Method for detecting bacterial endotoxin of xylitol injection
A technology of bacterial endotoxin and xylitol, which is applied in the direction of material analysis by observing the impact on chemical indicators, and analysis by making materials undergo chemical reactions, etc., can solve the problem that the test results take a long time, are easily contaminated, Problems such as adverse effects of test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 1 Materials and Instruments
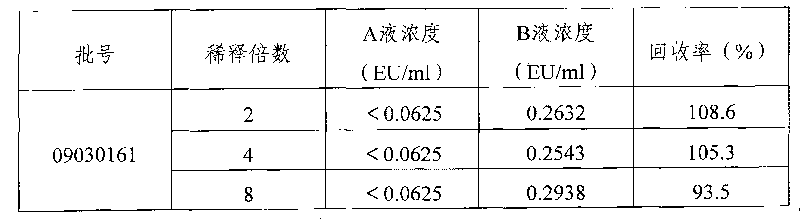
[0014] Bacterial endotoxin national standard product (batch number 200707, specification: 140EU / cart. National Institute for the Control of Pharmaceutical and Biological Products); dynamic turbidity Limulus reagent (batch number: 0807302, produced by Zhanjiang Andus Biological Co., Ltd., specification: λ=0.5EU / bottle; batch number: 08120512, produced by Fuzhou Xinbei Biochemical Industry Co., Ltd.; specification: λ=0.5EU / box); bacterial endotoxin test water (lot number: 0807100, specification: 5mL / box, Zhanjiang Andus Biological Co., Ltd.); 木糖醇注射液(规格:250ml:12.5g,批号:09030161、09030162、09030163,09030191,09030192,09030193;规格:500ml:25g,批号:09030151,09030152,09030153,09030194,09030195,09030196.安徽丰原 Pharmaceutical production); BET-32B Bacterial Endotoxin Tester (Tianjin University Radio Factory).
[0015] 2 Methods and results
[0016] 2.1 Determination of bacterial endotoxin limit according to the formula L=K / M, K is the prescribed route of admi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com