Method for measuring glycosylated hemoglobin content
A glycosylated hemoglobin and percentage technology, which is applied in the field of medical immunity, can solve the problems of short stable period of antibody working solution, unfavorable daily use, and difficulty in obtaining low-temperature refrigerators, and achieves the effect of simplifying operation steps and prolonging effective use time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The glycated hemoglobin detection reagent of this embodiment includes hemolysis, latex, antibody A reagent and antibody B reagent, wherein: hemolysis is H 2 O; latex concentration is 0.1%, suspended in the glycine buffer solution with a concentration of 15mmol / l; Antibody A is a goat anti-mouse IgG antibody with a concentration of 0.006mg / ml, mixed in a glycine buffer solution with a concentration of 60mmol / l; Antibody B is a mouse anti-human HbA1c monoclonal antibody with a concentration of 0.05 mg / ml, which is mixed in a glycine buffer with a concentration of 60 mmol / l; the amount of whole blood sample is 5 μl, the amount of hemolysis is 500 μl, and the amount of latex is 210 μl , the volumes of Antibody A and Antibody B were 50 μl and 70 μl, respectively. Wherein, the percentages of hemolysis, latex, goat anti-mouse IgG antibody and mouse anti-human HbA1c monoclonal antibody are 1.49%: 62.69%: 14.92%: 20.90%.
[0025] In this embodiment, if the concentration of the ...
Embodiment 2
[0027] In this embodiment, the method for directly measuring the percentage of glycosylated hemoglobin using the timing nephelometric method as the detection principle includes:
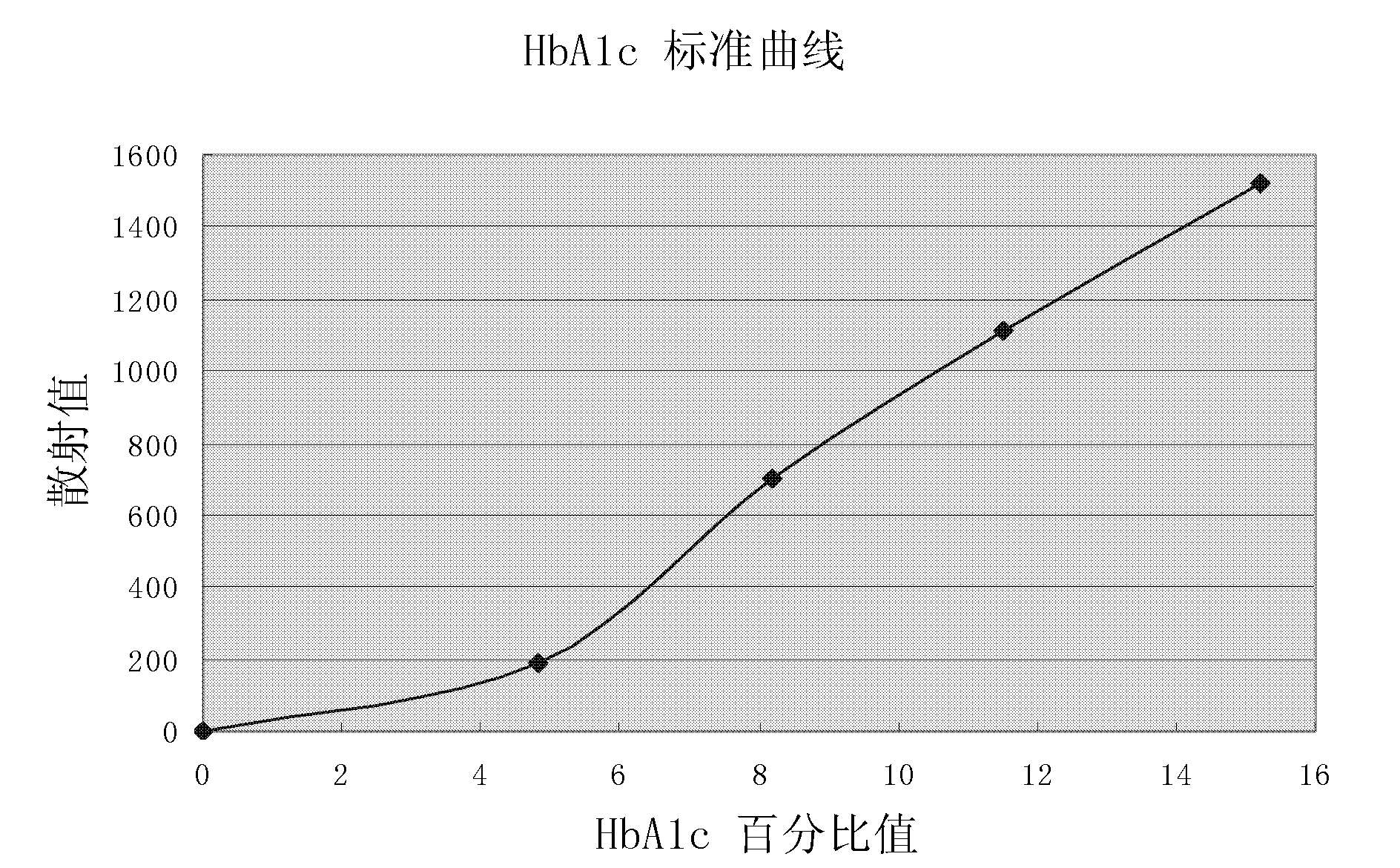
[0028] a. The making of the standard curve for the determination of glycated hemoglobin, that is, the corresponding database between the scattering value and the percentage of glycated hemoglobin-scattering value of multiple glycated hemoglobin standard products: the detection principle is based on the timing scattering turbidimetry, and the required materials include those described in the previous examples. glycosylated hemoglobin detection reagent, including 500 μl of hemolysis, 210 μl of latex, 50 μl of antibody A (goat anti-mouse IgG antibody with a concentration of 0.006 mg / ml), and 50 μl of antibody B (mouse anti-human HbA1c monoclonal antibody with a concentration of 0.05 mg / ml) Cloned antibody) 70μl; glycosylated hemoglobin standard and specific protein analyzer.
[0029] Sample processing: ...
Embodiment 3
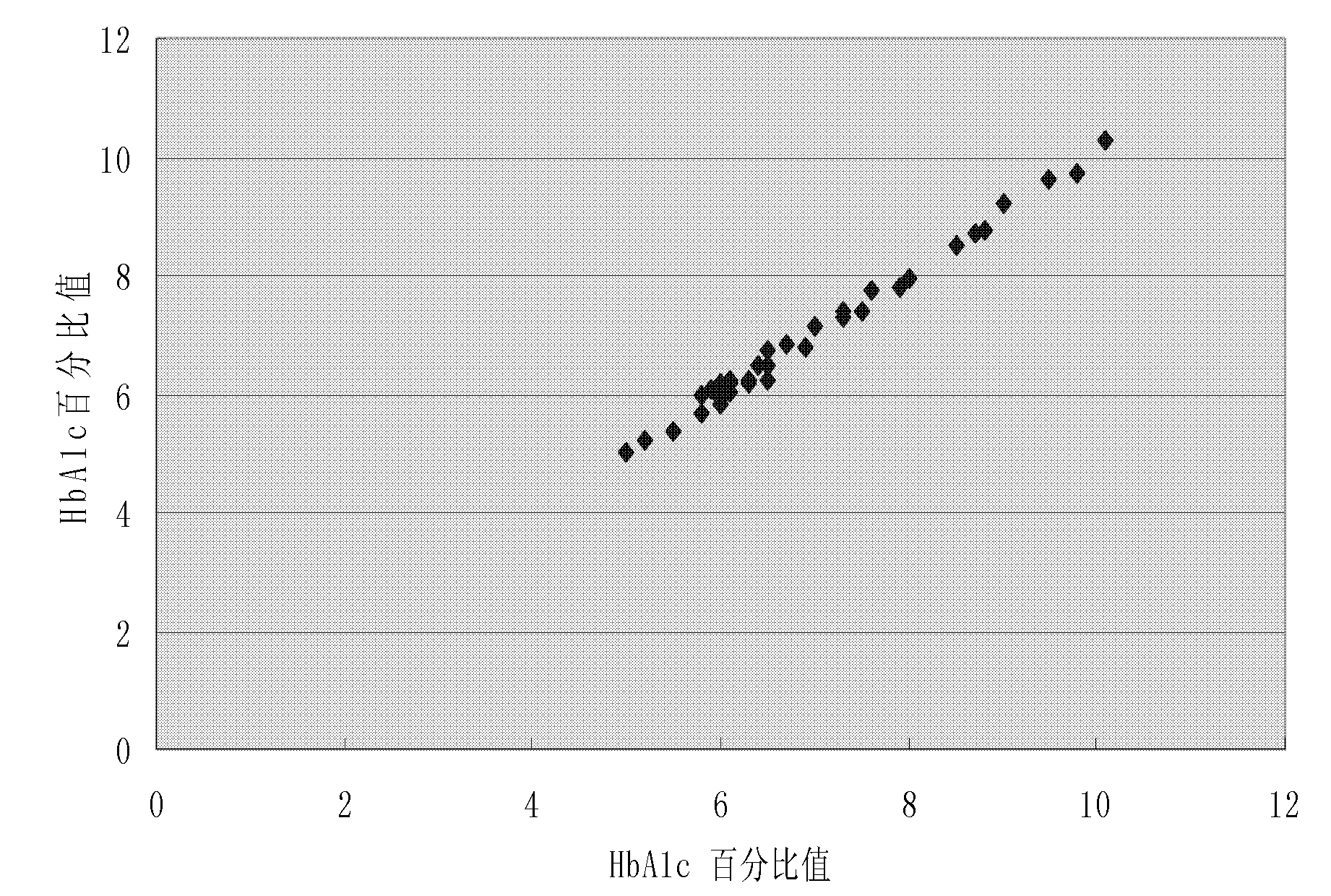
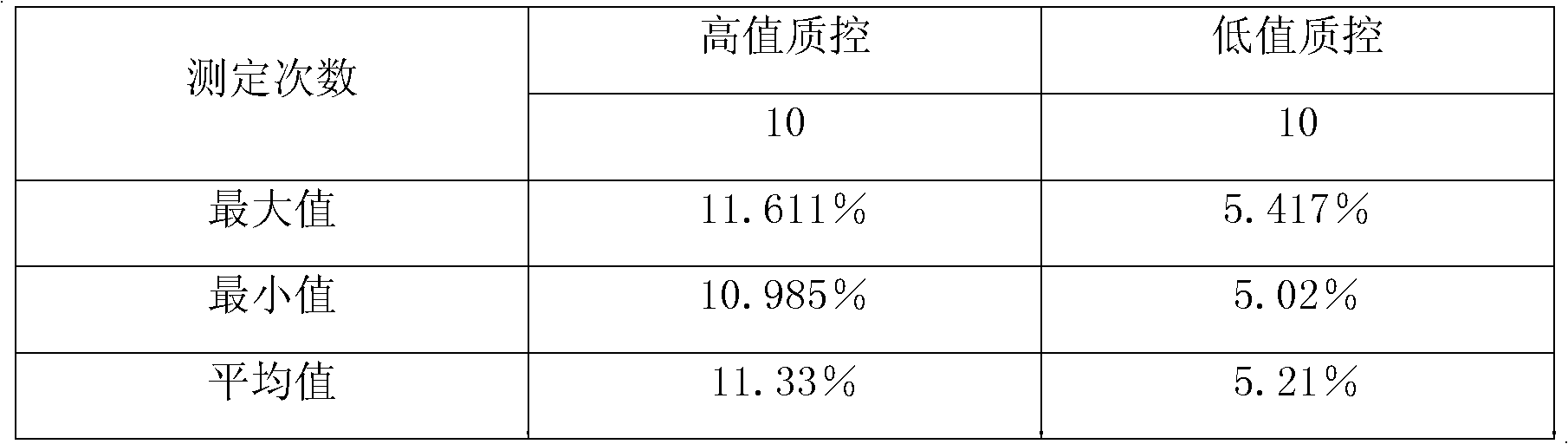
[0037] Example of sensitivity verification of rapid detection reagents for glycated hemoglobin: Prepare rapid detection reagents for glycated hemoglobin, such as the reagents described in the previous examples, high-value and low-value quality controls, and a specific protein analyzer. Take a traceable high-value quality control substance and a low-value quality control substance, perform 10 tests on each quality control substance, and calculate the average value, standard deviation and variation coefficient of the test results. The results are shown in Table 1:
[0038] Table 1
[0039]
[0040]
[0041] From the coefficient of variation in Table 1, it can be seen that the C-reactive protein detection method provided by the present invention has relatively high precision.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com