradiationTotal hemoglobin
The invention relates to a method for measuring glycosylated hemoglobin content, which comprises the following steps of: a, uniformly mixing a whole blood sample and hemolytic liquid; b, adding an appropriate amount of hemolyzed sample into latex suspending in glycine buffer solution; c, respectively adding an antibody B reagent and an antibody A reagent after preset time; d, uniformly mixing andreading a scattering value through a specific protein analyzer under the condition of single-wavelength light irradiation; and e, reading the glycosylated hemoglobin content from a standard curve diagram according to the scattering value. The glycosylated hemoglobin content of the blood sample can be directly determined without separately measuring the total hemoglobin content and the glycosylated hemoglobin content of the blood sample by taking fixed-time nephelometry as a detection principle; and antibody working solution does not need preparing in advance, so that the effective service time of the reagents is prolonged, the operating steps are simplified and the measuring time of the sample is shortened.
Owner:SHENZHEN GOLDSITE DIAGNOSTICS
Latex enhanced immuno-nephelometry kit for determining procalcitonin and preparation method and application of latex enhanced immuno-nephelometry kit for determining procalcitonin
ActiveCN104237525AMaterial analysis by observing effect on chemical indicatorBiological testingDiseaseMedicine
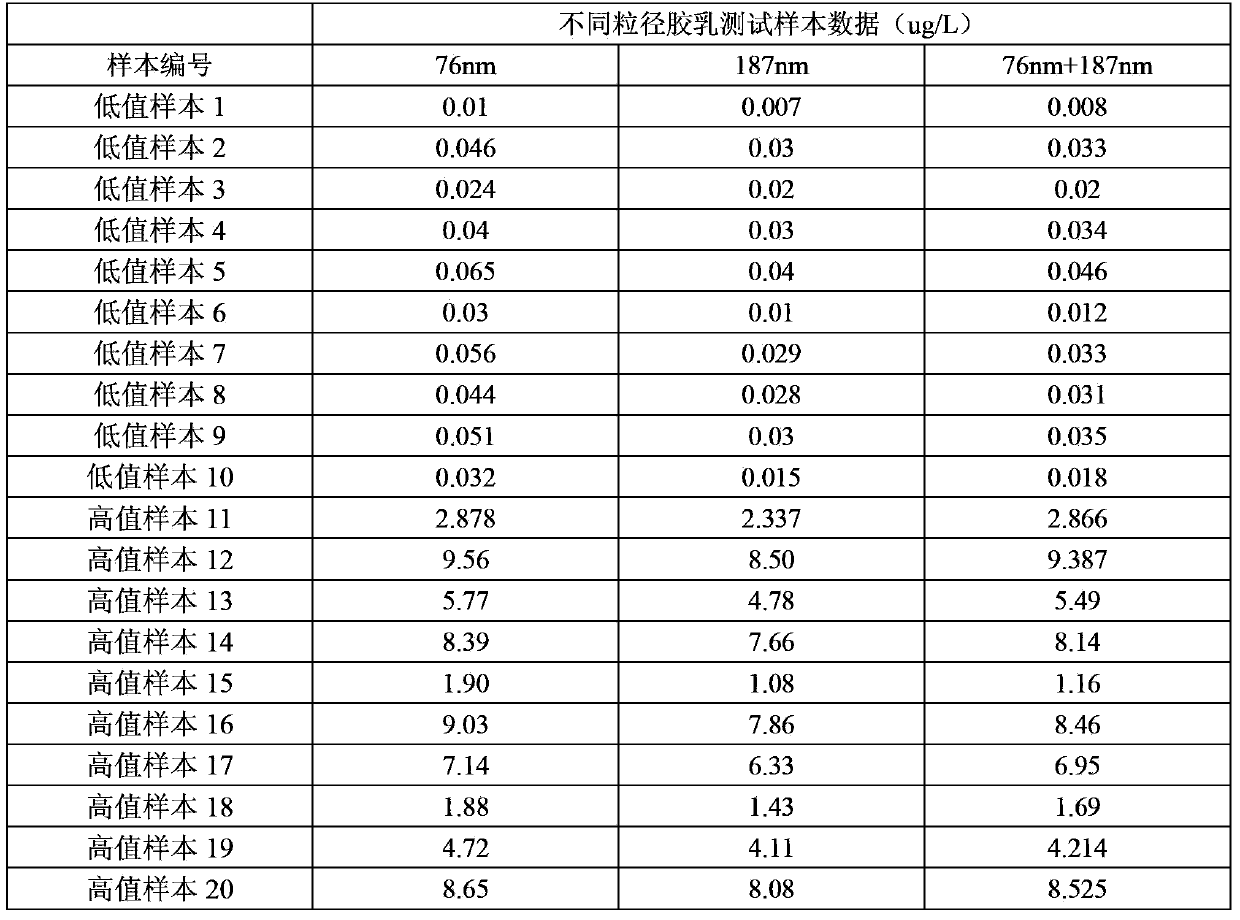
The invention discloses a latex enhanced immuno-nephelometry kit for determining procalcitonin and a preparation method and application of the latex enhanced immuno-nephelometry kit for determining procalcitonin, and belongs to the technical field of disease diagnosis and detection. The latex enhanced immuno-nephelometry kit for determining the procalcitonin comprises a diluent, a latex preparation, a blank liquid, a calibrator and a quality control material, wherein the latex preparation contains carboxylated polystyrene latex with different particle sizes and coupled with a PCT monoclonal antibody and a PCT polyclonal antibody respectively. According to the method, the PCT monoclonal antibody and the PCT polyclonal antibody are marked respectively, the latex with the large particle size can improve the detection sensitivity, and the latex with the small particle size can expand the linearity range, and therefore, the composite latex preparation can improve the detection sensitivity, can expand the detection linearity range, and has the advantages of high detection speed, high sensitivity, strong specificity and good accuracy for detection on the procalcitonin.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Method for measuring chlorine content of vanadium battery electrolyte solution
The invention relates to a method for measuring the chlorine content of a vanadium battery electrolyte solution. The concentration of chloride ions in the vanadium battery electrolyte solution is measured through the nephelometry. The method comprises the steps that (1), all vanadium ions in the vanadium battery electrolyte solution are converted into pentavalent vanadium ions, and an analysis sample is obtained and is diluted to be at multiples suitable for analysis; (2), a working curve is drawn out; (3), the absorbance of the diluted analysis sample is measured, and the corresponding chlorine concentration is obtained according to the measured absorbance and the working curve; (4), the chlorine concentration is multiplied by the dilution ratio, and finally the concentration of the chloride ions in the vanadium battery electrolyte solution is obtained. According to the method, the content of the chloride ions can be measured easily, rapidly and accurately, so that the content of the chlorine ions in the vanadium battery electrolyte solution is monitored in real time.
Owner:大力储能技术湖北有限责任公司
Kit for determining anti-cyclic citrullinated peptide (Anti-CCP) antibody
ActiveCN102507918AEnhanced detection signalHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsAntiendomysial antibodiesCitrulline
The invention discloses a kit for determining an anti-cyclic citrullinated peptide (Anti-CCP) antibody. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a buffer solution, inorganic salt, surfactant, preservative, interference removing protein, modified CCP-sensitized nano poly styrene (PS) microspheres and ultrapure water; the reagent R2 comprises a buffer solution, inorganic salt, surfactant, preservative, an anti Anti-CCP second antibody and ultrapure water; and the weight ratio of the reagent R1 to the reagent R2 is (50-90):(10-50). According to the method, the Anti-CCP is determined by adding the anti Anti-CCP second antibody by a latex booster immunization transmission turbidimetry, a detection signal is subjected to two-stage amplification, the detection sensitivity is improved, and the determination range is enlarged; and the kit can be applied to an automatic biochemical analyzer to shorten detection time, and improve detection efficiency.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
IgM (Immune Globulin M) immune turbidimetry test kit
ActiveCN104407159AHigh analytical sensitivityDoes not affect accuracyBiological testingMagnetite NanoparticlesBovine serum albumin
The invention discloses an IgM (Immune Globulin M) immune turbidimetry test kit, and belongs to the technical field of clinical in-vitro test reagents. The kit disclosed by the invention comprises a reagent R1, a reagent R2 and a calibration material. The stability and the analysis sensitivity of the kit are effectively improved by adding a certain amount of silicon dioxide-cladded magnetic nano-particles into the reagent R1 and adding a certain amount of bovine serum albumin and Kathon-CG into the reagent R2; the linear range of the kit is also larger; the accuracy of each reagent is high; popularization and application of the kit in markets are facilitated.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Method for detecting distribution uniformity of colloid in colloid battery
ActiveCN102353606AThe detection process is fastHigh sensitivityWeighing by removing componentElectrical batteryGas phase
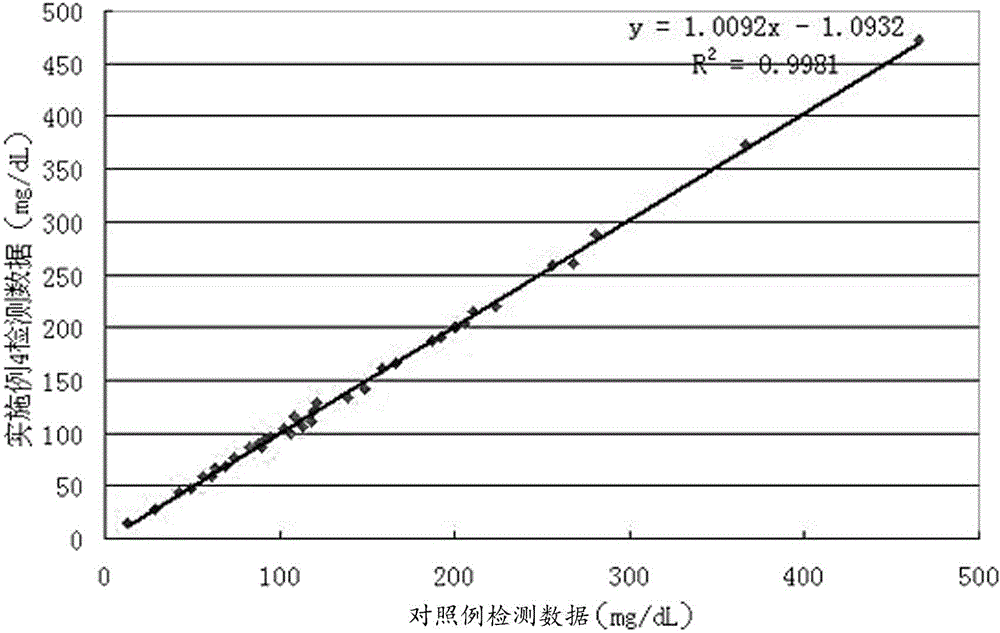
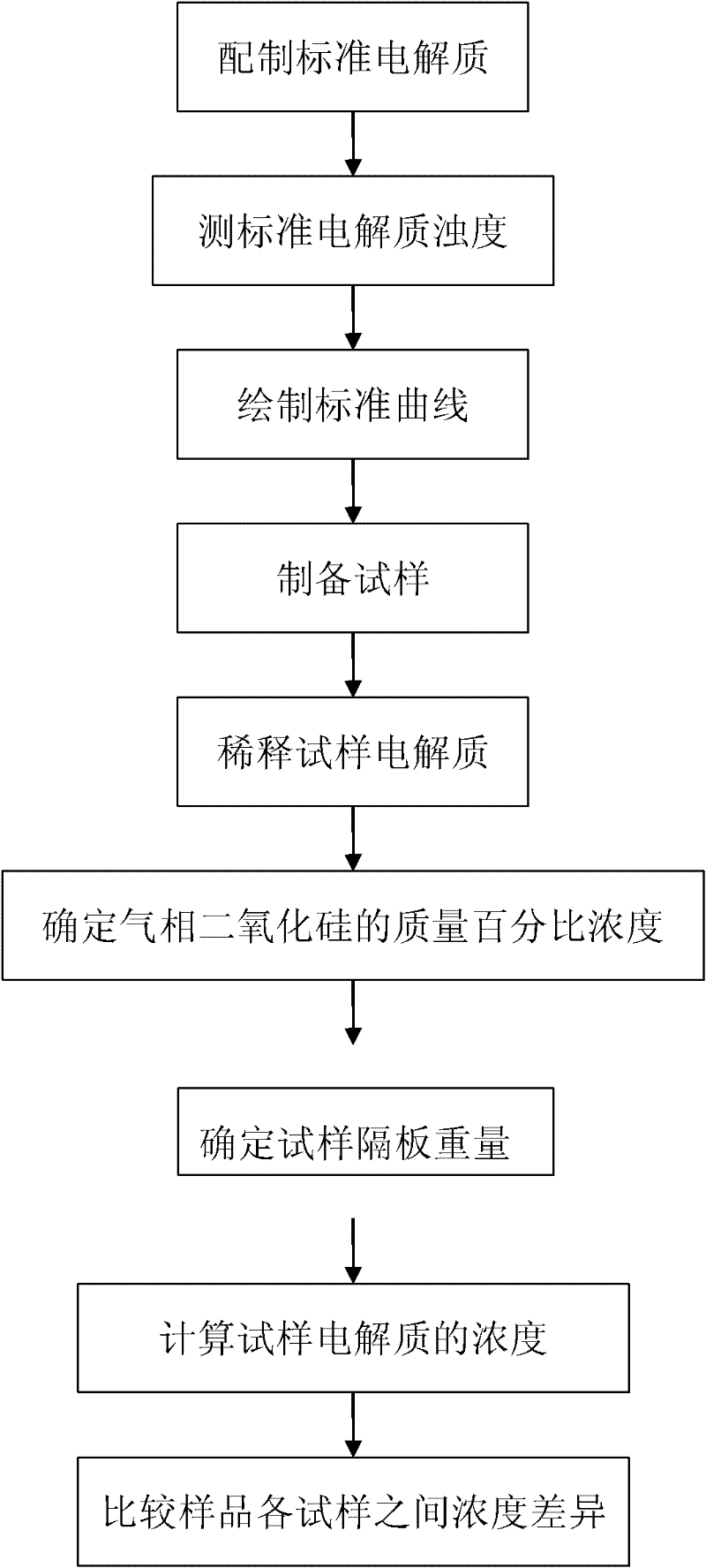
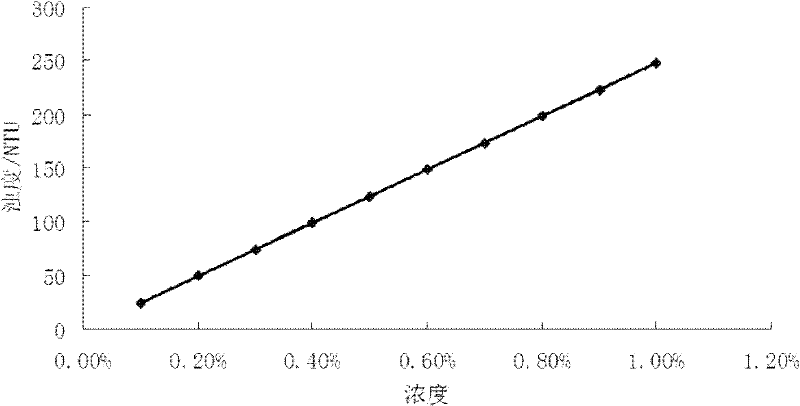
The invention discloses a method for detecting the distribution uniformity of colloid in a colloid battery, comprising the following steps of: measuring turbidity values of standard liquids of different concentrations by nephelometry, drawing a standard curve corresponding to the relationship between concentration and turbidity; dividing a sample to be measured into samples at different positionsand detecting their electrolytes' gas phase silica concentration, uniformly dispersing the electrolytes fixed in separator plates into added distilled water by the use of an ultrasonic machine, filtering out a filtrate, measuring the turbidity value of the filtrate, finding a corresponding concentration of gas phase silica in the diluted electrolytes from the standard curve, measuring the dry weight of each sample separator plate as filter residue, calculating the concentration of gas phase silica in the undiluted colloidal electrolytes in the separator plates, comparing the concentration difference between the samples at different positions, and representing the distribution uniformity of colloid after colloid pouring of the colloid battery.
Owner:CHAOWEI POWER CO LTD
Immuno-nephelometry of lipoprotein (a) and reagent therefor
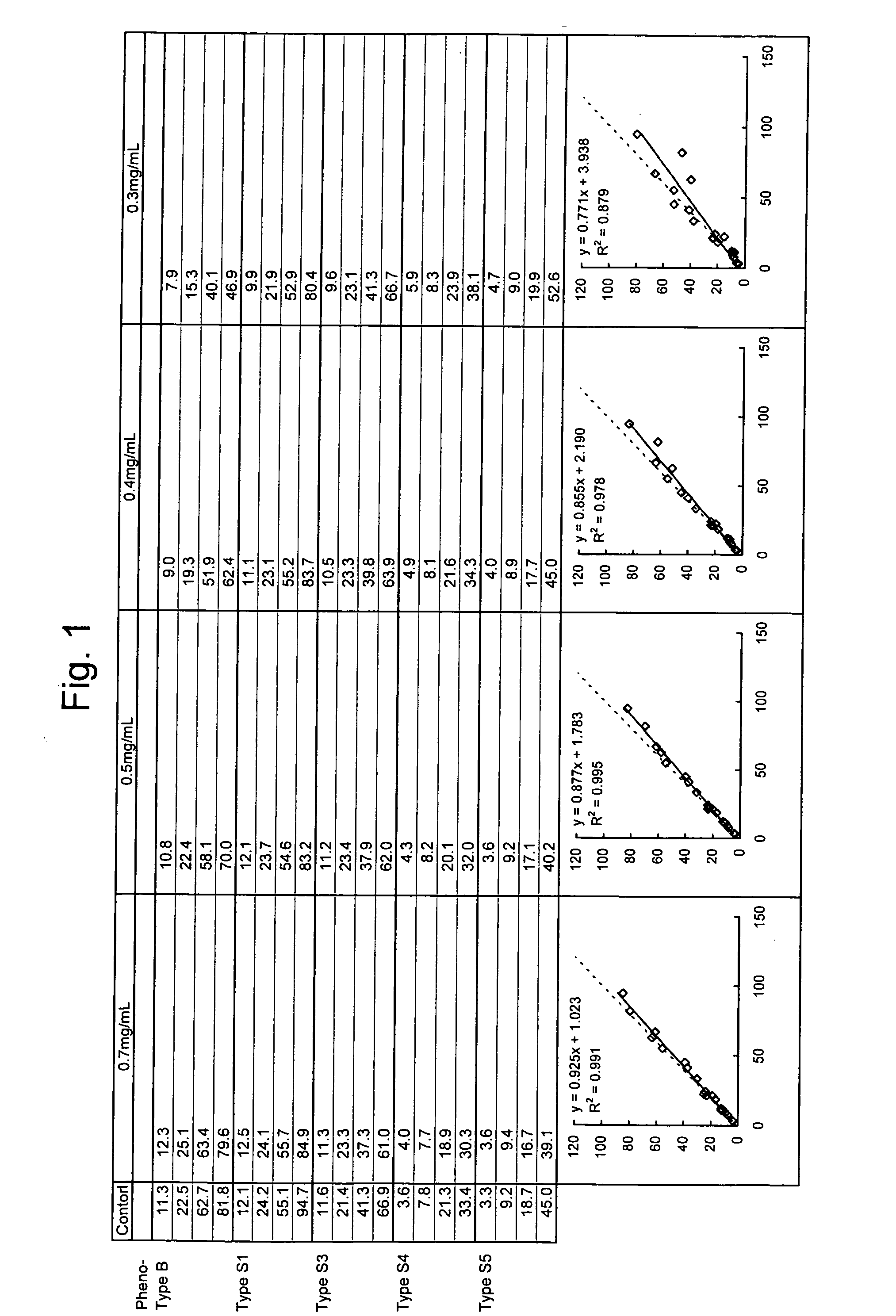
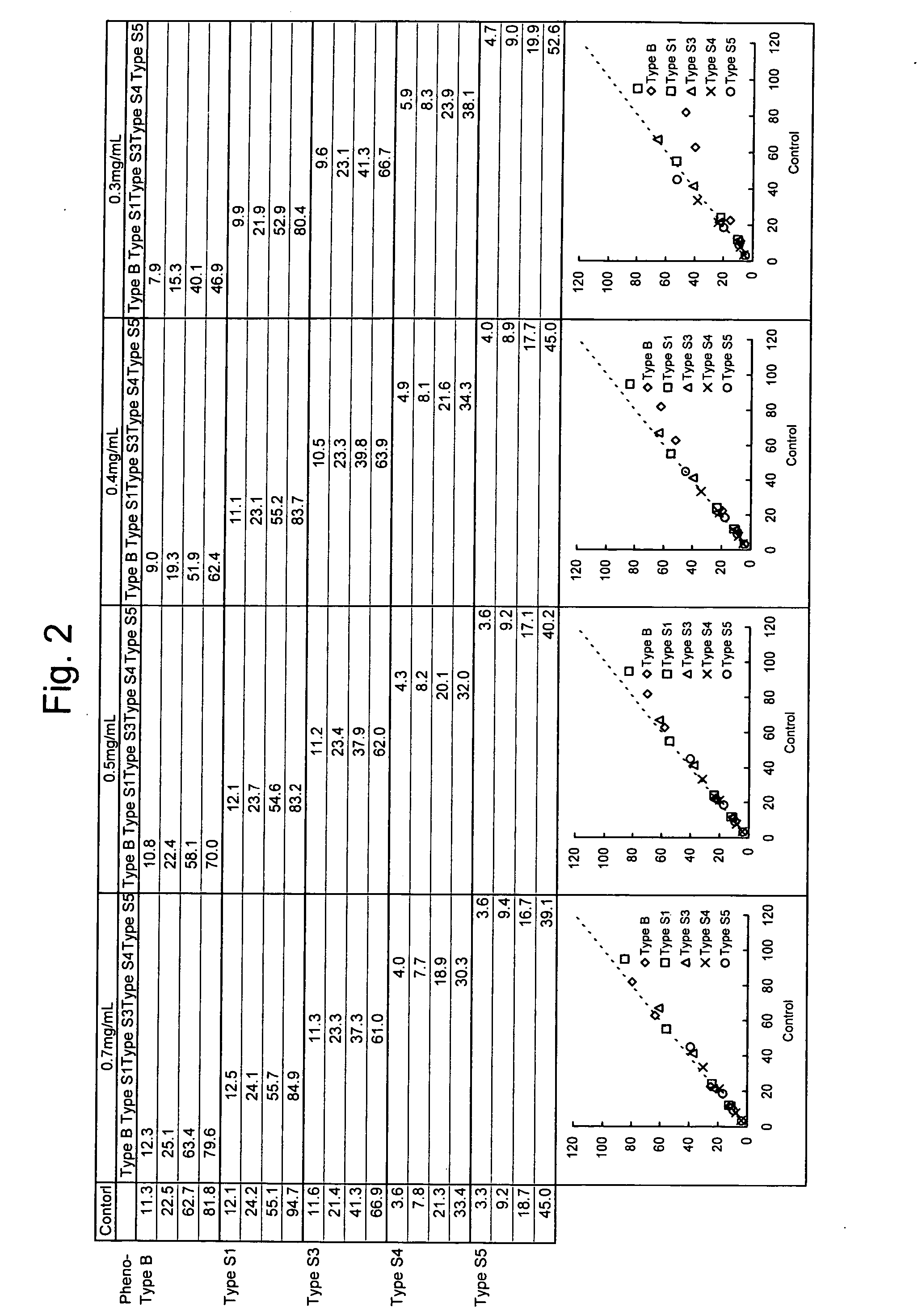
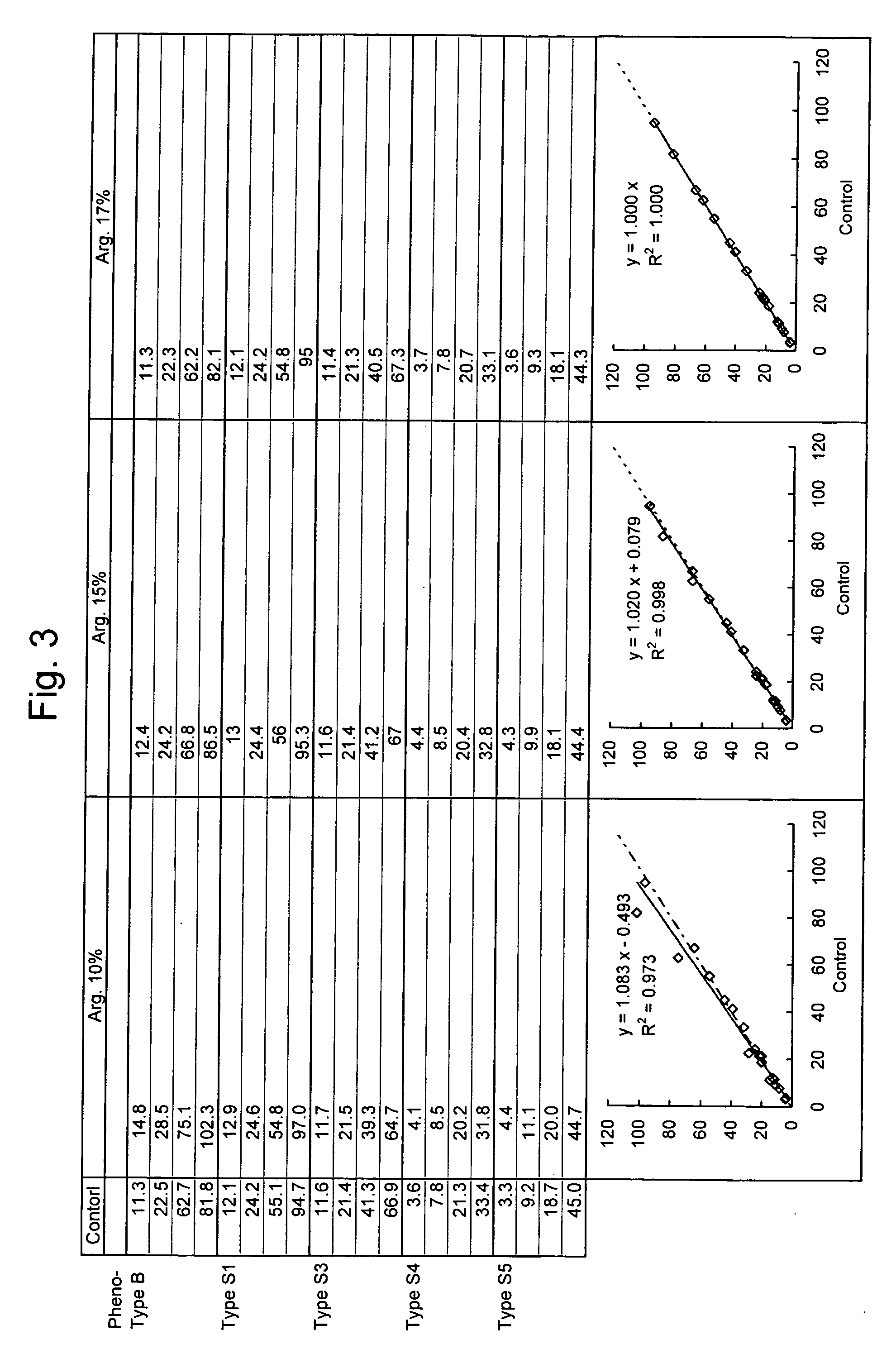
Provided is a method for quantitatively measuring an antigen having diverse phenotypes with accuracy in immunoassay. The present invention is particularly intended to latex turbidimetric immunoassay using the antigen-antibody reaction of an antigen having phenotypes, wherein, in detection utilizing the immunoassay, the amount of an antibody against the antigen added to an assay system is adjusted and a basic amino acid is added to the assay system, thereby circumventing the variability of a measurement value attributable to phenotype variety and obtaining a measurement value having a high correlation with a measurement value of the antigen in a biological sample that is measured on a molecular basis.
Owner:DENKA SEIKEN CO LTD
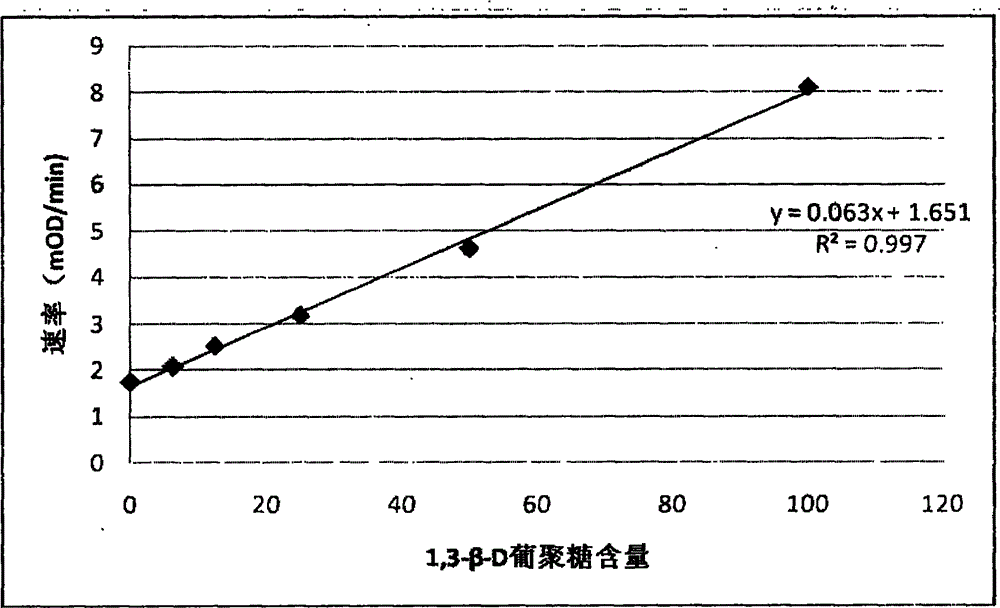
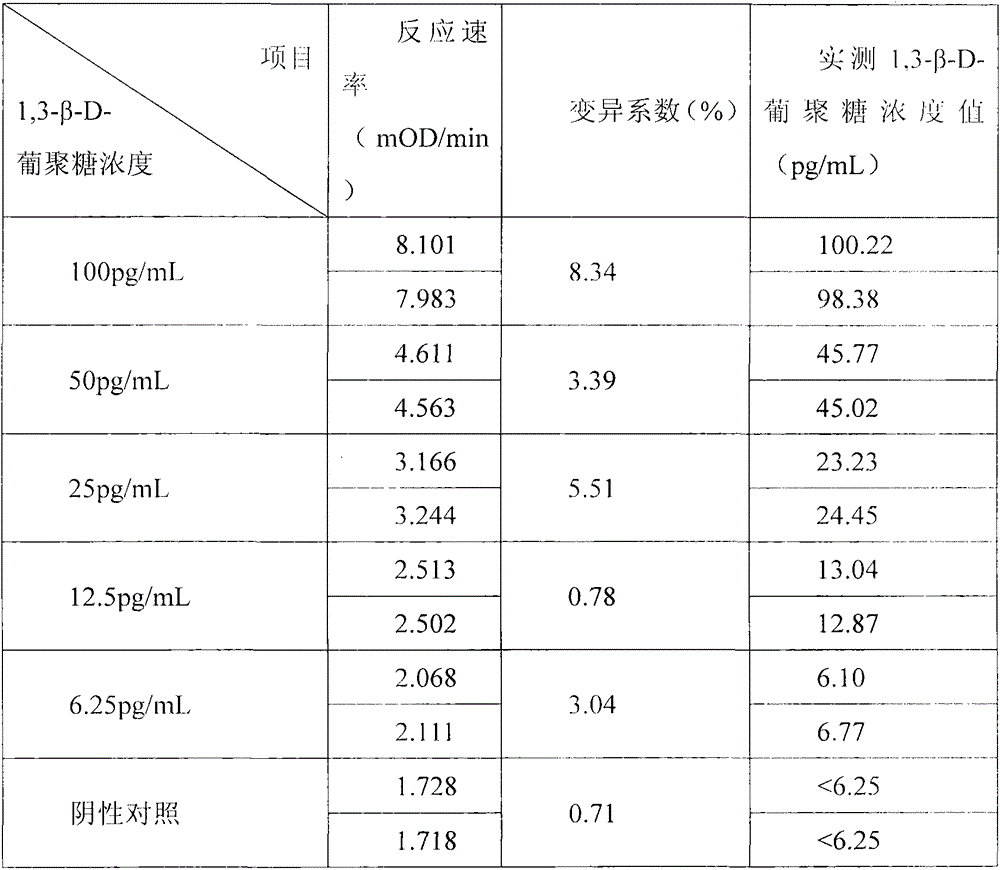
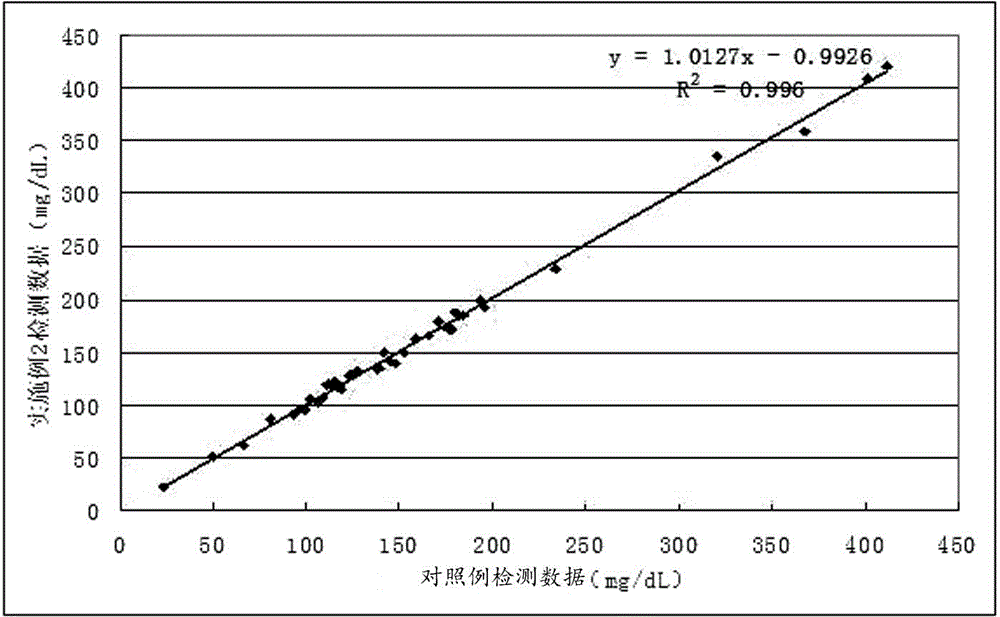
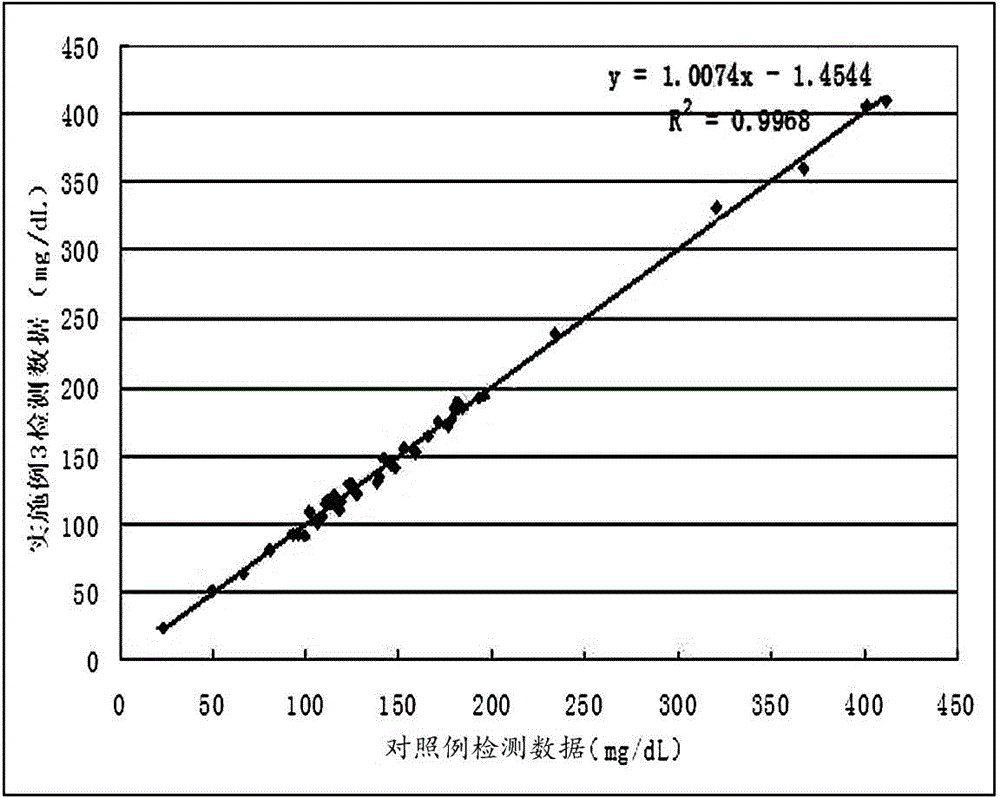
Developing-process fungus 1,3-beta-D-glucan detection kit for human body fluid
ActiveCN105021817AReduce false positive rateLess susceptible to interferenceColor/spectral properties measurementsBiological testingZymogenEnzyme digestion
The invention relates to a developing-process fungus 1,3-beta-D-glucan detection kit for human body fluid. The developing-process fungus 1,3-beta-D-glucan detection kit comprises a reaction main agent, a main agent compound solution, a sample treatment solution, heat-source-free water, a standard product and a quality control product, wherein the reaction main agent takes horseshoe crab blood cells as a main raw material and contains G factors, coagulase, coagulase zymogen and a polypeptide developing substrate; the polypeptide developing substrate is synthesized tripeptide or tetrapeptide with a Gly-Arg tail end connected with a PNA; the polypeptide developing substrate is subjected to enzyme digestion by adopting the coagulase; after the free paranitroaniline (PNA) is generated, a microplate reader is used for directly detecting so that a detection route is shortened and the cost is reduced; the microplate reader is used for carrying out a velocity-method enzyme kinetics detection method so that the sensitivity is relatively high when being compared with a nephelometry detection method; and the reaction main agent is not easily interfered by protein in a body fluid sample and medicines to generate non-specific turbidity, so that the probability of a false positive detection result is reduced and the detection accuracy is relatively high.
Owner:DYNAMIKER BIOTECH TIANJIN
Alpha1-AT (antitrypsin) immunoturbidimetry detection kit
ActiveCN104374924AHigh analytical sensitivityImprove stabilityBiological testingMagnetite NanoparticlesEngineering
The invention discloses a Alpha1-AT (antitrypsin) immunoturbidimetry detection kit and belongs to the technical field of clinical external detection reagents. The detection kit comprises a reagent R1, a reagent R2 and a calibrator. A certain amount of magnetic nano-particles covered with silicon dioxide are added in the reagent R1, a certain amount of BSA (bovine serum albumin) and Proclin-300 are added in the reagent R2, and accordingly analytical sensitivity and stability of the detection kit are improved, linear range thereof is expanded, reagents accuracy is higher, and further application and promotion in the market is facilitated.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Immunological latex turbidimetry method and reagent therefor
InactiveUS20020182752A1Easy and rapid and high-sensitivity analysisImprovement of such an avoidanceBiological material analysisBiological testingAntigenProteinase activity
An immunological latex turbidimetry method for analyzing an antigen or antibody in a sample, comprising steps of: (1) bringing a sample which may contain the antigen or antibody to be analyzed into contact with a protease-treated albumin; and (2) bringing a mixture obtained in the above step (1) into contact with latex particles carrying an antibody or antigen specifically reacting with the antigen or antibody to be assayed, and analyzing a turbidity caused by a latex agglutination reaction, is disclosed. Further, an immunological latex turbidimetry reagent comprising (1) a first component containing a protease-treated albumin, and (2) a second component containing latex particles carrying an antibody or antigen specifically reacting with an antigen or antibody to be assayed is also disclosed.
Owner:MITSUBISHI CHEM MEDIENCE
Troponin I competition turbidimetry detecting kit
The invention relates to a troponin I competition turbidimetry detecting kit. Three minitype peptide fragments of human cardiac troponin I (cTnI) needed by setting artificial synthesis are used, the peptide fragments and inert carrier protein are coupled, and the peptide fragments and polystyrene latex microballoons prepared by antibodies having an effect on the minitype peptide fragments form reagents. The kit prepared by the immune latex reagents can be used by a semiautomatic or full-automatic developing analyzer for detecting cardiac troponin content in serum.
Owner:温州煦棠生物科技有限公司
Device, system and method for in-situ optical monitoring and control of extraction and purification of plant materials
ActiveUS20180306708A1Distillation regulation/controlPolarisation-affecting propertiesPhotoluminescenceTurbidity
In-situ optical spectroscopic monitoring, characterization and feedback control of extraction and purification processes of compounds such as oils, alkaloids, flavonoids, terpenes and cannabinoids derived from plant material are described. Liquids from an extraction or purification process flow down an optically transparent tube, such as one made of glass. An in-situ optical monitoring assembly is configured to be mounted onto the tube to measure optical properties of the liquid extract or liquid condensate as it flows in the tube. Optical properties of the flowing liquid may include optical transmittance, reflectance, photoluminescence, scattering, absorbance, turbidity, nephelometry, polarimetry and colorimetry. The output of the optical monitoring assembly can be used to display spectral and other about the flowing liquid, set alarms to notify an operator of a predetermined condition such as a set point, and used to control extraction or purification process.
Owner:AROMETRIX INC
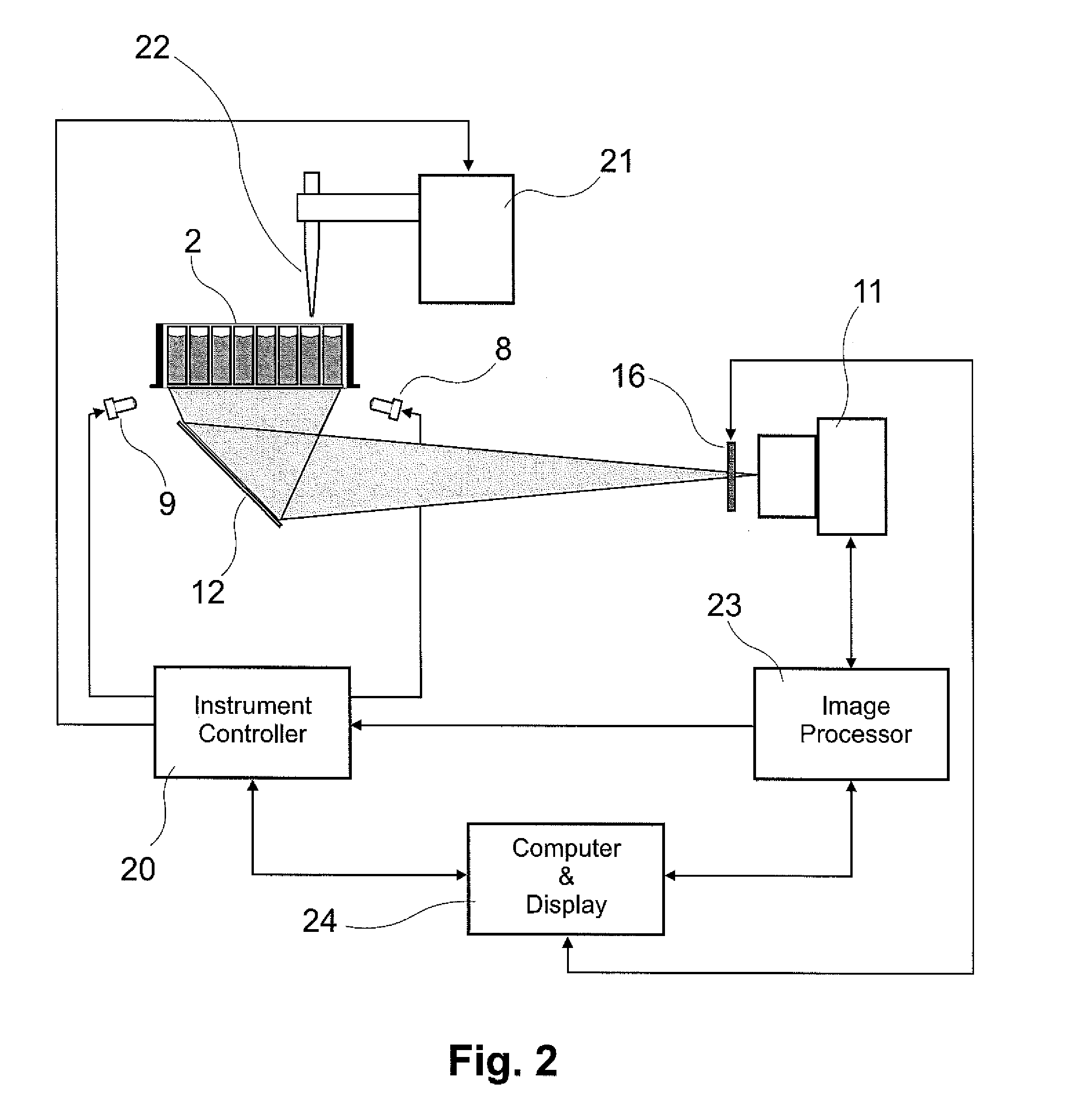
Nephelometry method and apparatus for determining the concentration of suspended particles in an array of sample containers
ActiveUS20160209328A1Concentration adjustableScattering properties measurementsParticle suspension analysisSuspended particlesSingle vessel
Described are devices, methods, and systems that are suitable for rapidly and simultaneously determining the concentration of suspended particles in a sample. The devices, methods, and systems allow for the rapid and simultaneous interrogation of a large number of sample wells in a single vessel, for example, samples contained in a two-dimensional array or micro-titer plate, without the need for moving reading heads or moving the sample vessel. The nephelometry system allows the user to rapidly and simultaneously measure the concentration of the particles in numerous samples, adjust the concentration of the particles in the sample with a sample handling system, and re-measure the concentration of the samples in order to achieve a desired concentration.
Owner:BECTON DICKINSON & CO
Method for detecting endotoxin content
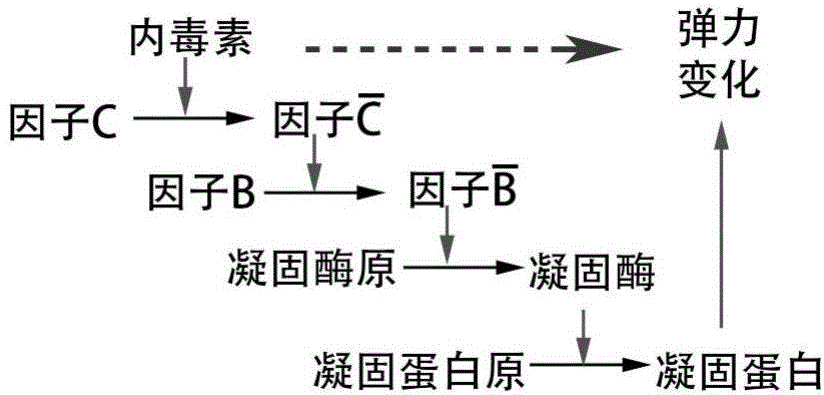
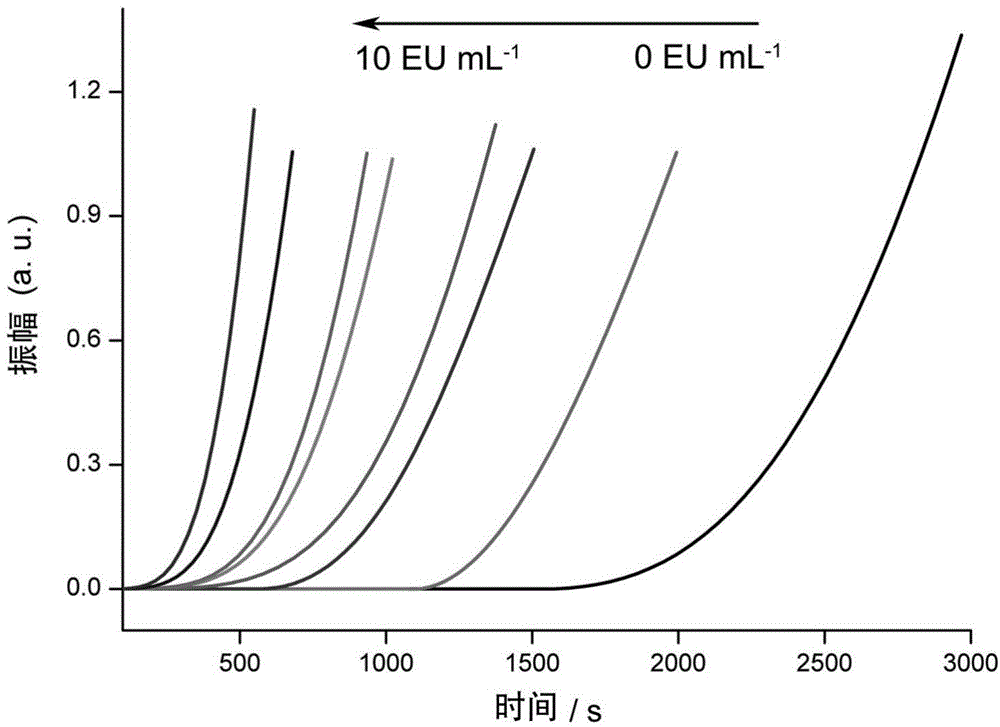
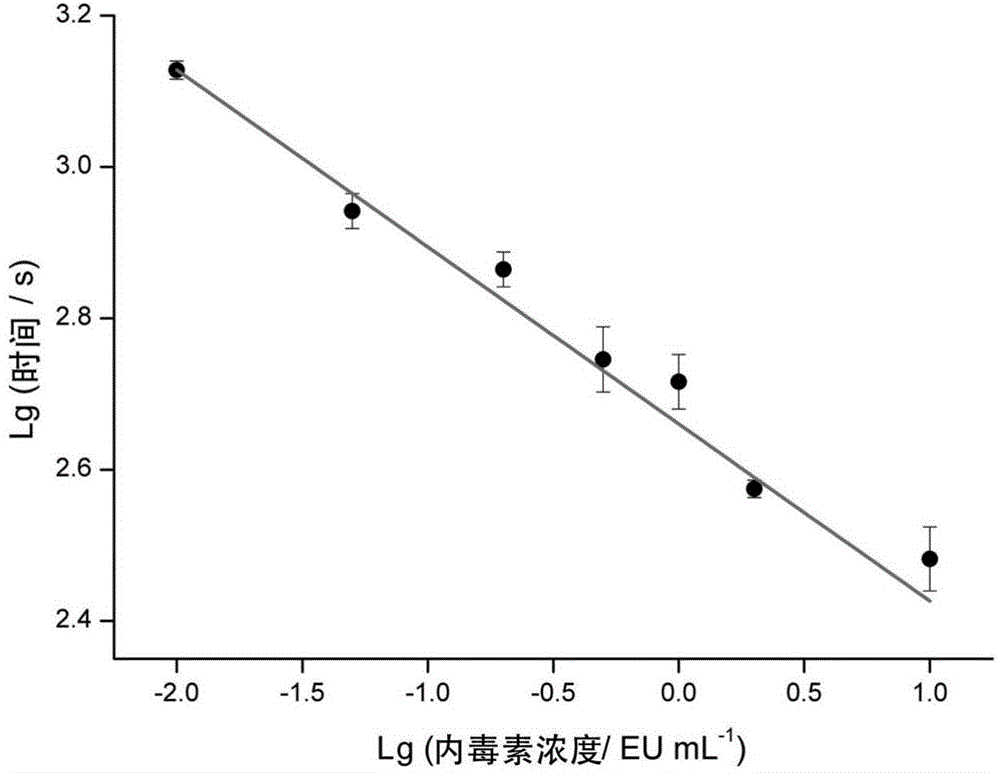
ActiveCN104698159AComprehensive detectionConvenient researchBiological testingElectrochemical biosensorDiluent
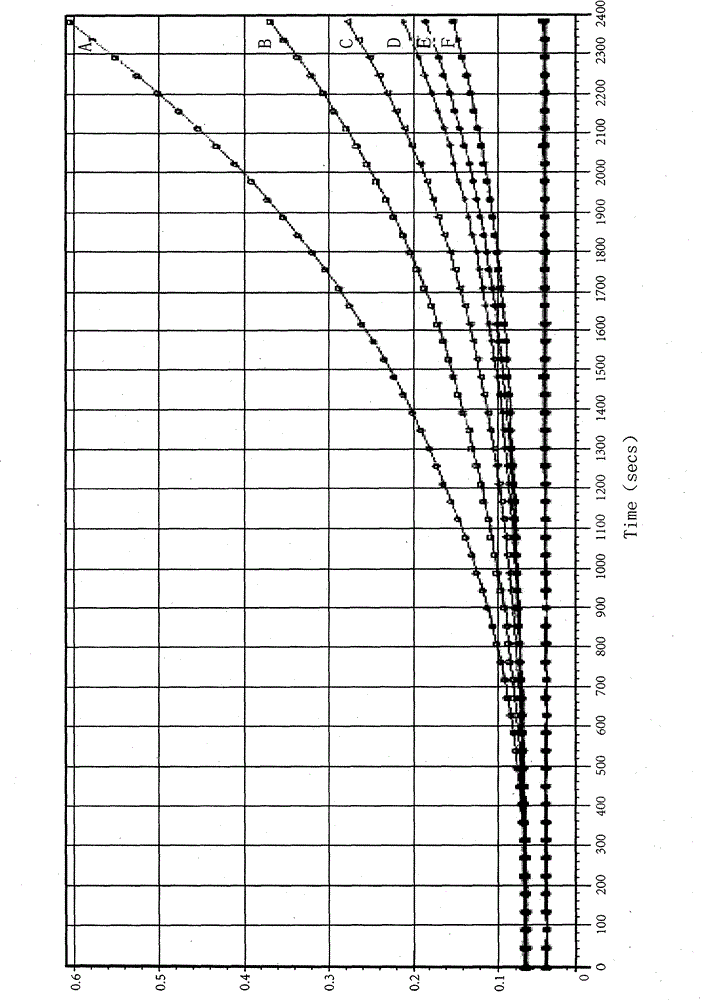
The invention discloses a method for detecting endotoxin content. The method comprises the following steps: S1. preparing at least five endotoxin standard solutions containing different concentrations; S2. respectively mixing a tachypleus amebocyte lysate diluent with each endotoxin standard solution at the ratio; S3. respectively detecting the time required when the elasticity of each mixed liquid reaches 0.1 amplitude by virtue of a thrombelastogram instrument, and drawing a bilogarithmic graph of time-endotoxin concentration to obtain a standard curve graph; and S4. mixing the tachypleus amebocyte lysate diluent with a to-be-detected sample liquid according to a specific ratio, detecting the time required when the elasticity reaches 0.1 amplitude by virtue of the thrombelastogram instrument, and comparing with the standard curve graph, so as to obtain the endotoxin content in the to-be-detected sample liquid. Compared with traditional nephelometry and electrochemical biosensor, relatively comprehensive detection can be carried out on the overall process; research on the special physiological process is facilitated; the detection time is greatly shortened; and the detection sensitivity and accuracy are synchronously improved.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Detection method of polyacrylamide concentration in tertiary oil recovery liquid
ActiveCN103226104AImprove concentration detection accuracyEliminate errorsMaterial analysis by observing effect on chemical indicatorTurbidityOsmolar Concentration
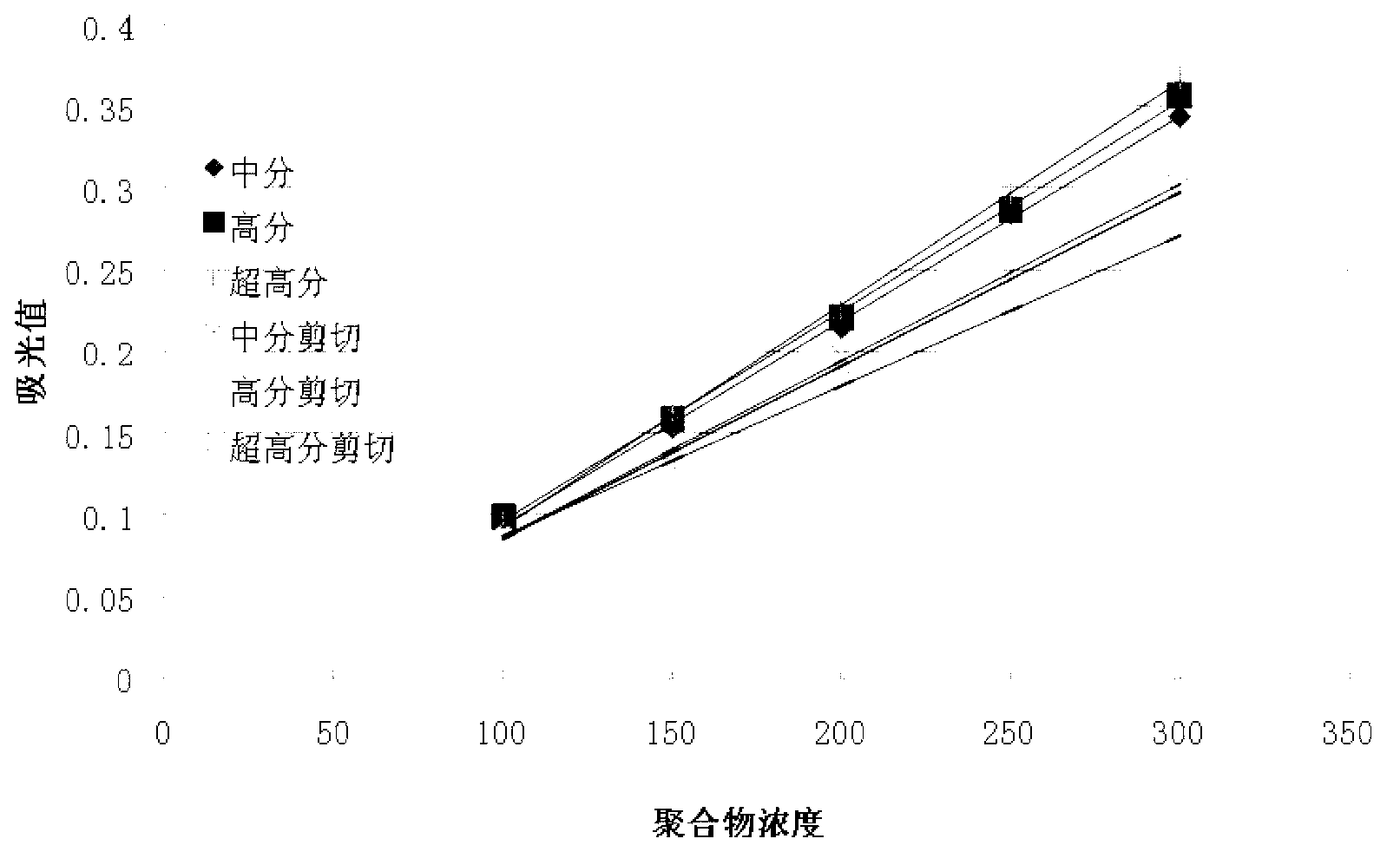
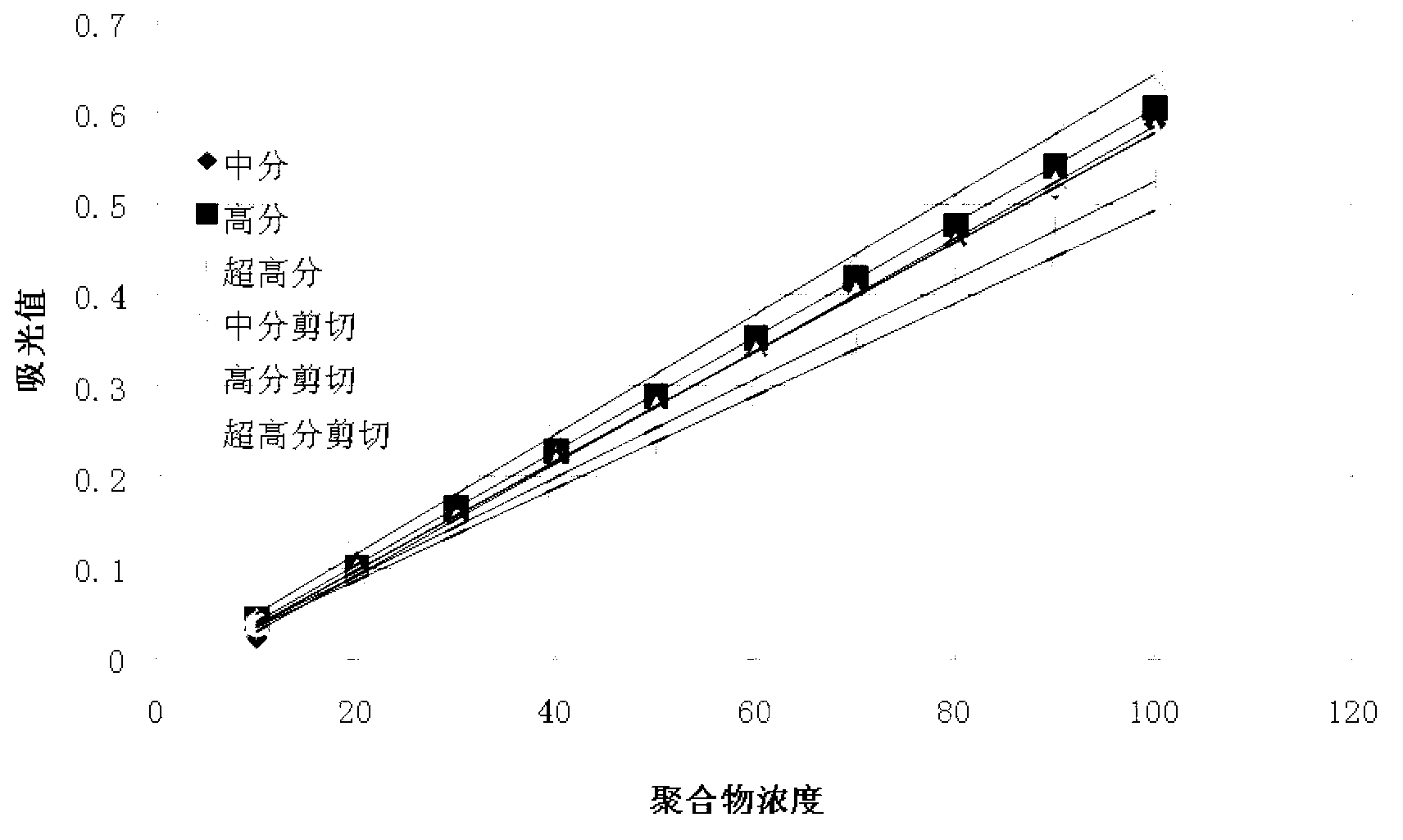
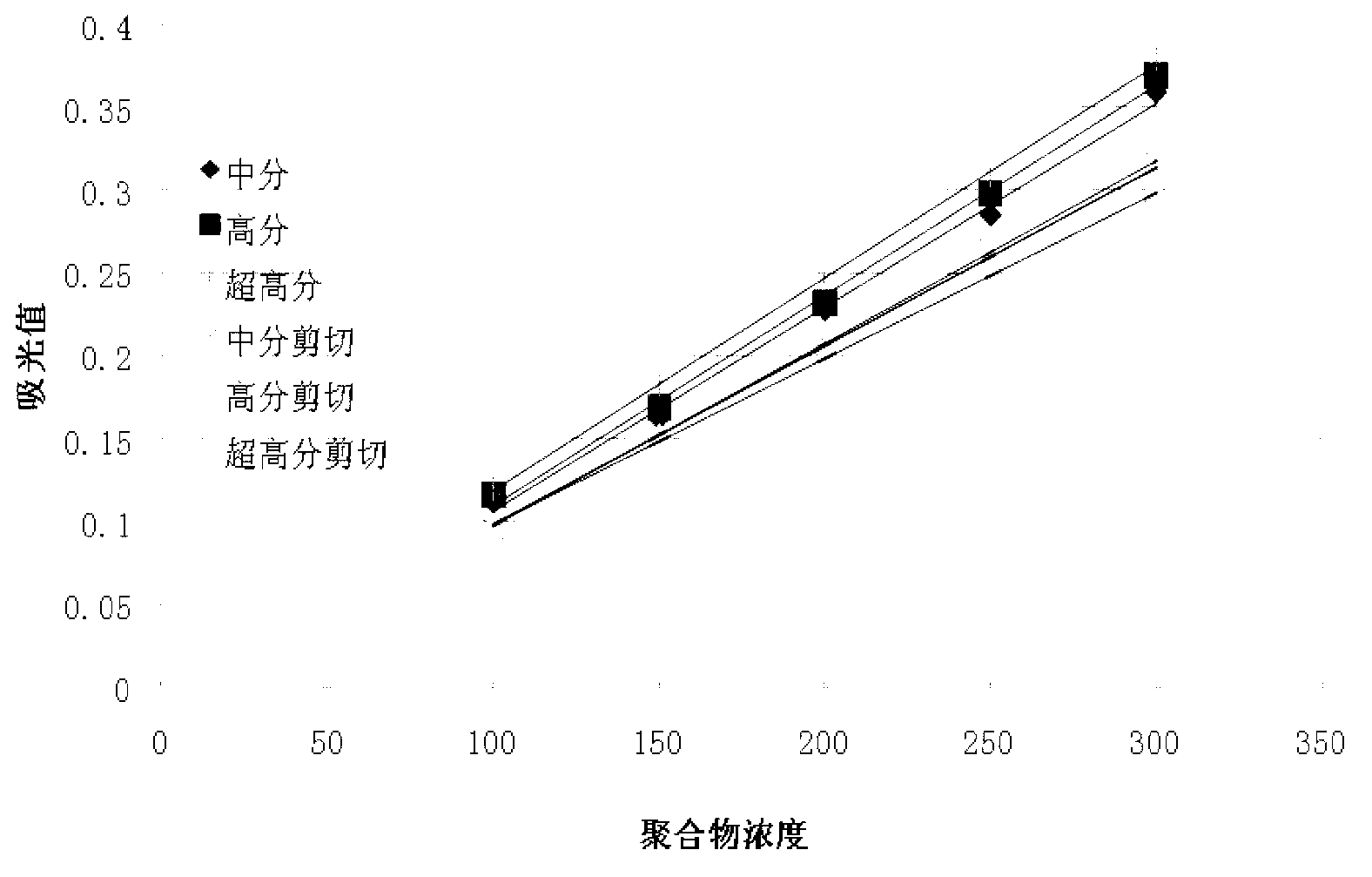
The invention provides a detection method of polyacrylamide concentration in a tertiary oil recovery liquid. The method comprises: drawing a standard curve, preparing standard polyacrylamide solutions of different concentrations, shearing for at least 30 seconds under rotate speeds of 11000-22000 r / min, determining light absorption values of the solutions, drawing a standard curve, detecting to-be-detected samples, determining light absorption values of the to-be-detected samples, and determining the concentration of the polyacrylamide of the samples referring to the drawed standard curve, wherein a nephelometry or a starch-cadmium iodide method is used to determine the light absorption values of the solutions. According to the invention, the method can eliminate relatively large errors of polymer concentrations of the recovery liquid determined by the traditional nephelometry and the starch-cadmium iodide methods, thereby increasing a detection precision of the polymer concentrations of the recovery liquid.
Owner:PETROCHINA CO LTD +1
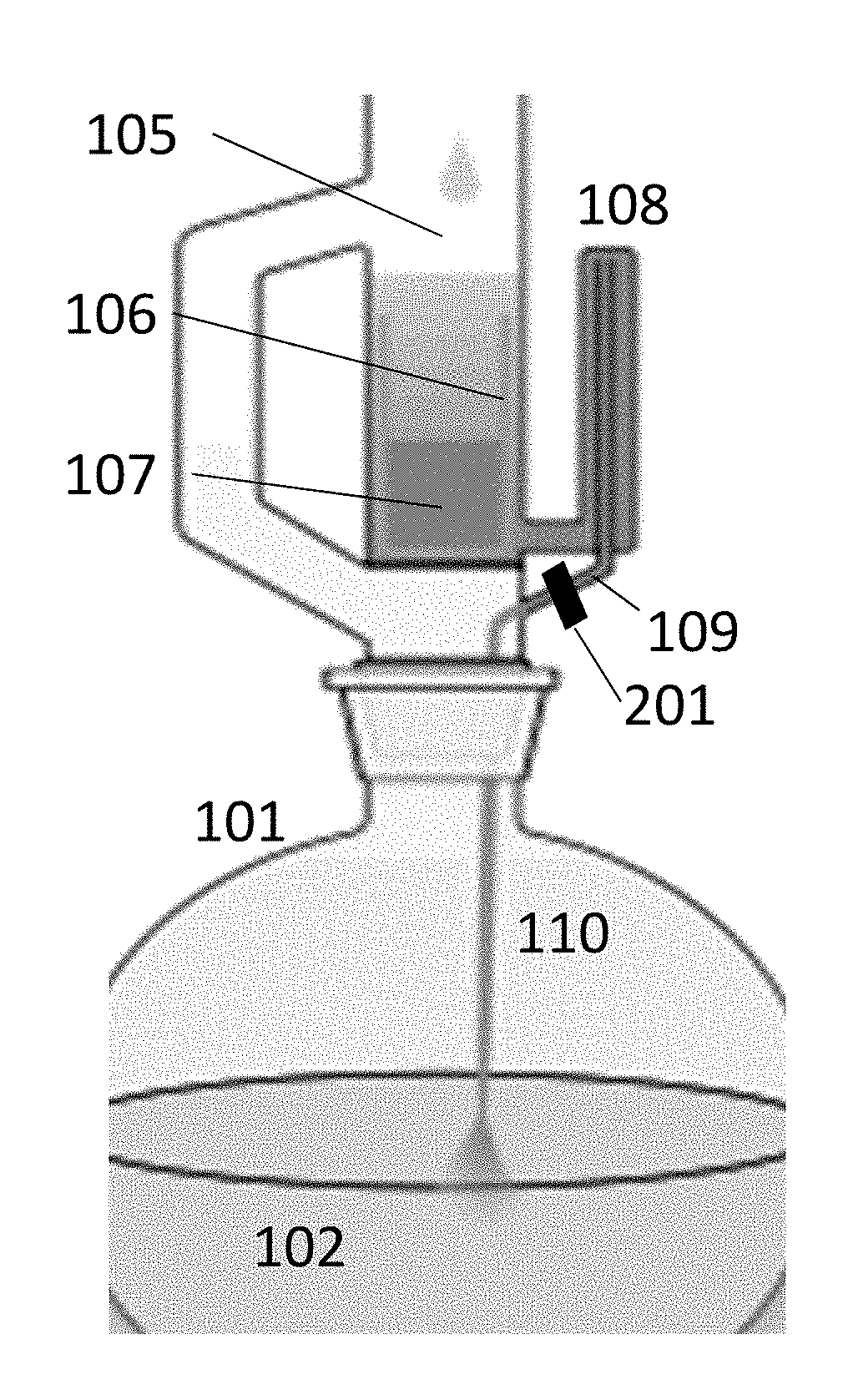
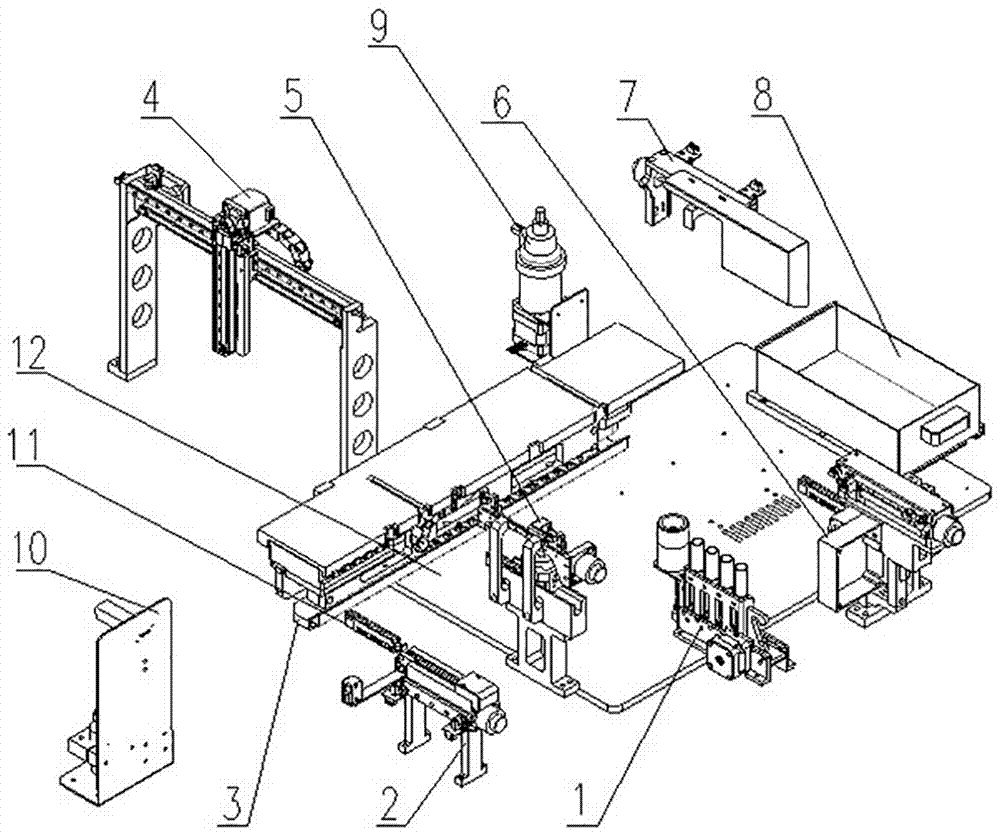
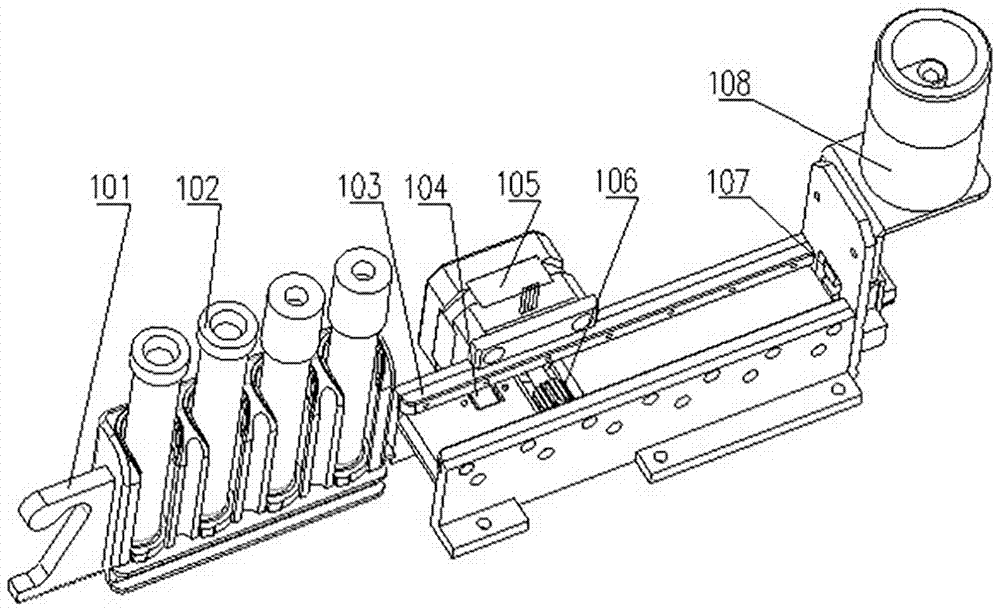
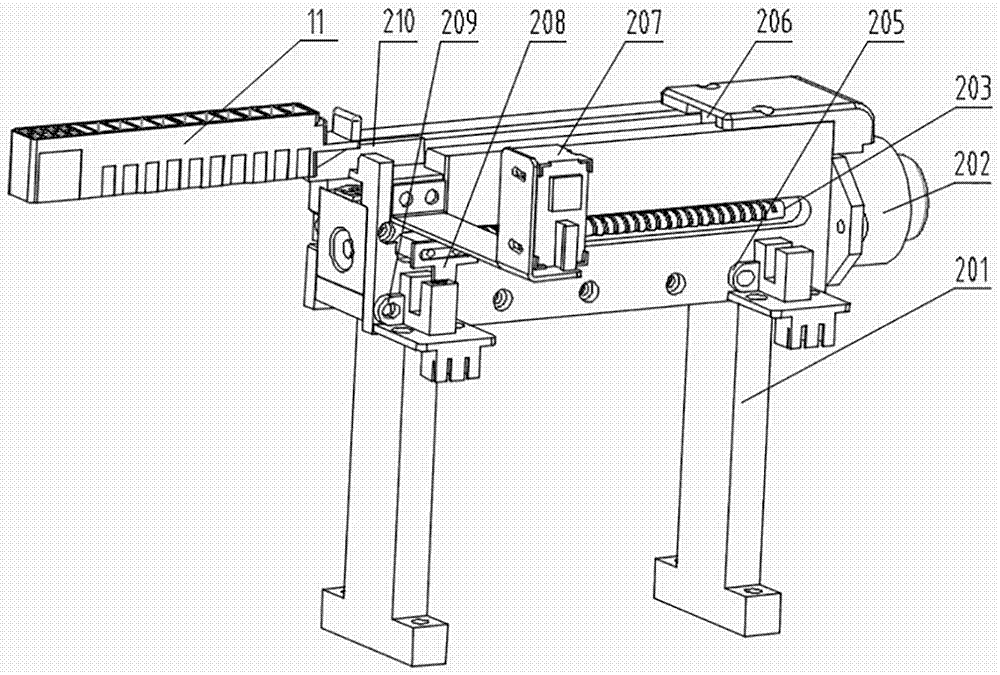
Biochemical analyzer and detection method thereof
The invention relates to a biochemical analyzer and a detection method thereof. The biochemical analyzer comprises a base (12), a sampling module (4), an incubation device (3) and a detection device (6) are fixed on the base (12), the incubation device (3) consists of a linear guide rail 305 and an incubation tank 310, and the incubation tank 310 can move along the linear guide rail 305. The biochemical analyzer also includes a mobile device and a detection component, the mobile device enables the movement of a chip box (11) in the incubation tank 310 along the vertical direction of the linear guide rail 305, and the chip box (11) can be detected through the detection component. Through colorimetry and nephelometry dual detection, the analyzer provided by the invention can rapidly detect multiple items of one object or multiple items of multiple objects, and has the characteristics of single part, multiple targets, multiple channels, full automation, high flux and convenient use, etc.
Owner:SHENZHEN LIVING WATER POCT CO LTD
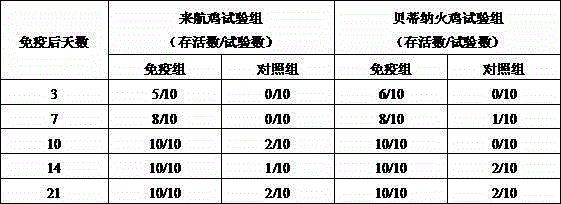
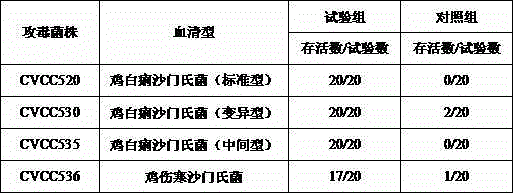
Preparation method and application of chicken white diarrhea salmonella inactivated vaccine
ActiveCN105031635AIncrease productionGood immune protectionAntibacterial agentsAntibody medical ingredientsBiotechnologySalmonella diarizonae
The invention discloses a chicken white diarrhea dyed condensation antigen, a preparation method and an application thereof and belongs to the technical field of veterinarian diagnosis. The antigen is prepared by following steps: performing recovery passage to chicken white diarrhea salmonella strains comprising SP7220, SP7701, SP8441 and SP9905 to obtain a seed bacterial liquid; inoculating the seed bacterial liquid on a solid culture medium for proliferation; inactivating the bacteria in formalin; regulating the concentration of the bacterial liquid through turbidimetry; and finally adding a crystal violet solution to dye the bacterial liquid, fully mixing uniformly the bacterial liquid and packaging the bacterial liquid to obtain the antigen. The preparation method is simple and reasonable, is scientific, is stable in production and is low in cost. The chicken white diarrhea condensation antigen production strain is good in antigenicity and is low in mutation rate. A prepared chicken white diarrhea dyed condensation antigen product is high in sensitivity, is strong in specificity, is quick in diagnosis and is clear in condensation image, and is an excellent dyed condensation antigen for detecting chicken white diarrhea.
Owner:JIANGSU INST OF POULTRY SCI
Means of Achieving a Lambertian Distribution Light Source via a Light Emitting Diode Array
InactiveUS20050180134A1Accurate measurementQuick changeScattering properties measurementsSpectral modifiersTurbidityDiscrete circuit
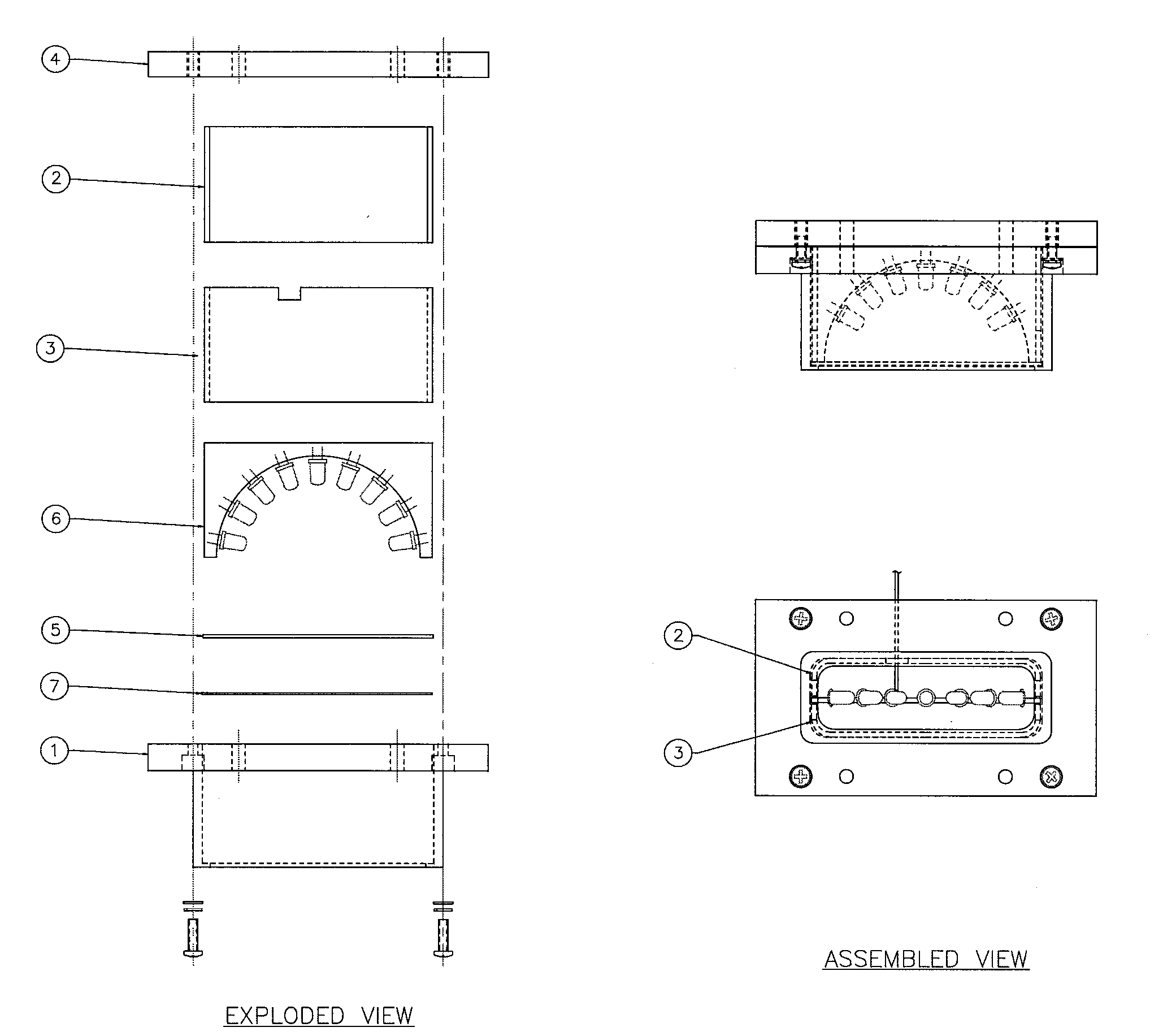
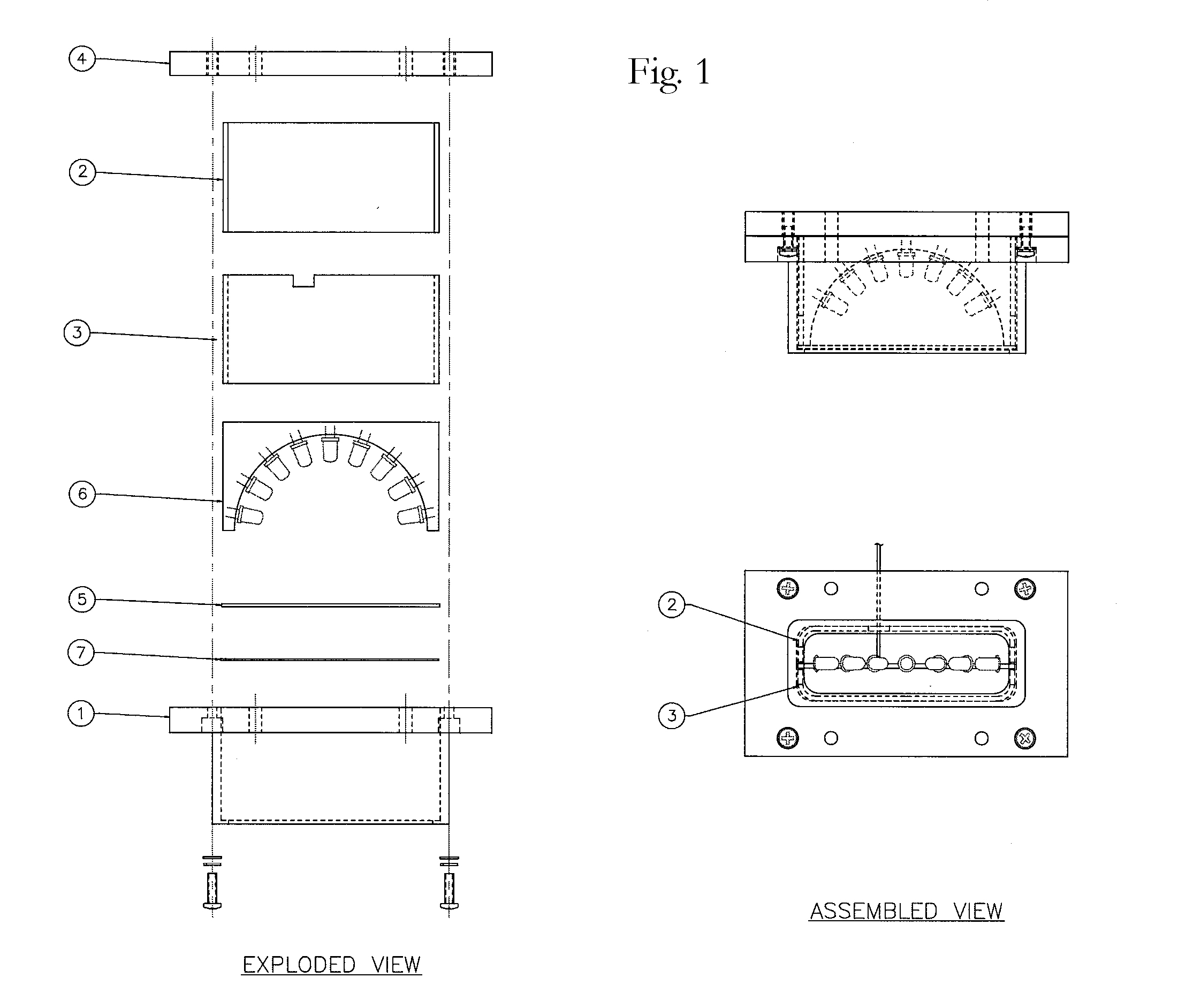
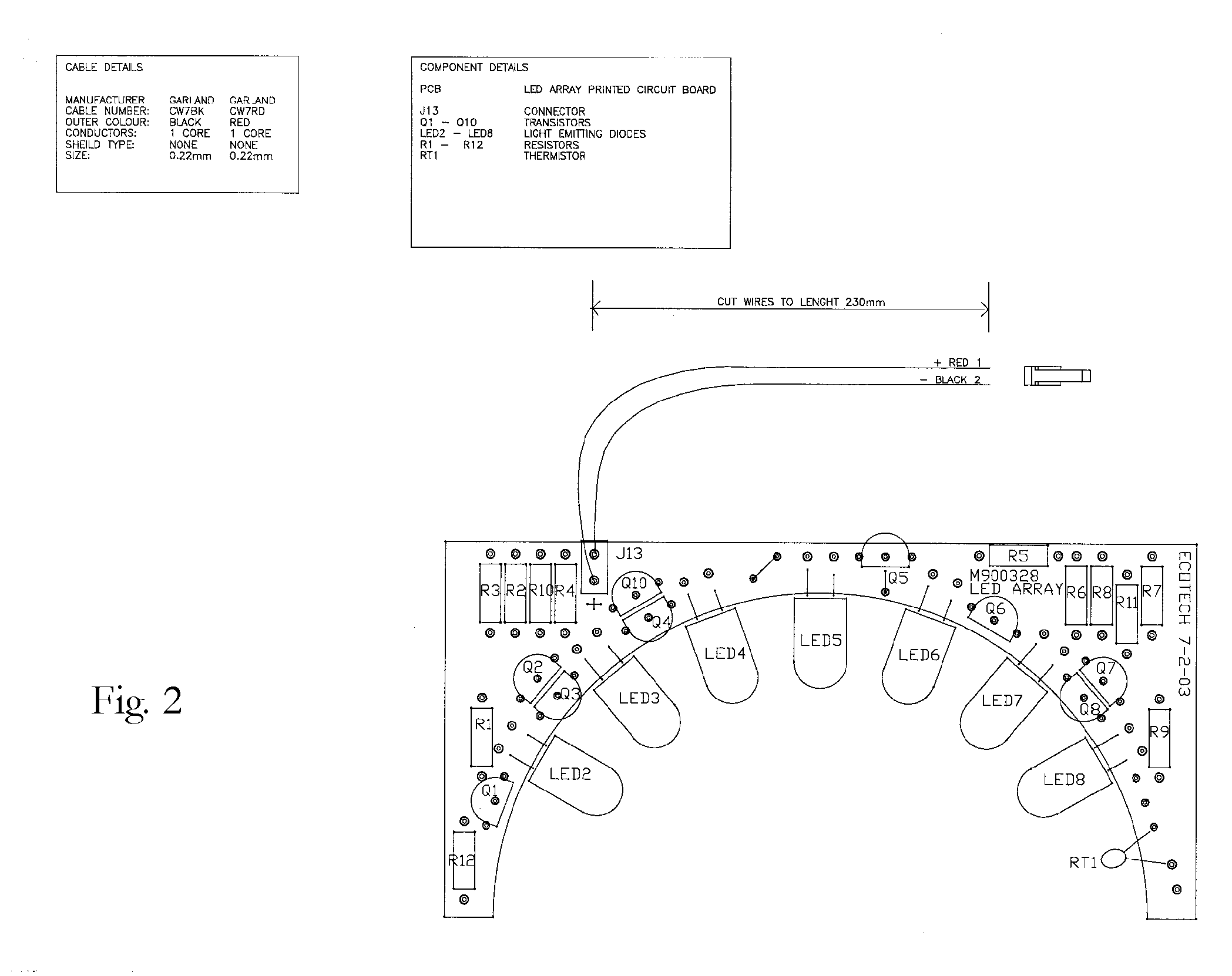
A semi-circular LED array that is comprised of independent high intensity LED components and which in combination, will emit a focused single wavelength of highly accurate and precise frequency, in accordance with the Lambertian principle. The resultant combined light source can then be used in combination with an integrating nephelometer, for purposes of precise ambient air quality monitoring. The entire LED array and housing chamber can be easily interchanged to facilitate separate and different wavelength light sources, as required. The user can, when desired, easily remove the entire LED array, and replace with another LED array of different total wavelength capability. Thus a considerably more versatile and accurate light source is provided for use in nephelometry, that can be automatically adjusted via discrete circuitry to provide a precise and accurate specific wavelength light source, which removes any requirement for external light filters, and which provides significantly increased long life and significantly reduced maintenance requirements. The overall effect is a very stable Light Source which produces a fixed wavelength of light with a distribution that is Lambertian.
Owner:DAL SASSO ROBERT +2
Method for detecting bacterial endotoxin of xylitol injection
InactiveCN101718709AKnow the actual contentMaterial analysis by observing effect on chemical indicatorBacteroidesToxin
The invention relates to a method for detecting bacterial endotoxin of a xylitol injection, which comprises the step of detecting the xylitol injection by using a tal reagent through a dynamic nephelometry, wherein the xylitol injection to be detected is diluted for 2 to 4 times, the pH of the xylitol injection to be detected is adjusted to be between 6.2 and 7.2, and the detection temperature is controlled to be 37.2+ / -0.2 DEG C. The method can overcome the defects of an animal pyrogen test, is time-saving and economical for detecting the bacterial endotoxin, can more quickly and more clearly obtain the actual content of the bacterial endotoxin in the xylitol injection, and has a positive significance for discharging a finished product.
Owner:蚌埠丰原涂山制药有限公司
Microfluidic chip for scattering turbidimetry determinationand biochemical immunization machine using same
PendingCN108760686AImprove convenienceGuaranteed to be fully chargedScattering properties measurementsLaboratory glasswaresDiluentTurbidimetry
The invention provides a microfluidic chip for scattering turbidimetry determinationand a biochemical immunization machine using same, which belong to the field of determination equipment. The microfluidic chip for scattering turbidimetry determination comprises a body, a mixing slot, and reaction holes, the body is provided with a sample adding slot, a sample quantification slot, and a sample overflow slot, the sample adding slot is communicated with the sample quantification slot, the sample quantification slot is communicated with the sample overflow slot, the body is also provided with a diluent adding slot, a diluent quantification slot, and a diluent overflow slot, the diluent adding slot is communicated with the diluent quantification slot, the diluent quantification slot is communicated with the diluent overflow slot, both the sample quantification slot and the diluent quantification slot are communicated with the mixing slot, the mixing slot is communicated with the reaction holes through a flow passage assembly, one side, which is close to the circle center of the body, of each reaction hole is planar, and the parts, which correspond to the outer circles of the reaction holes, of the body are convex arcs. According to the invention, the scattering of scattering turbidimetry determination light can be effectively controlled, determination light intensity sensitivity isimproved, and the invention can be used in colorimetry determination and transmission turbidimetry assay.
Owner:天津诺迈科技有限公司
Method for measuring content of ayfivin and its preparations by nephelometry
InactiveCN101271117AIncrease supplyPromote growthMaterial analysis by optical meansBiological testingTurbidityTest organism
The invention discloses a method for measuring bacitracin and the content of the preparation thereof by the turbidity method, and the method contains the following steps: the preparation of standard solution, the preparation of test solution, the preparation of test organism suspension, the content measurement, etc. The antibiotics-microbial test and multi-dose turbidity method of the invention are adopted for measuring the bacitracin and the content of the preparation thereof, thus the test method is accurate and effective and has low cost, and test results can truly reflect antibacterial activity of the bacitracin and the preparation thereof; the method has stronger practicality and operability in the production and the quality control of the drug and the preparation thereof.
Owner:高燕霞
Anti-Cyclic Citrullinated Peptide Antibody Assay Kit
ActiveCN102507918BEnhanced detection signalHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsAntiendomysial antibodiesCitrulline
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
Solutions of mannoproteins and their use
InactiveUS20130309721A1Reduced activityShelf lifeAlcoholic beverage preparationPeptidesTurbidityTartrate
The present invention describes a mannoprotein solution which has a turbidity, when measured by nephelometry at a concentration of 200 g / l of mannoprotein and at a pH in the range of pH 4 to pH 8 lower than 70 NTU, preferably lower than 60 NTU, more preferably lower than 50 NTU, most preferably lower than 40 NTU. This solution can be advantageously used in the stabilisation of wine against tartrate salt precipitation because it is very clear and can be added directly to the wine without causing any turbidity.
Owner:DSM IP ASSETS BV
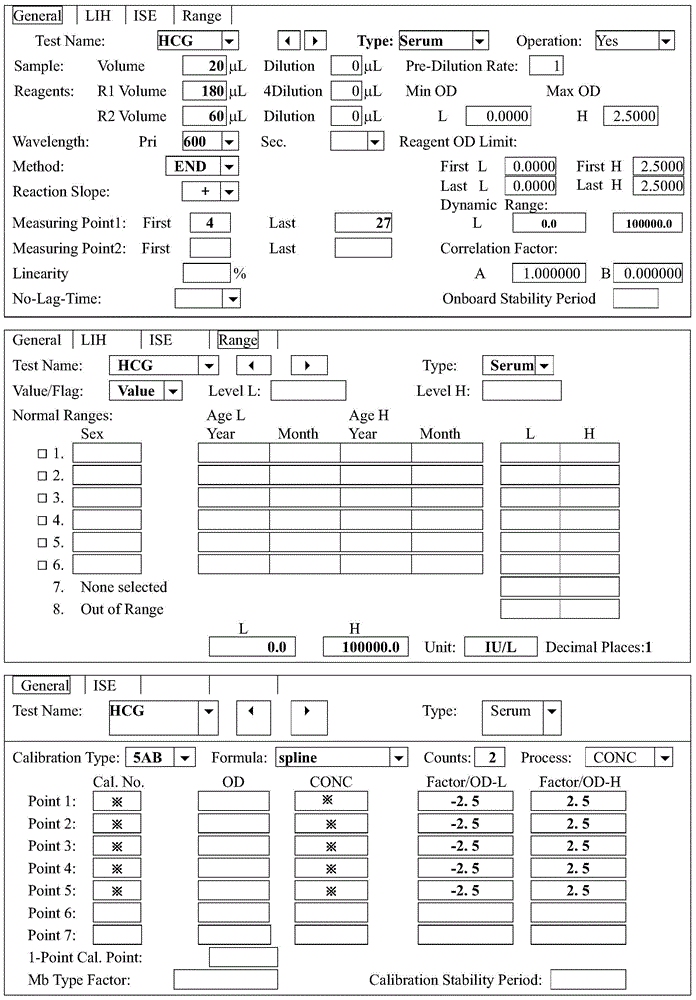
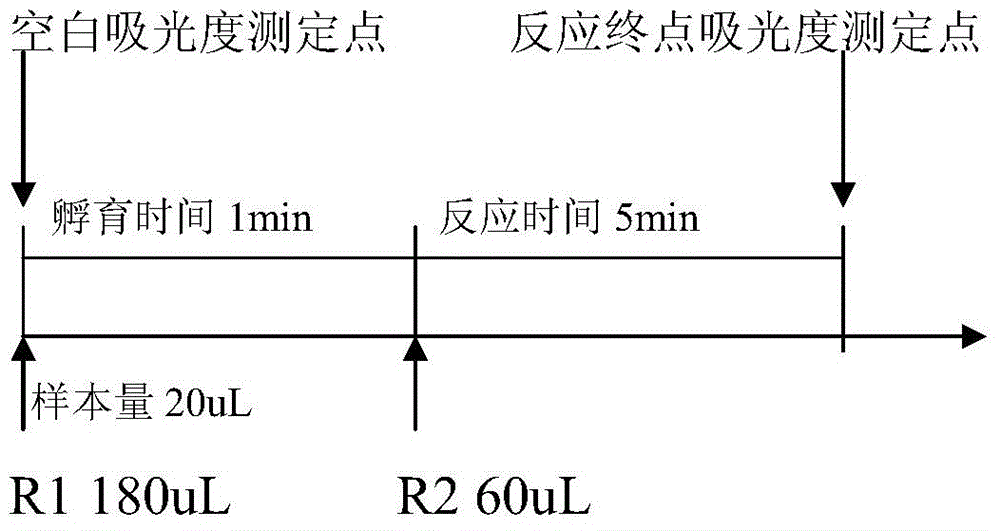
Quantitative detection kit for human chorionic gonadotropin and application of quantitative detection kit
The invention discloses a quantitative detection kit for human chorionic gonadotropin. The quantitative detection kit comprises a reagent R1, a reagent R2 and a calibration material, wherein the reagent R1 comprises a Tris buffer solution, a beta-human chorionic gonadotropin monoclonal antibody and coarse nano latex particles; the reagent R2 comprises a Tris buffer solution, a beta-human chorionic gonadotropin monoclonal antibody, and thin nano latex particles; the calibration material is a beta-human chorionic gonadotropin calibration solution. According to the quantitative detection kit, the concentration of the human chorionic gonadotropin in serum is quantitatively determined by adopting a latex enhanced immune nephelometry; measurement results are accurate; the detection process is convenient and quick; determination results are stable; by adopting a dual-nano-latex technology, the determination linear range is wide and can reach 100,000IU / L, while the determination linear range of existing other methods does not exceed 20,000IU / L; moreover, the quantitative detection kit is suitable for existing various fully automatic biochemical analyzers, low in detection cost, and suitable for developing project determination in batches clinically.
Owner:XINCHANG COUNTY MEIDI BIOLOGICAL TECH
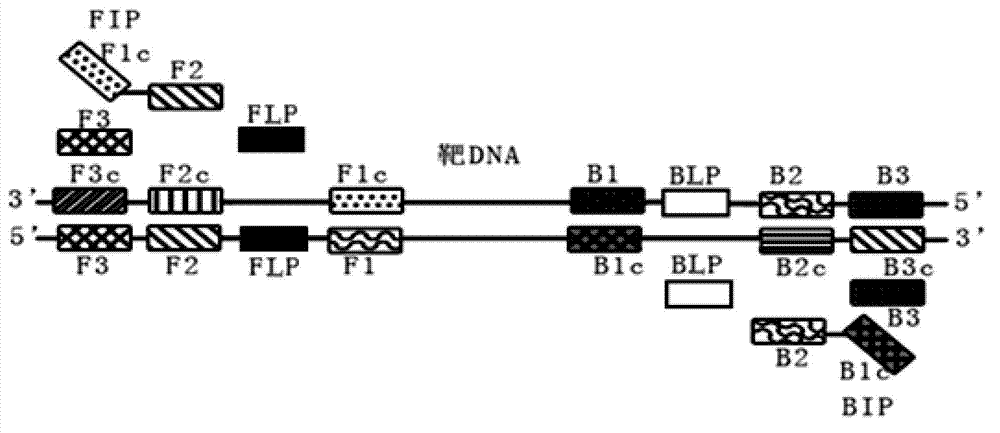
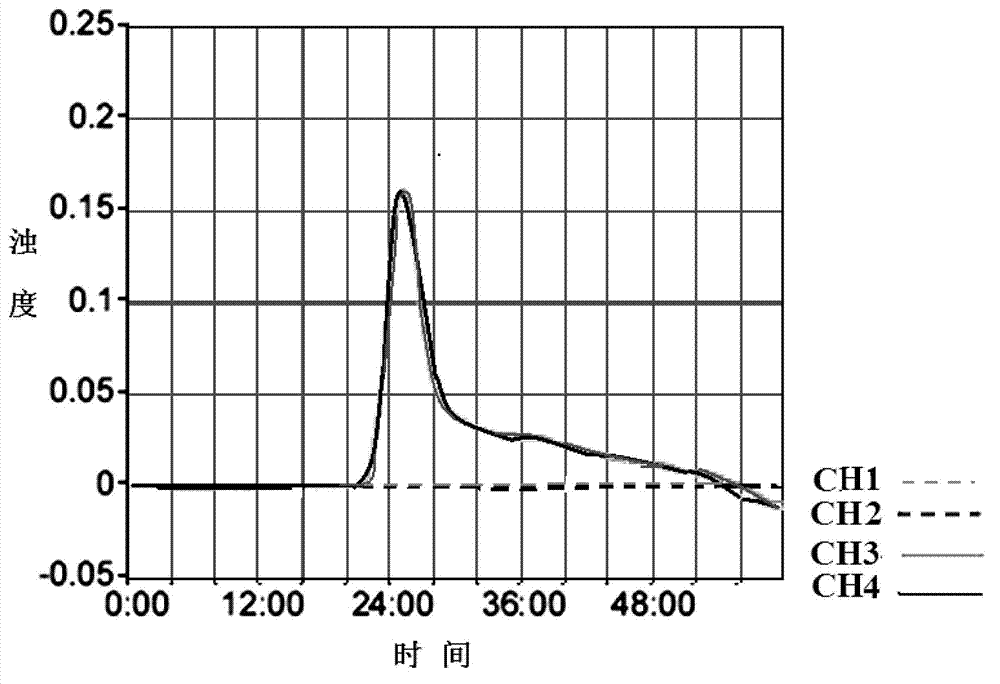
Loop-mediated isothermal amplification (LAMP) primer, kit and application of kit for T25 strain of transgenic maize
InactiveCN102816859AHigh amplification efficiencyKeep orangeMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceLoop-mediated isothermal amplification
The invention discloses a primer which is used in LAMP detection, suitable for specificity detection of the T25 strain of transgenic maize, and provided with a base sequence such as SEQ ID NO: 2-7. The LAMP detection technique is adopted and nephelometry or a development process is combined to establish a standard method suitable for the specificity detection of the T25 strain of the transgenic maize in food. The method is simple in operation; expensive instruments are not dependent under a constant temperature condition; the detection time is about 1 hour, the method sensitivity reaches 0.5%, and the detection efficiency can reach that of existing polymerase chain reaction (PCR) method or fluorescence quantitative PCR method; the result is simple to judge, and the detection cost can reduced greatly; and the method can be applied to practical inspection and quarantine work, particularly to preliminary screening of mass samples and food quality assurance in various basic level laboratories, and can also serve as beneficial supplement of the existing PCR method.
Owner:徐君怡 +3
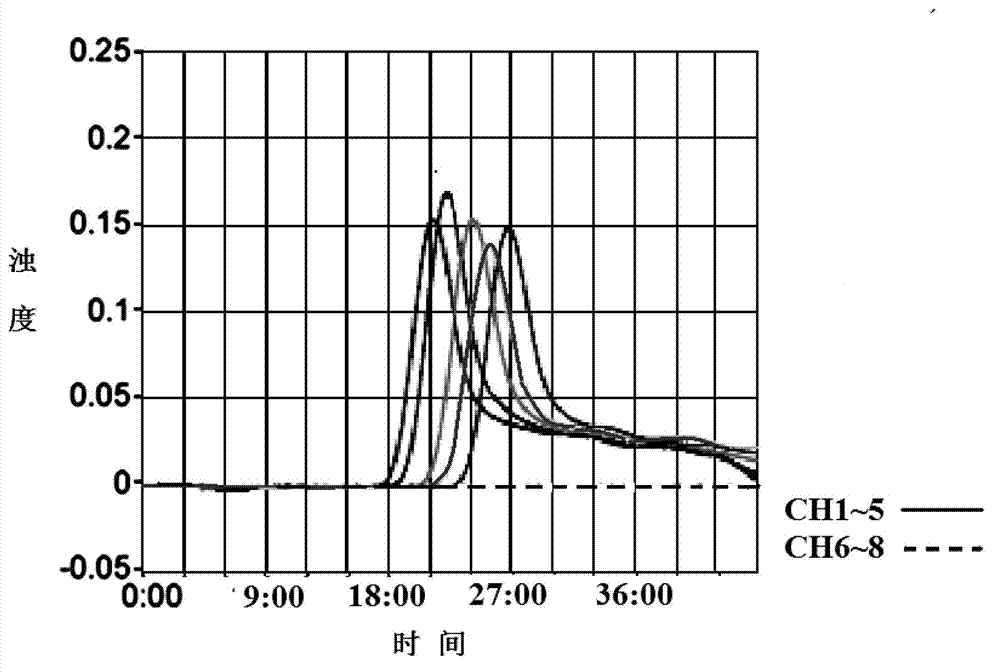
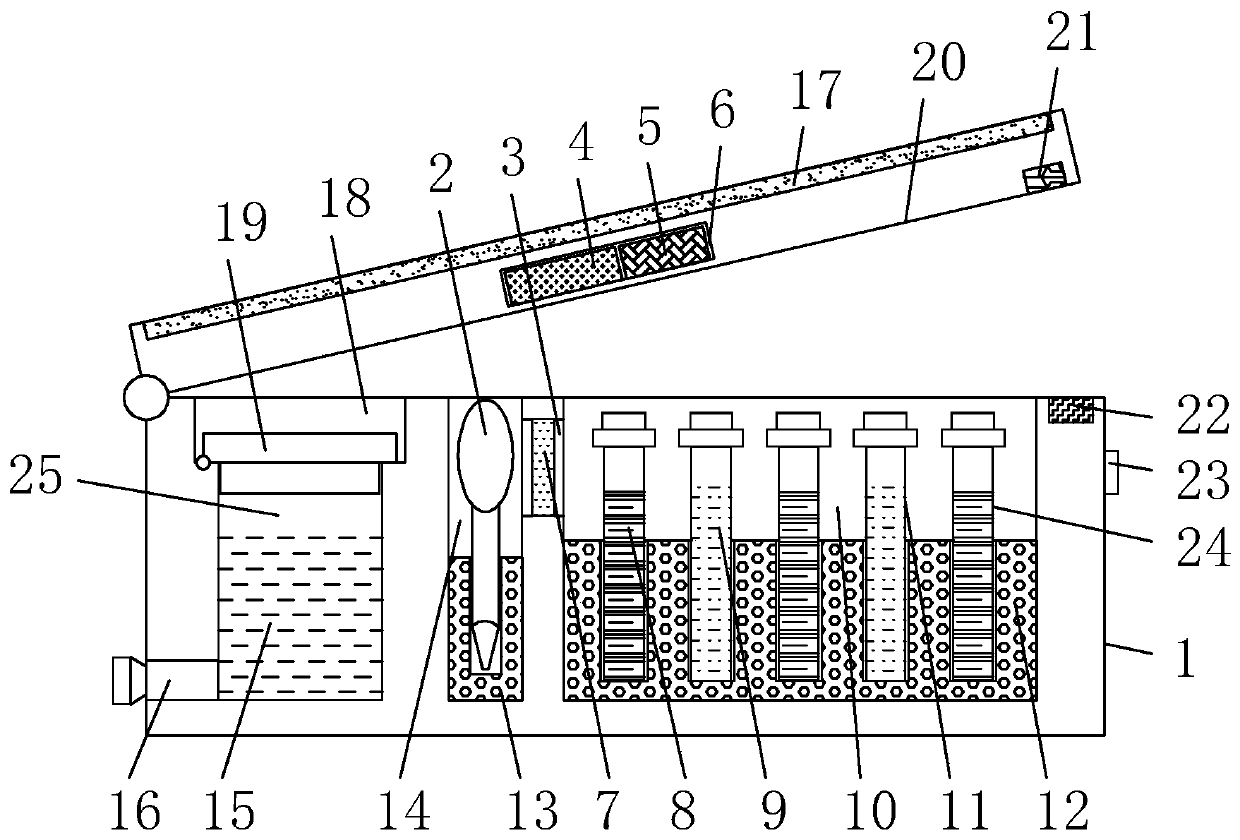
Apolipoprotein E test kit
The invention discloses an apolipoprotein E test kit. The kit comprises a box body, wherein from left to right, the top of the box body is provided with a groove, a dropper placement groove and a placement groove in sequence, the left of the bottom of the inner cavity of the groove is actively connected with a second cover plate through a hinge, a standard solution placement groove is arranged at the lower end of the groove in the inner cavity of the box body, a standard solution is injected in the inner cavity of the standard solution placement groove, the bottom of the inner cavity of the dropper placement groove is fixedly connected with a second placement plate, a dropper is inserted in the inner cavity of the second placement plate, and the bottom of the inner cavity of the placement groove is fixedly connected with a first placement plate. The method adopts nephelometry and comprises steps of fusing ApoE antigen in a sample with a R2 reagent 9 in a solution, so as to form an antigen-antibody compound immediately and cause certain turbidity, wherein the degree of the turbidity is directly proportional to content of ApoE antigen when a certain amount of antibody exists, comparing the solution with a calibration solution treated the same way and calculating content of ApoE in unknown samples.
Owner:浙江伊利康生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com