Preparation method and application of chicken white diarrhea salmonella inactivated vaccine
A Salmonella and inactivated vaccine technology, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, antibacterial drugs, etc., can solve problems such as weak cross-protection, slow immunity, and weak cellular immune protection, and achieve The product is safe and reliable, with good protection ability and high output effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Separation, purification and staining microscopy
[0022] Salmonella pullorum SP7220 strain was isolated from the liver of Bailaihang chickens that died in a chicken farm in Qingdao City, Shandong Province in November 1972. The specific isolation method is as follows: aseptically collect the liver of the dead chick, place it in nutrient broth for overnight cultivation at 37° C., then inoculate it on Martin agar medium, and cultivate it at 37° C. for 24 hours. Pick a colony with a diameter of 1.0mm-2.0mm, off-white transparent, and smooth surface, inoculate it on Martin agar medium, and incubate at 37°C for 24 hours. The purely cultured bacteria were subjected to Gram staining microscope inspection, and then observed with an optical microscope, showing elongated bacilli with slightly rounded ends.
[0023] Salmonella pullorum sp8441 strain was isolated from the oviduct of 6-month-old white legion laying hens in a chicken farm in Beijing in December 1984. The specifi...
Embodiment 2
[0041] Salmonella pullorum strains SP7220, SP8441 and SP9905 were resuscitated and multiplied respectively, and typical colonies were selected for expansion and culture to make seed bacterial liquid. Inoculate the seed bacterial liquid at 3.0% of the total volume of the culture medium, collect the bacterial liquid after deep aeration culture at 37°C for 12 hours in a fermenter, remove the supernatant by centrifuging at 5000r / min for 15 minutes, and resuspend the bacteria with normal saline containing 0.3% formaldehyde Mud, inactivated at 8°C for 12h. Fully mix the inactivated bacteria solution with an equal volume of adjuvant containing 0.5mg / ml chitosan oligosaccharide, 0.75mg / ml Acanthopanax polysaccharide and 20mg / ml propolis, and aseptically subpackage to make the inactivated Salmonella pullorum vaccine .
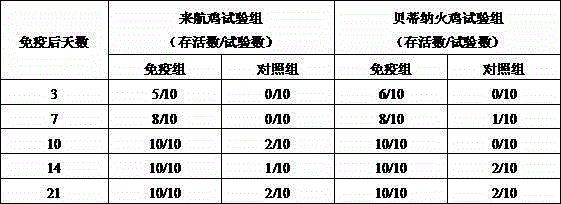
[0042] The vaccine was injected intramuscularly into 3-day-old healthy Laihang chickens and 3-day-old healthy Betina turkeys (0.2ml per bird, containing 2 billion bact...
Embodiment 3
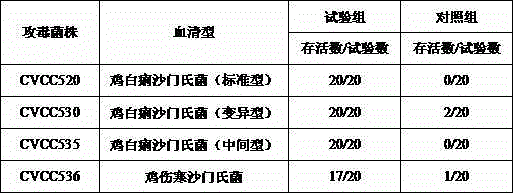
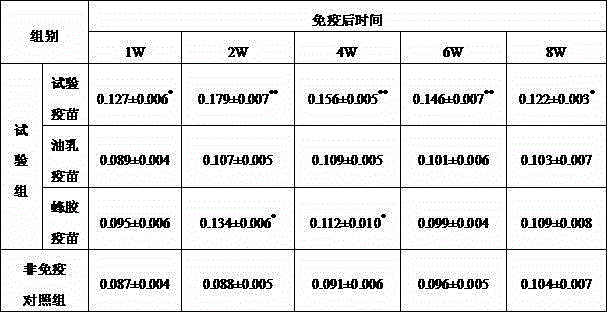
[0047] According to the preparation method described in Example 2, the Salmonella pullorum inactivated vaccine was made, and the inactivated vaccine semi-finished product was used at the same time, according to "Practical Veterinary Biological Products Technology" (Zhang Zhenxing, etc., China Agricultural Science and Technology Press, 1996, 53-54) The manufacture method of the oil-emulsion adjuvant inactivated vaccine and the propolis adjuvant inactivated vaccine is respectively made into the oil-emulsion adjuvant inactivated vaccine and the propolis adjuvant inactivated vaccine (the antigen concentration is 10 billion bacteria per milliliter). Eighty 7-day-old healthy Bailaihang chicks were selected and divided into 4 groups. The first group of test chickens was intramuscularly injected with Salmonella pullorum inactivated vaccine (0.2ml per feather) prepared by the preparation method described in Example 1, and the second group was injected intramuscularly with oil emulsion a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com