Troponin I competition turbidimetry detecting kit
A detection kit and troponin technology, applied in the medical field, can solve the problems of specificity that is difficult to meet clinical detection requirements, single antibody action site, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] (1) Peptide synthesis: Tribute dual-channel peptide synthesizer (Protein Technologies) was used for solid-phase peptide synthesis.
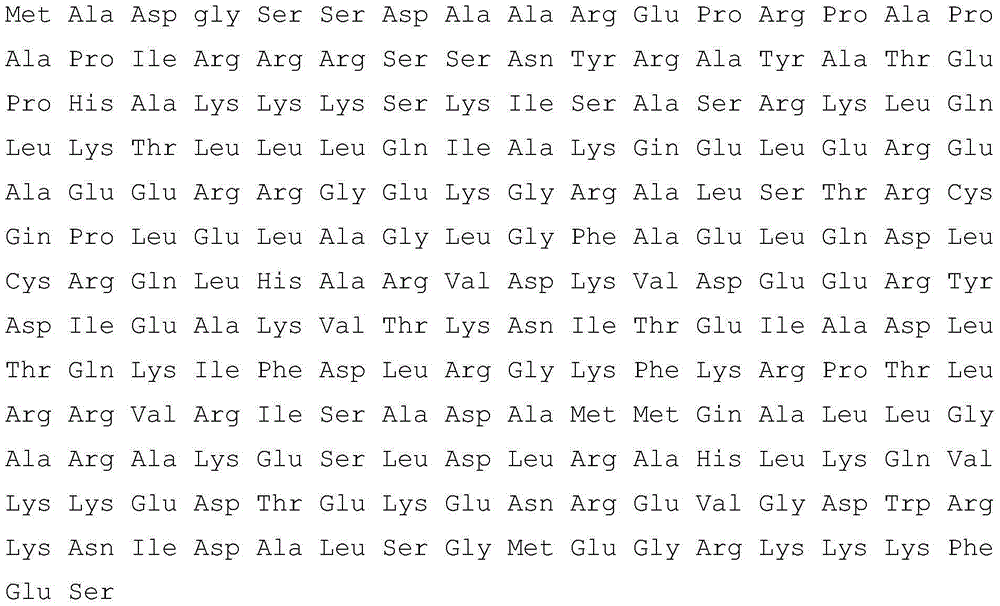
[0095] (2) Peptide screening: The enzyme-linked immunosorbent overlay experiment was used for peptide screening, and amino acid sequence 8; sequence 9; sequence 10 were selected.
[0096] (3) Carrier protein labeling
[0097] 1) Mix the amino-terminal peptide, central region peptide and carboxy-terminal peptide at 2:3:1 (mass ratio), take 10 mg of the mixed peptide and dissolve it in phosphate buffer with pH=6.5, add 100 mg After EDC was reacted at room temperature for 1 hour, 6 mg of bovine serum albumin was added to shake and react overnight.
[0098] 2) Separating and purifying the reacted bovine serum albumin mixture with a Sephadex G-100 chromatographic column, discarding proteins with a molecular weight lower than 100KD, and retaining cTnI-BSA with a molecular weight above 100KD.
[0099] (4) Immunolatex microsphere labeling
[01...
Embodiment 2
[0111] (1) Peptide synthesis: Tribute dual-channel peptide synthesizer (Protein Technologies) was used for solid-phase peptide synthesis.
[0112] (2) Peptide screening: Peptide screening was performed by enzyme-linked immunosorbent overlay experiment, and sequence 8 was selected.
[0113] (3) Carrier protein labeling
[0114] 1) Dissolve 10 mg of the amino-terminal peptide in phosphate buffer at pH = 6.5, add 100 mg of EDC, react at room temperature for 1 hour, add 6 mg of bovine serum albumin and shake and react overnight.
[0115]2) Separating and purifying the reacted bovine serum albumin mixture with a Sephadex G-100 chromatographic column, discarding proteins with a molecular weight lower than 100KD, and retaining cTnI-BSA-1 with a molecular weight above 100KD.
[0116] (4) Immunolatex microsphere labeling
[0117] Carbodiimide was used to activate the carboxyl group of the microspheres to obtain cTnIAb-PS-1 labeled microspheres with cTnI polyclonal antibody at the ami...
Embodiment 3
[0129] (1) Peptide synthesis: Tribute dual-channel peptide synthesizer (Protein Technologies) was used for solid-phase peptide synthesis.
[0130] (2) Peptide screening: Peptide screening was performed by enzyme-linked immunosorbent immunoassay overlay experiment, and sequence 9 was selected.
[0131] (3) Carrier protein labeling
[0132] 1) Dissolve 10 mg of the peptide in the central region in phosphate buffer at pH = 6.5, add 100 mg of EDC, react at room temperature for 1 hour, then add 6 mg of bovine serum albumin to shake and react overnight.
[0133] 2) Separating and purifying the reacted bovine serum albumin mixture with a Sephadex G-100 chromatographic column, discarding proteins with a molecular weight lower than 100KD, and retaining cTnI-BSA-2 with a molecular weight above 100KD.
[0134] (4) Immunolatex microsphere labeling
[0135] Carbodiimide was used to activate the carboxyl group of the microspheres to obtain cTnIAb-PS-2 labeled microspheres with polyclonal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com