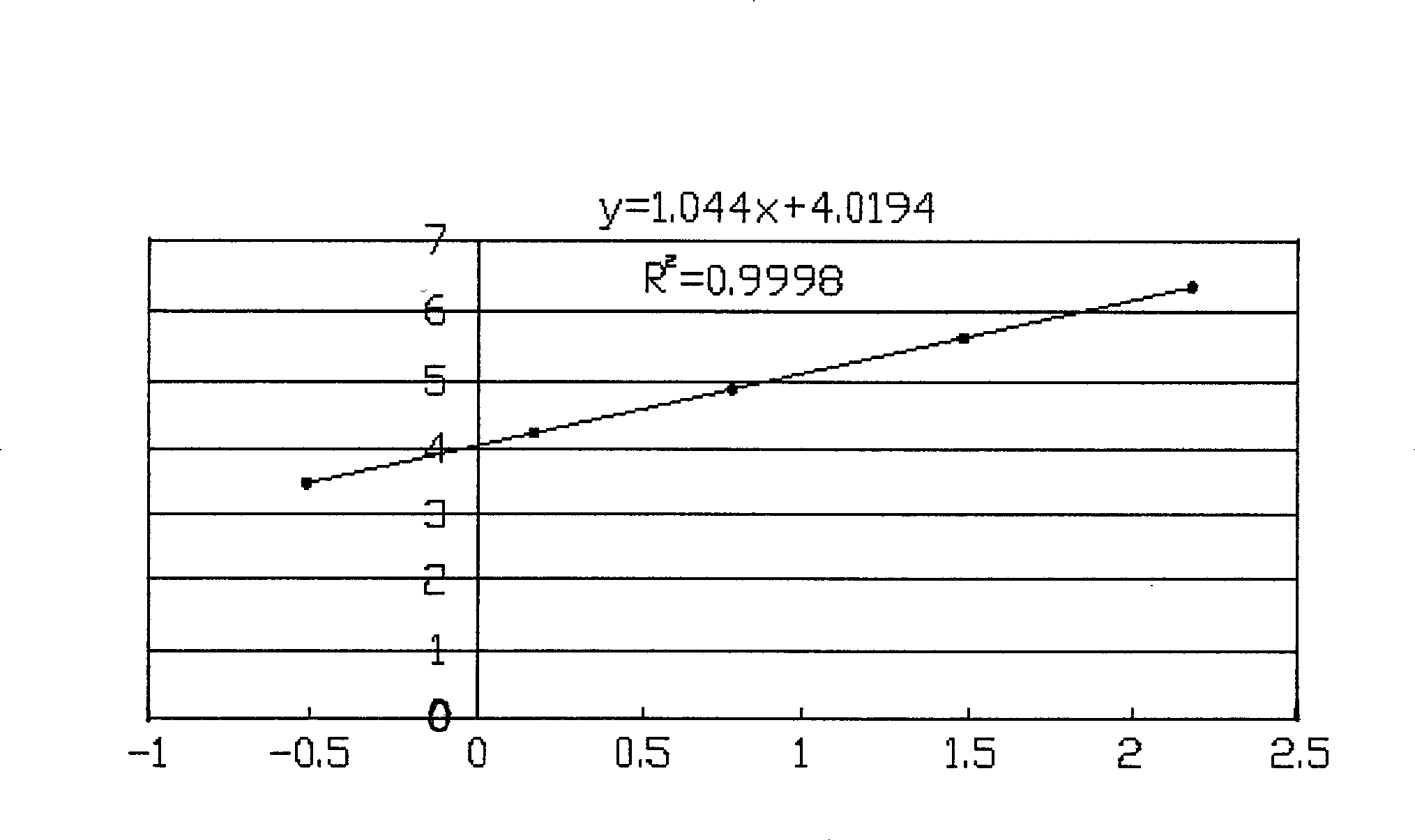
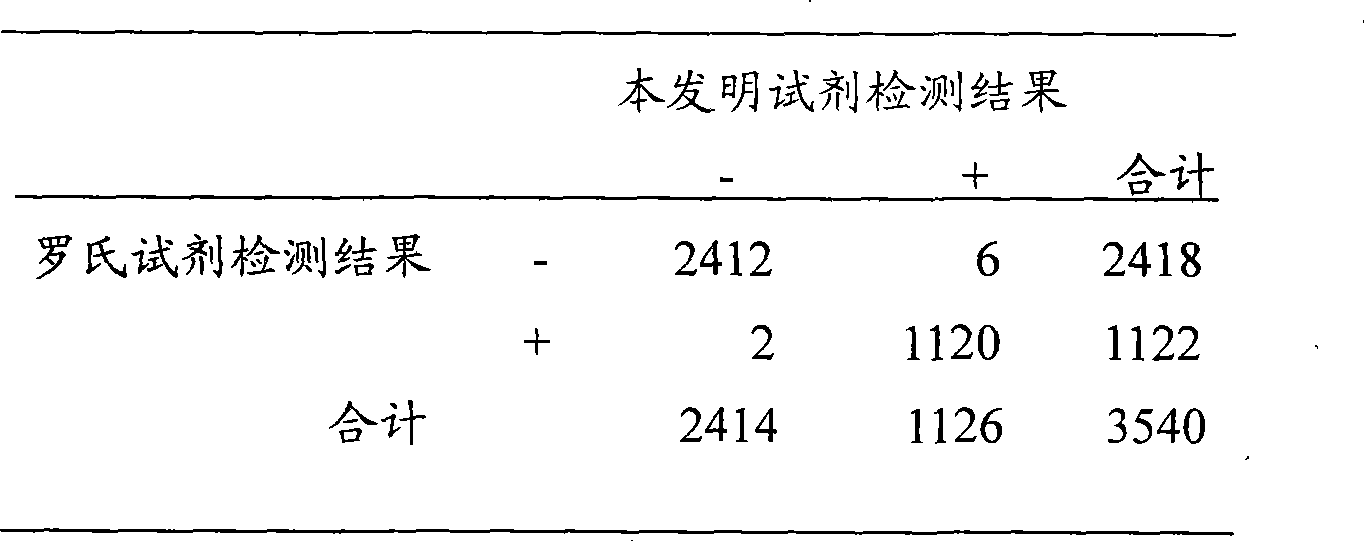
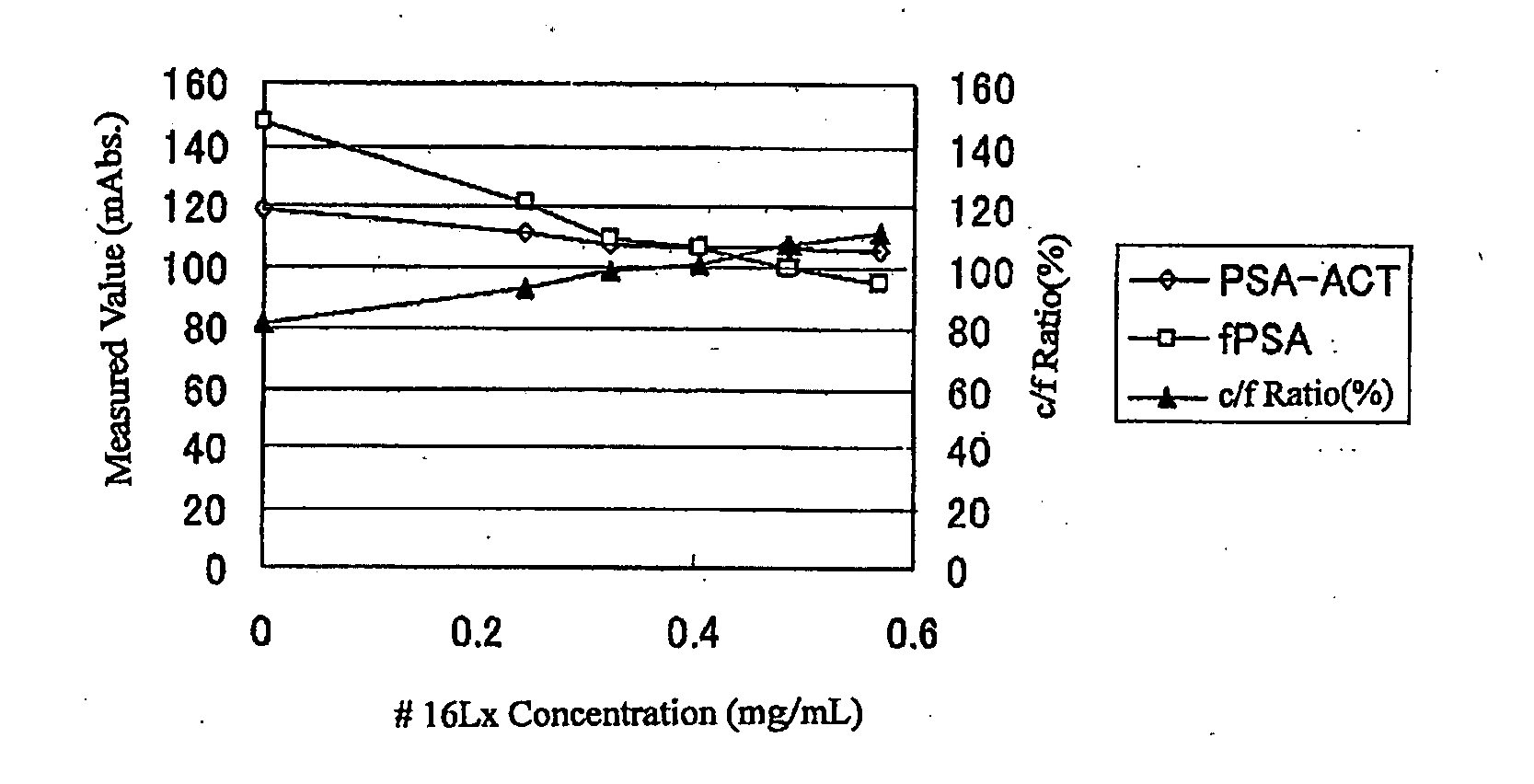
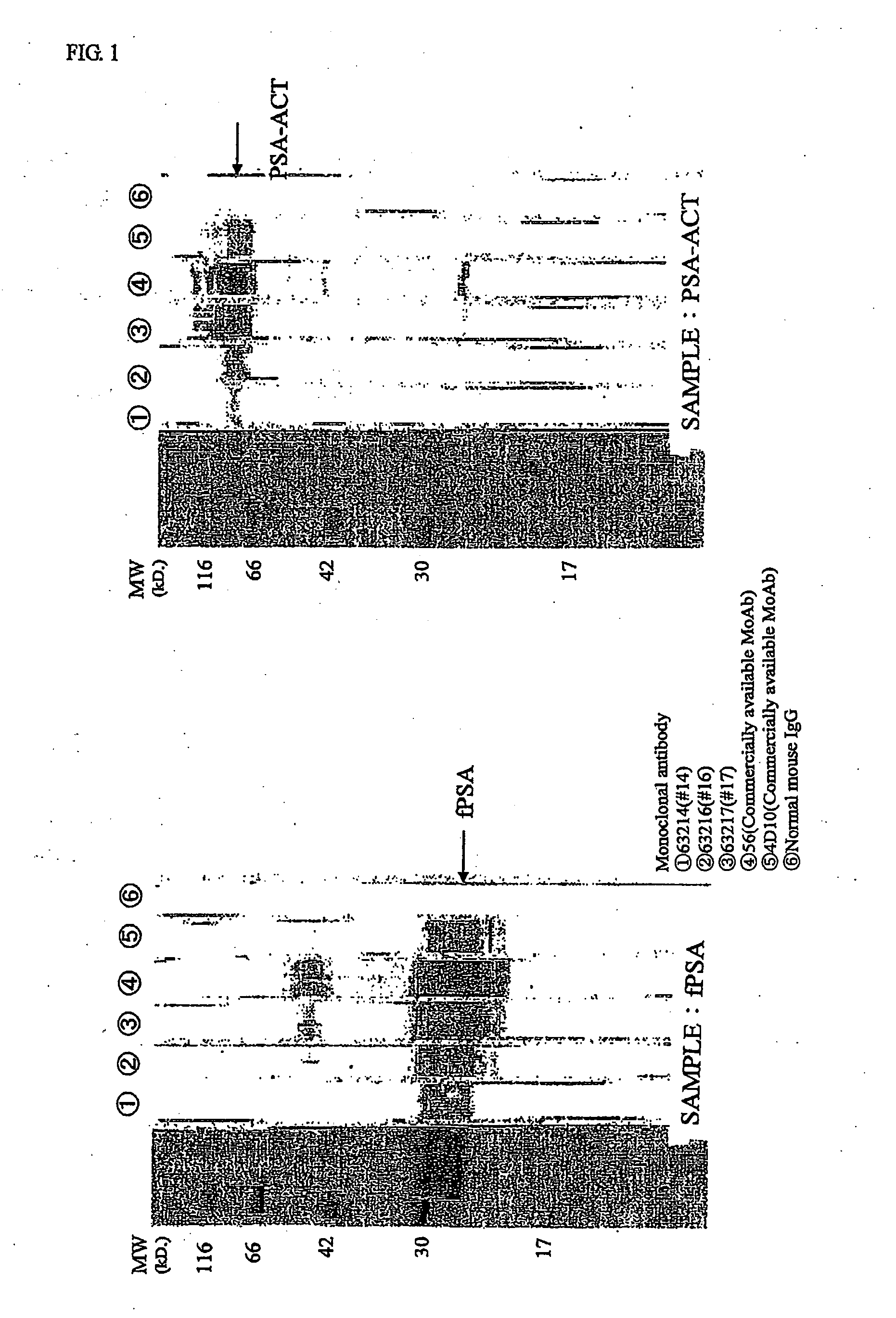
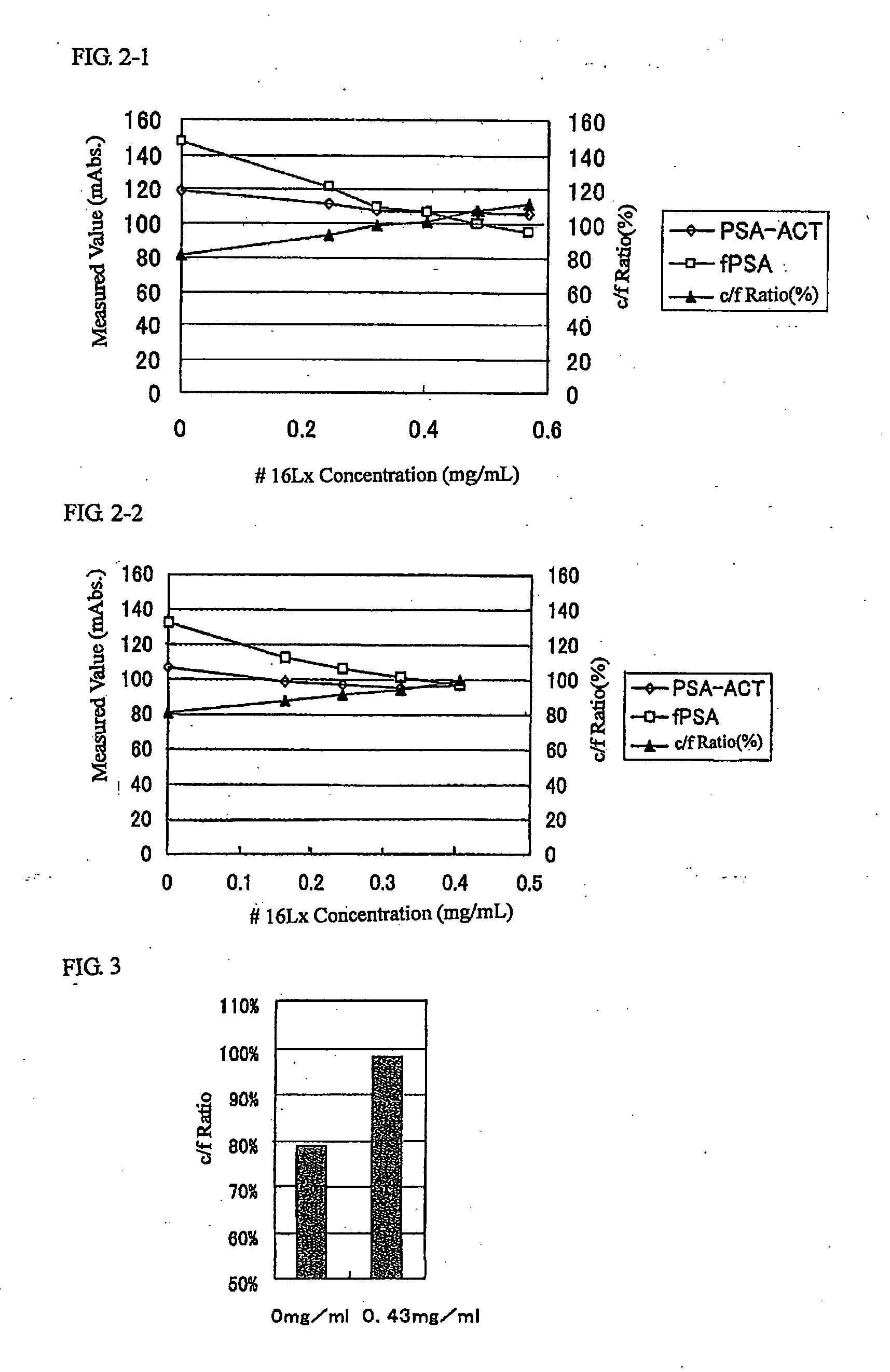
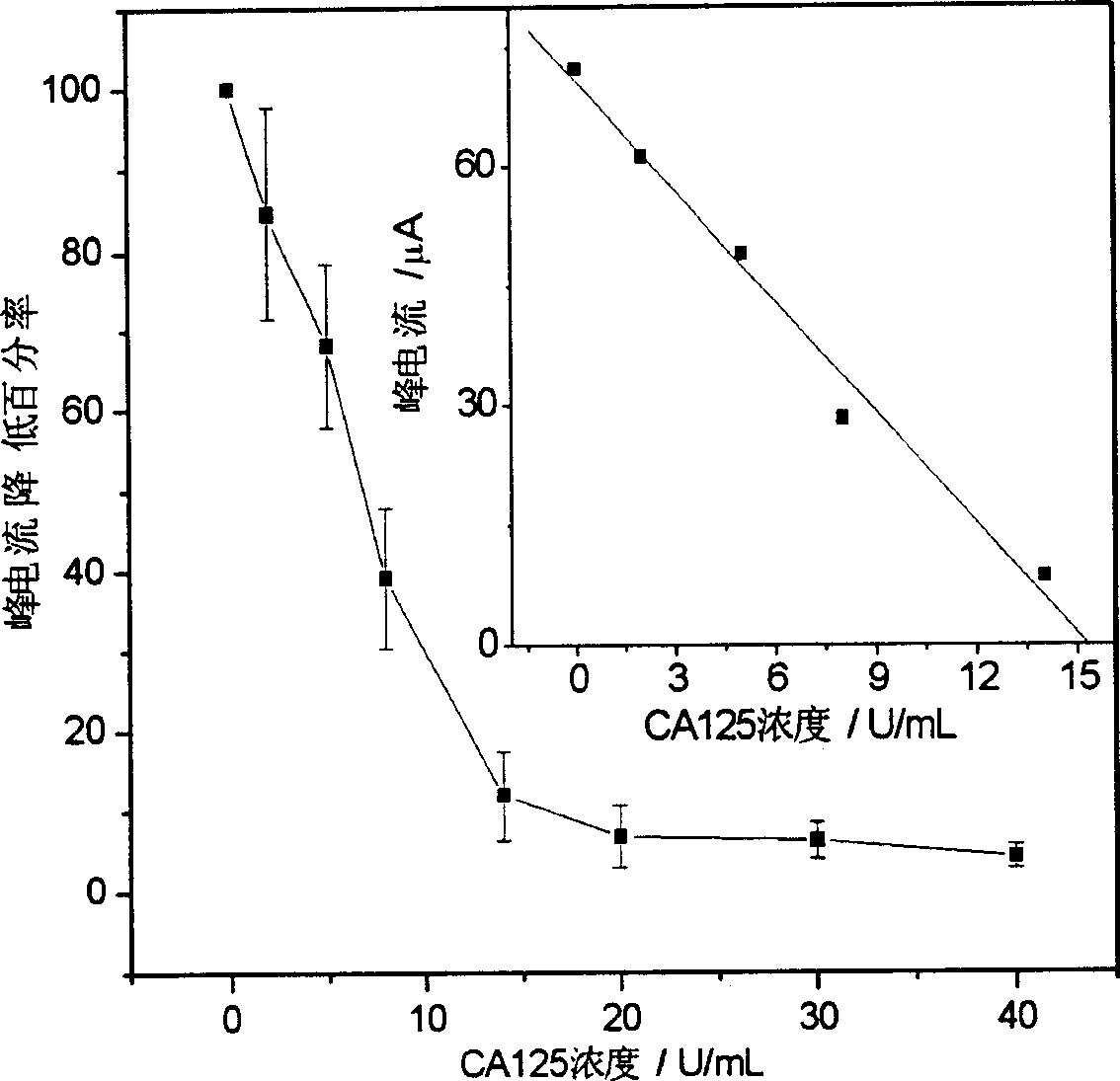
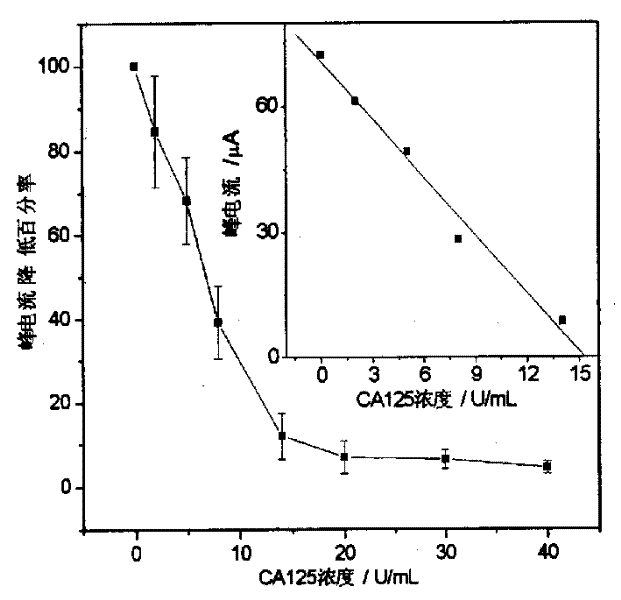
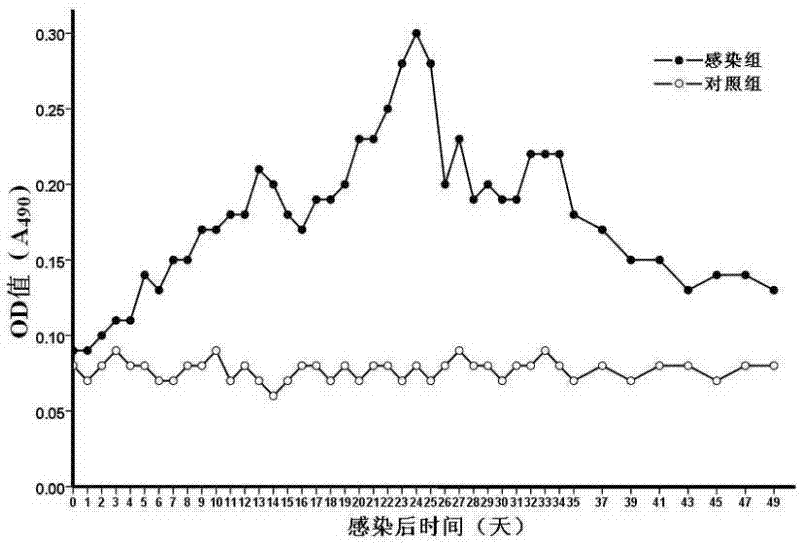
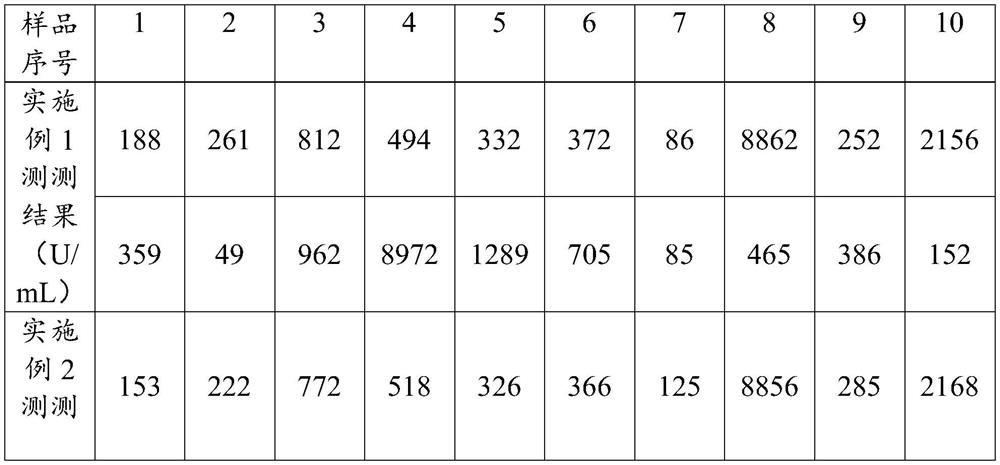
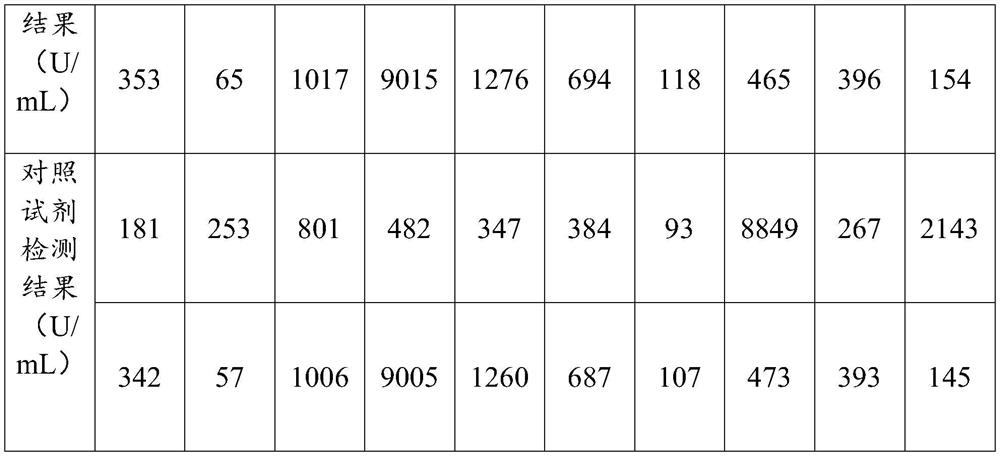
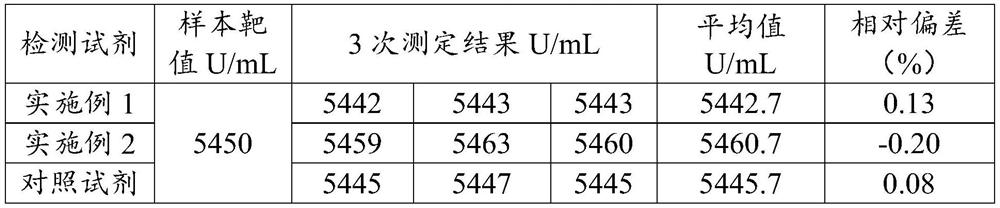
Patents
Literature
38 results about "Antigen assays" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
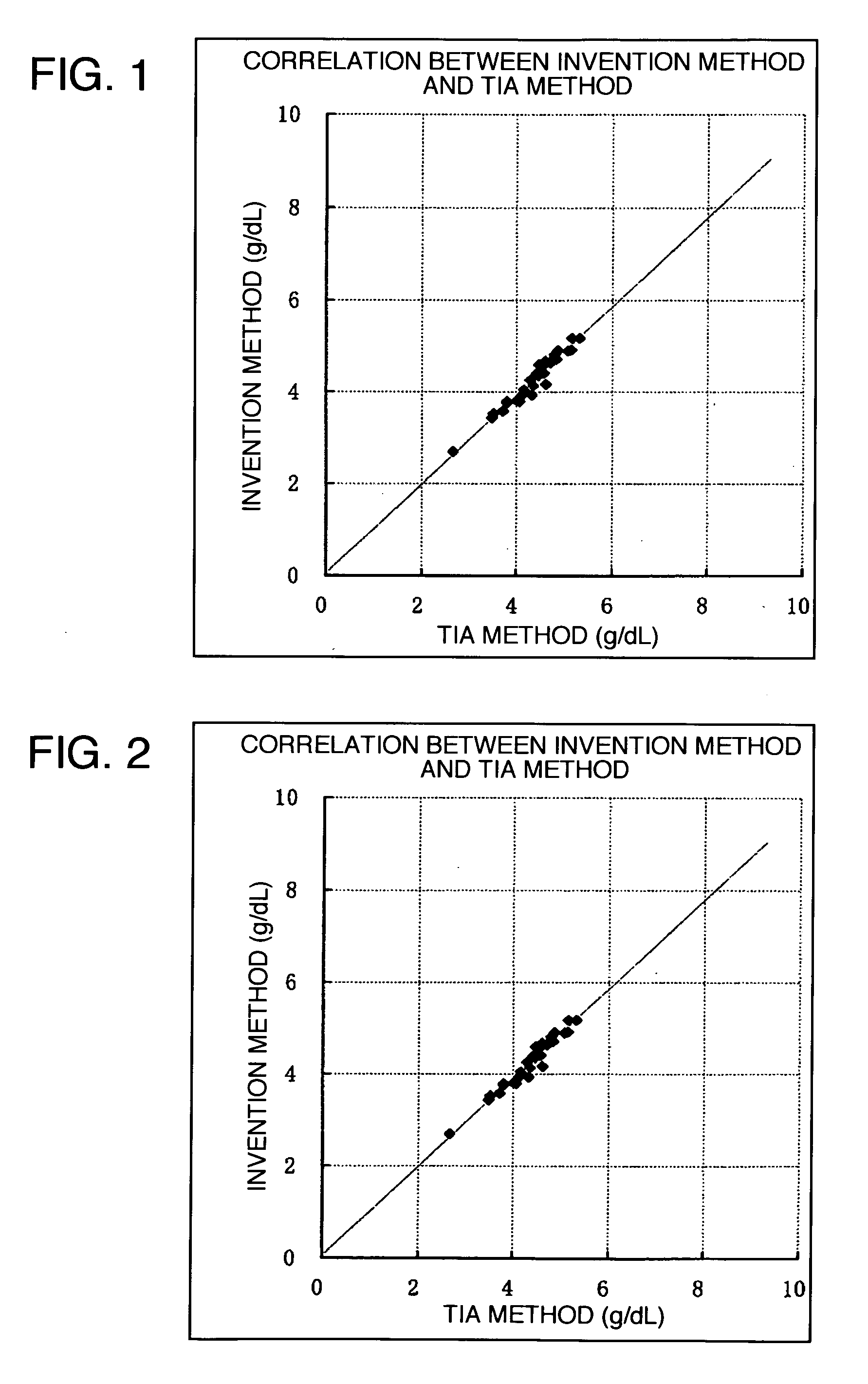
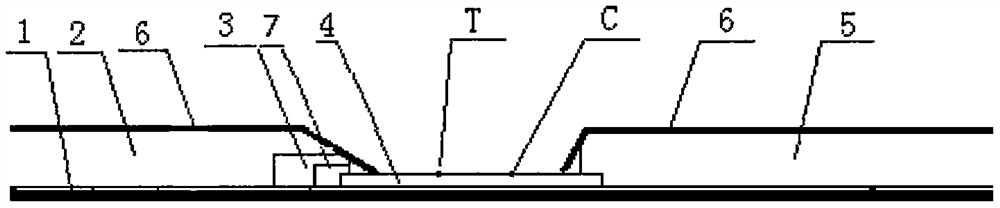
ELISA is a popular format of "wet-lab" type analytic biochemistry assay that uses a solid-phase enzyme immunoassay (EIA) to detect the presence of a substance, usually an antigen, in a liquid sample or wet sample.
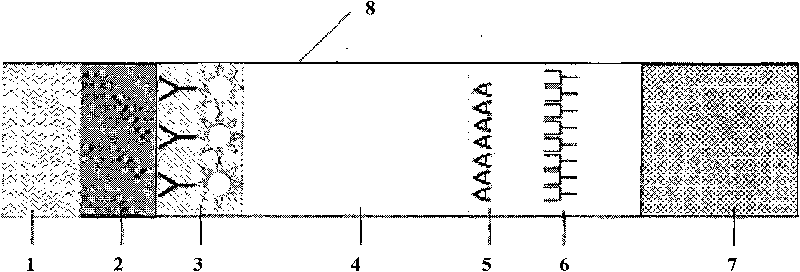
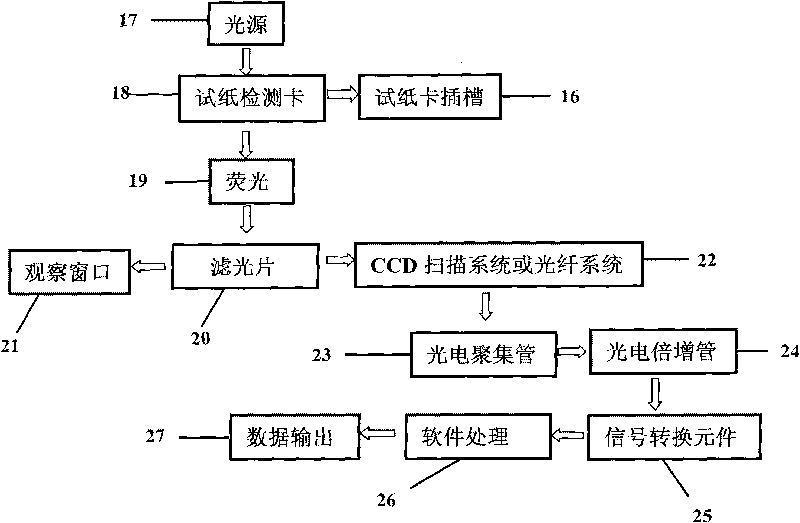
Fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and method for preparing same
InactiveCN101726596ASimple and fast operationHigh sensitivityBiological testingLuminescent compositionsCelluloseHepatitis B virus
The invention discloses a fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and a method for preparing the same. The testing card comprises a hepatitis b surface antigen test paper strip, a hepatitis b e surface antigen test paper strip, a hepatitis b surface antibody test paper strip, a hepatitis b e surface antibody test paper strip, and a hepatitis b core antibody test paper strip. Each test paper strip is formed by overlapping and bonding filter paper, a sample pad, a glass fiber film spray-coated with fluorescent microspheres, a cellulose nitrate film and water absorption paper on a bottom plate by glue in sequence, wherein the cellulose nitrate film is coated with antigens serving as a testing area and anti-rabbit antibodies serving as a quality control area; and during a test, after emitted fluorescent light passes a filter, the emitted spectrum is collected, accumulated and multiplied by the CCD scanning technology and then converted into a numerical signal, the numerical signal is multiplied by a correction factor, and the strength of the corrected fluorescent light is substituted in a standard curve of a fluorescence analyzer, so that the concentrations of the five indexes of hepatitis b of the sample can be automatically worked out. The test of hepatitis b viruses by the testing card has the characteristics of specificity, sensitivity, simpleness and accuracy.
Owner:WUXI ZODOLABS BIOTECH
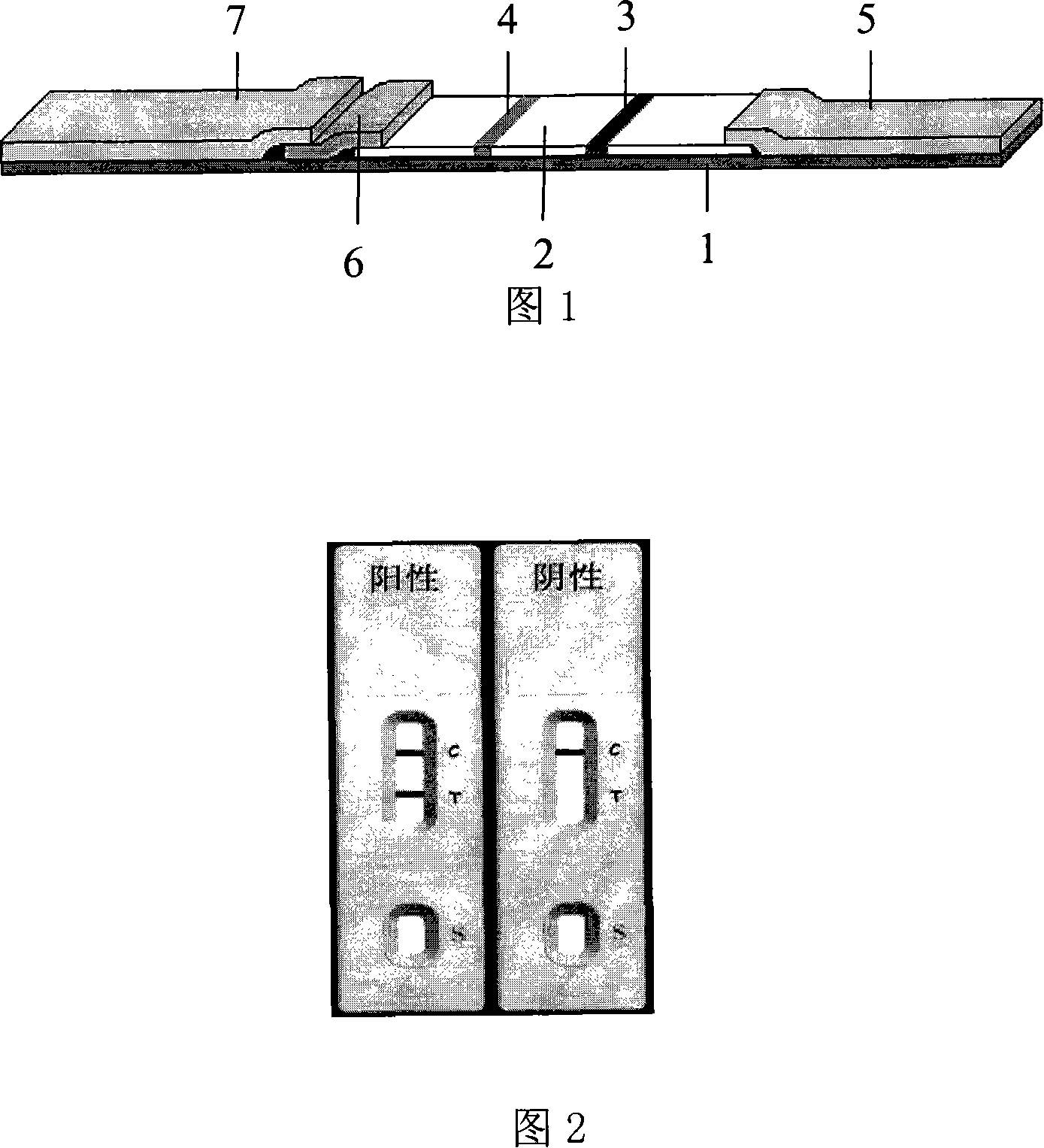
Mycobacterium tuberculosis antibody rapid diagnosis reagent kit and detecting method thereof
The present invention relates to a method to prepare a reagent kit to quickly diagnose mycobacterium tuberculosis antigens jointly packaged by 38KD and 16KD antigens and a test method. The blood antigen test paper of the present invention jointly packages 38 KD and 16KD of TB antigens into a lower part of a NC film to form a test line (T). Goat anti-mouse IgG is packaged into an upper part of the NC film to form a quality control line (C). In addition, anti-human IgG (mouse anti-human IgG, goat anti-human IgG or SPA) marks nanometer coloring particles (colloidal gold particles or sol particles or nanometer beads) to prepare a labeling pad. The NC film, the labeling pad, a sampling pad and a sample adsorption pad are glued and assembled onto a plastic board to form the test paper. During test, few tested sample is added onto the NC film of the test paper. And then, sample diluents are added through a sampling end. After sampling, it is necessary to observe result of immunoreaction, thus realizing quick auxiliary TB antigen diagnosis.
Owner:天津中新科炬生物制药股份有限公司
Hepatitis b virus surface antigen chemiluminescence immune assay determination kit and method for preparing same
InactiveCN101363861AGuaranteed SensitivityEasy to produce and operateChemiluminescene/bioluminescencePolyclonal antibodiesBiotin-binding proteins
The invention particularly relates to a kit for determining the surface antigen of the hepatitis B virus, and a preparation method thereof, which belongs to the medical field of immunological analysis. The kit comprises (1) a hepatits B virus surface antigen calibration material; (2) an avidin-coated carrier; (3) the polyclonal antibody or monoclonal antibody of a biotinylated hepatitis B virus surface antibody; (4) the polyclonal antibody or monoclonal antibody enzyme-labeled of the surface antibody; and (5) a chemiluminescent primer. Furthermore, the preparation method comprises the following steps of (1) preparing a calibration material from the pure surface antigen; (2) coating the carrier with avidin; (3) performing biotinylation of the polyclonal antibody or monoclonal antibody of the surface antibody; labeling the polyclonal antibody or monoclonal antibody of the surface body with an enzyme; (4) sub-packaging the calibration material and the chemiluminescent primer; and (5) assembling. The kit has the advantages of simpliness, rapidness, sensitiveness, stability and the like.
Owner:北京科美东雅生物技术有限公司
Reagent for Assaying Antigen and Method of Assaying Antigen
It is intended to provide a reagent and an assay method whereby an antigen can be conveniently and accurately assayed without resorting to any special antibody. Using two or more carriers having different particle sizes, a carrier having a smaller particle size is sensitized with at least one monoclonal antibody, which is selected from among three monoclonal antibodies being reactive with both of a free antigen and a complex antigen and having different recognition sites from each other, while a carrier having a larger particle size is sensitized with the remainder monoclonal antibodies, thereby providing an assay reagent and an assay method whereby the reactivities of a free antigen and a complex antigen can be controlled.
Owner:SEKISUI MEDICAL CO LTD
Colloidal gold labeling lap gene monoclonal antibody measuring Listeria monocytogenes kit
The invention is a colloidal gold labeling lap gene monoclonal antibody measuring Listeria monocytogenes kit, belongs to the technical field of the hygiene analysis and medical test, and is characterized by comprising the following steps: a. primer designing; b. reaction conditions for PCR amplification; c. reaction conditions of agarose gel electrophoresis (AGE); e. applying heat shock conversion method; f. preparing plasmid by SDS cracking process clean up kit; g. induction expression of protein; h. SDS-PAGE electrophoresis; j. cutting the required strips from the SDS-PAGE electrophoresis gel; and k. preparing colloidal gold by using a trisodium citrate reduction method with little improvement. The invention has the beneficial effects of improving the limitation that only single legionella single antigen can be measured. The invention also features pure antibody and high valence and can improve accuracy and sensitivity. Compared with ELISA method and PCR method, the measurement for Listeria monocytogenes in the invention is rapid and convenient by colloid gold-immunochromatography assay.
Owner:何成彦 +3
Carbohydrate antigen CA19-9 assay kit and detection method using the same
InactiveCN107389946ALong validity periodHigh Luminescence Quantum YieldBiological testingCarbohydrate antigenMagnetic bead
The invention discloses a carbohydrate antigen CA19-9 assay kit. The kit comprises a magnetic separation reagent R1, a labeling reagent R2, a CA19-9 calibration product and a CA19-9 quality control product. The invention also provides a preparation method of the kit. The magnetic separation reagent R1 in the kit is prepared through coupling a CA19-9 monoclonal antibody to the surface of a carboxyl magnetic bead. The labeling reagent R2 is prepared through coupling a luminescent substance acridinium ester to a paired CA19-9 monoclonal antibody. The reactions occur under nearly homogeneous conditions. Through use of an excitation solution, the reaction product antibody-antigen-antibody sandwich complex can immediately release photons at 430 nm and can release strong photons without use of a luminescent enhancer and a catalyst so that luminescence detection can be directly carried out. Through optimizing the experimental processes of magnetic bead coating with an antibody and antibody labeling with acridinium ester, the stability and the detection sensitivity of the kit are greatly improved. The kit has a wide linear detection range, is easy to operate and has a fast detection rate.
Owner:北京健安生物科技有限公司
Hepatitis B virus pre S1 antigen chemiluminscence immunoassay kit and preparation method thereof
InactiveCN101539576AEasy to detectQuick checkChemiluminescene/bioluminescenceAntigenMonoclonal antibody
The invention provides a hepatitis B virus pre S1 antigen chemiluminscence immunoassay kit and a preparation method thereof, belonging to the medical field of immune analysis. The kit comprises: (1) hepatitis B virus pre S1 antigen calibrator; (2) carrier of avidin; (3) hepatitis B virus pre S1 monoclonal antibody of biotinylation; (4) anti-HBs monoclonal antibody marked with acridinium ester; (5) chemiluminescence initiating agent; and (6) concentrated cleaning solution. The method for preparing the kit further comprises the following steps: (1) the calibrator is prepared by using the hepatitis B virus pre S1 pure product; (2) the carrier is coated with the avidin; (3) the biotinylation is performed to the hepatitis B virus pre S1 monoclonal antibody; (4) the anti-HBs monoclonal antibody is marked with the acridinium ester; (5) the chemiluminescence initiating agent is prepared; (6) the concentrated cleaning solution is prepared; (7) the calibrator, the biotinylated antibody, the acridinium ester marker, the chemiluminescence initiating agent and the concentrated cleaning solution are subpacked; and (8) the finished product is assembled. The kit is simple, convenient, rapid, sensitive, stable, and the like.
Owner:北京科美东雅生物技术有限公司
Immuno-nephelometry of lipoprotein (a) and reagent therefor
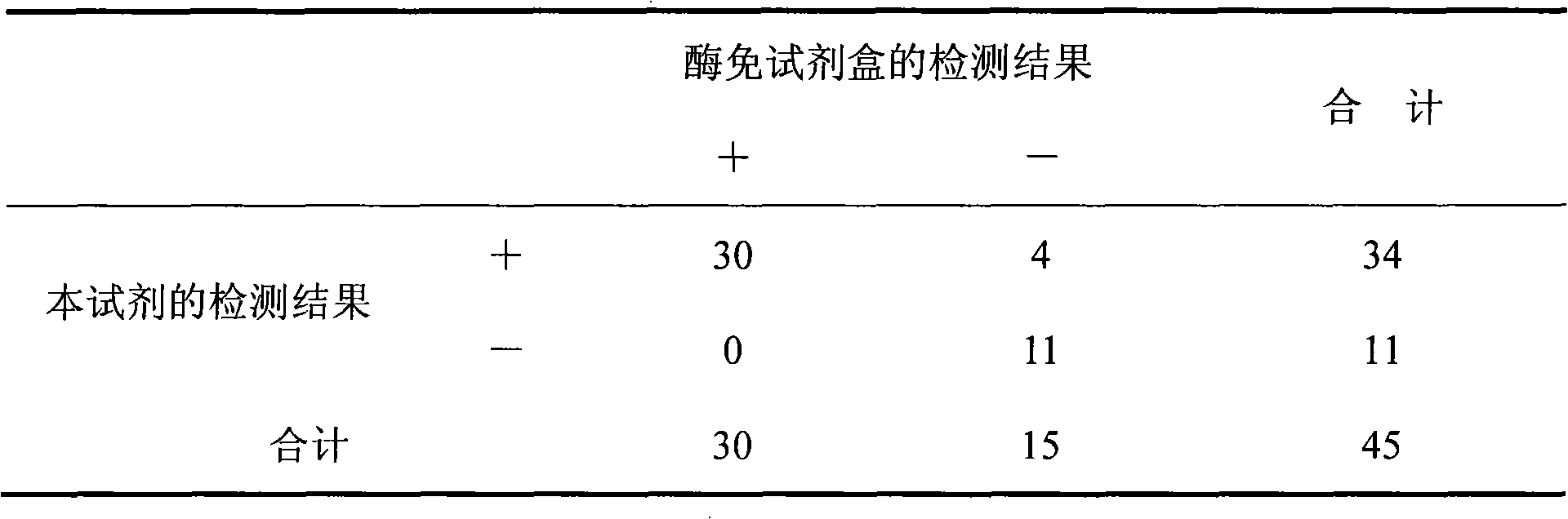
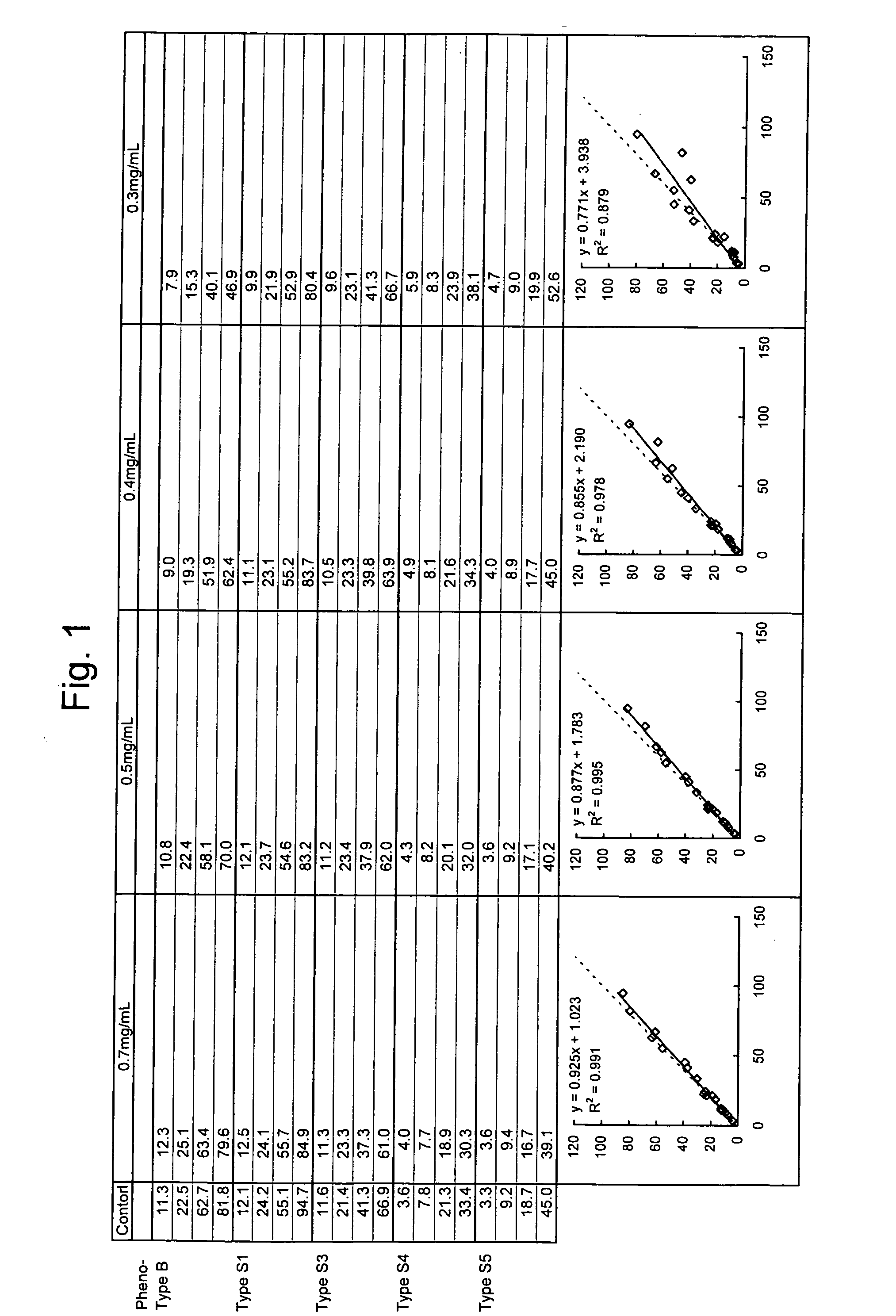
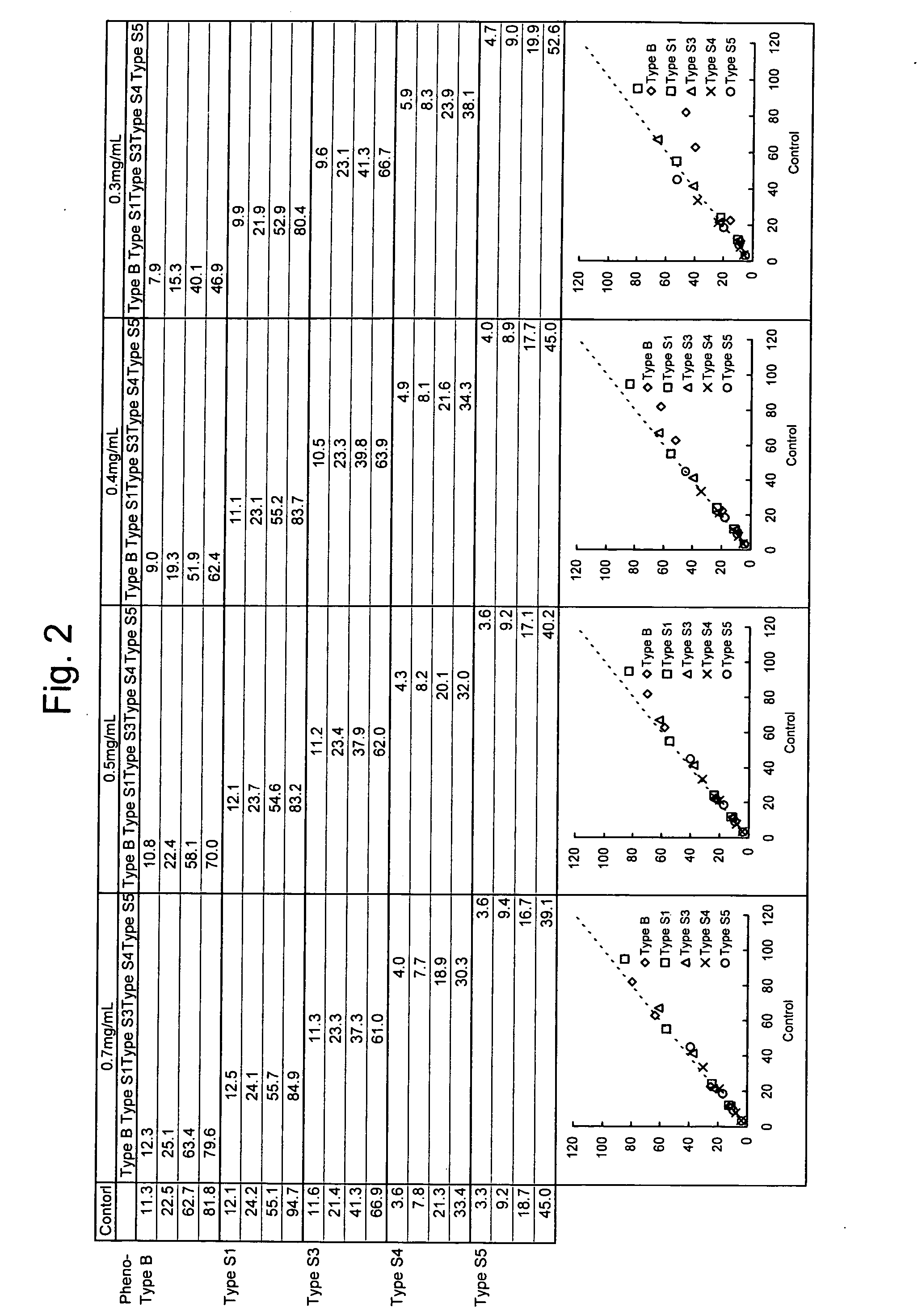
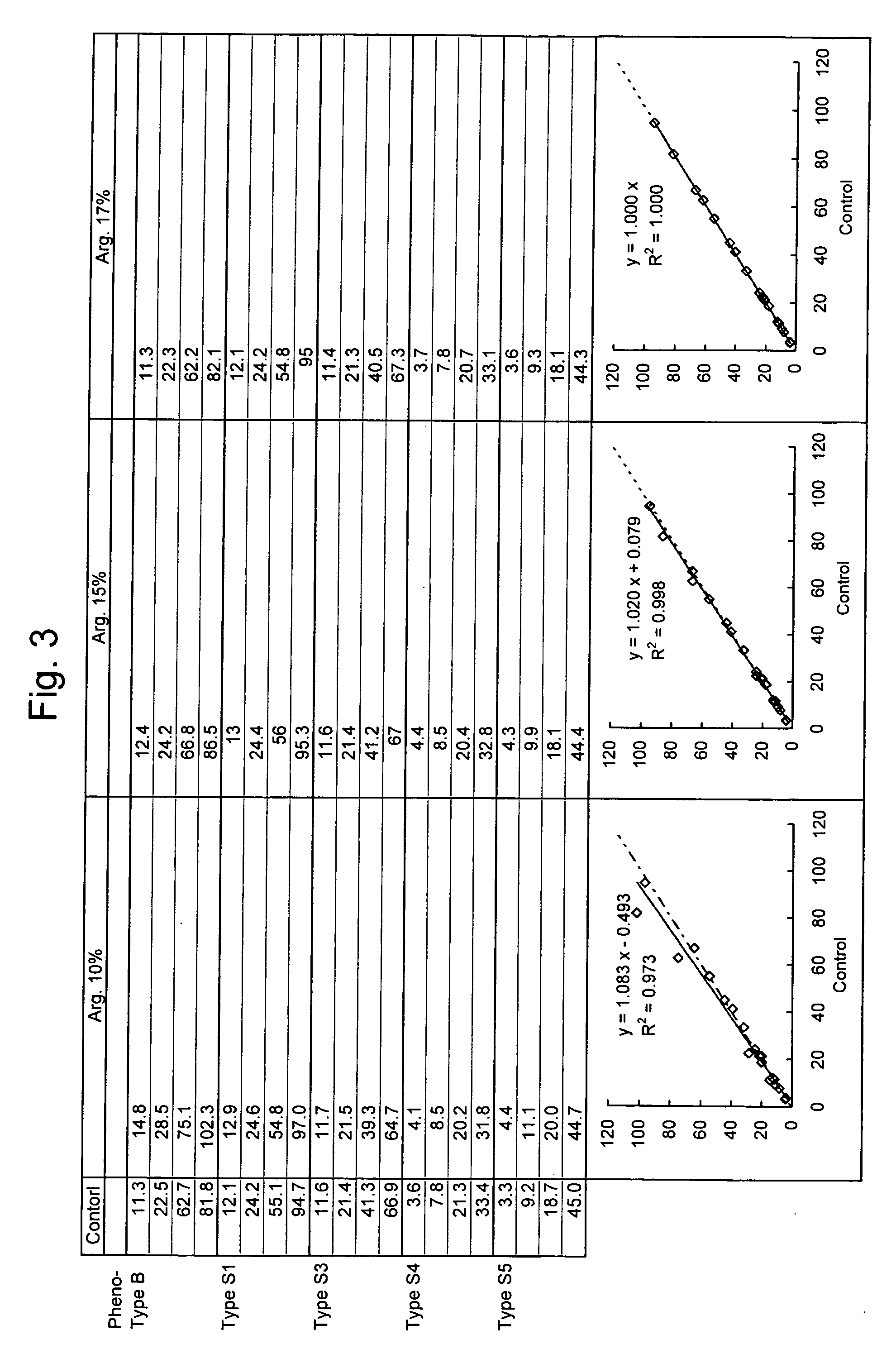
Provided is a method for quantitatively measuring an antigen having diverse phenotypes with accuracy in immunoassay. The present invention is particularly intended to latex turbidimetric immunoassay using the antigen-antibody reaction of an antigen having phenotypes, wherein, in detection utilizing the immunoassay, the amount of an antibody against the antigen added to an assay system is adjusted and a basic amino acid is added to the assay system, thereby circumventing the variability of a measurement value attributable to phenotype variety and obtaining a measurement value having a high correlation with a measurement value of the antigen in a biological sample that is measured on a molecular basis.
Owner:DENKA SEIKEN CO LTD
Multi-item respiratory tract antigen detection card and kit
ActiveCN112362869AAdequate responseHigh sensitivityBiological testingImmunoassaysAntibody conjugateAntigen testing
The invention relates to a multi-item respiratory tract antigen detection card which comprises an influenza A virus antigen test strip, an influenza B virus antigen test strip, a respiratory tract adenovirus antigen test strip, a respiratory tract syncytial virus antigen test strip and a mycoplasma pneumoniae antigen test strip, wherein each of the influenza A virus antigen test strip, the influenza B virus antigen test strip, the respiratory tract adenovirus antigen test strip, the respiratory tract syncytial virus antigen test strip and the mycoplasma pneumoniae antigen test strip comprisesa colloidal gold conjugate pad, and each colloidal gold conjugate pad comprises a streptavidin conjugate pad and a double-nanoparticle double-labeled antibody conjugate pad; the test strip in the detection card enables biological raw materials to react fully, improves the sensitivity of antigen detection, effectively reduces missing detection, and meanwhile, the blocking agent is added into the sample pad to improve the specificity, so that influenza A virus, influenza B virus, respiratory adenovirus, respiratory syncytial virus and mycoplasma pneumoniae antigen can be detected at the same time.
Owner:山东康华生物医疗科技股份有限公司
Three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol and preparation method thereof
ActiveCN104237521AAchieving Simultaneous DetectionRapid Field DetectionMaterial analysisCellulosePolyvinyl chloride
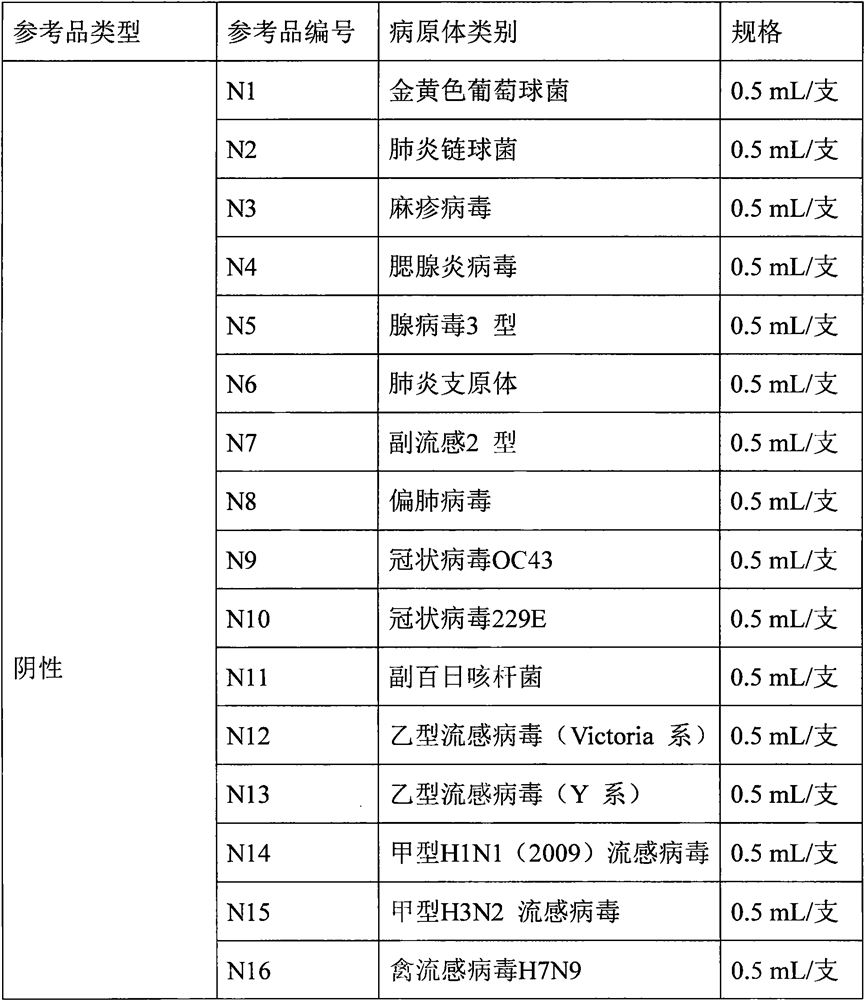
The invention provides a three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol and a preparation method thereof, and belongs to the technical field of immunological detection. The preparation method comprises two parts, namely, the preparation of a monoclonal antibody and the preparation of a colloidal gold chromatographic test strip, wherein the monoclonal antibody can be used for recognizing thiamphenicol, chloramphenicol and florfenicol at the same time and is high in sensitivity; the colloidal gold chromatographic test strip comprises a polyvinyl chloride backing; a sample pad is arranged at the front end of the polyvinyl chloride backing and is connected with the front end of a nitrocellulose membrane; the rear end of the nitrocellulose membrane is connected with a water absorbing pad; the monoclonal antibody marked by colloidal gold is used as a combining pad; the nitrocellulose membrane is sequentially wrapped with a chloramphenicol sodium succinate-BSA (Bovine Serum Albumin) antigen test ray T and a goat-anti-mouse IgG control ray C. The three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol is fast to detect, and the detection needs only 3 to 5 minutes; the test strip is convenient to carry, and suitable for detection on site; the operation is simple and convenient and does not need a professional technical person.
Owner:JIANGNAN UNIV
Preparation of reagent-free ampoul immuno sensor and use thereof
InactiveCN1438482AAvoid pollutionSimplify the analysis systemBiological testingMaterial electrochemical variablesAntigenThermostat
The antigen to be tested is dissolved in the buffer solution. After the pretreatments of carrier surface, said solution is dropped and coated on said surface which is then over alkoxy compound. The immunity sensor can be obtained by sealing the aqueous thermostat of the system. With the condition of measuring antigen to be tested being opimized, the standard curve for measuring the antigen to be tested can be obtained after a series of treatments are carried out. Through the degradation of the electrochemical signal, the correspondent concentration can be looked up from the standard curve. The method does not need to add the additional intermediate or reagent into the solution of the specimen to be measured so as to prevent the pollution of the electrodes as well as reduce the operation steps.
Owner:JIANGSU CANCER HOSPITAL +1
Reagent kit for detecting antiuninuclear cell proliferation Listeria bacteria by colloidal gold Hly gene monoclonal antibodies
The invention relates to a reagent kit for detecting antiuninuclear cell proliferation Listeria bacteria by colloidal gold Hly gene monoclonal antibodies and belongs to the technical field of hygiene inspection and medical inspection. The reagent kit is characterized by comprising the steps of: 1, primer design; 2, polymerase chain reaction (PCR) amplification; 3, agarose gel electrophoresis reaction conditions; 4, target deoxyribonucleic acid (DNA) segment cutting; 5, heat shock conversion method application; 6, reagent kit purification and plasmid preparation by a sodium dodecyl sulfate (SDS) cracking method; 7, protein induction expression; 8, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE); 9, required strip cutting from the SDS-PAGE; and 10, colloidal gold preparation. The regent kit has the beneficial effect that the limitation aiming at the single-clostridium and single-antigen test in the prior art is avoided. The antibody is pure, the valence is high, and the sensitivity and the accurate degree are improved. The single added Listeria bacterium is detected. Compared with an enzyme-linked immuno sorbent assay (ELISA) method and a PCR method, the reagent kit has the advantage that the Listeria bacterium detection is faster and more convenient by the immune colloidal gold chromatography.
Owner:何鑫 +4
Anti-H7N9 subtype avian influenza virus monoclonal antibody epitope as well as screening method and application thereof
The invention provides an anti-H7N9 subtype avian influenza virus monoclonal antibody epitope as well as a screening method and application thereof, belonging to the technical field of immunodetection. The screening method comprises the following steps: mixing wild type H7N9 subtype avian influenza virus liquid with a corresponding monoclonal antibody with a neutralizing property for incubation, and inoculating the mixture to an SPF chick embryo, so as to obtain allantoic fluid with a positive hemagglutination titer; and carrying out gradient dilution on the positive allantoic fluid, mixing the positive allantoic fluid with the monoclonal antibody for incubation, inoculating the mixture to the SPF chick embryo, determining a hemagglutination inhibition titer of the monoclonal antibody by selecting the allantoic fluid with the positive hemagglutination titer as an antigen, when the determined hemagglutination inhibition titer is lower than the hemagglutination inhibition titer of a wild type virus by 8log2, determining the positive allantoic fluid as an escape mutant of the wild type H7N9 subtype avian influenza virus, measuring an HA gene sequence of the positive allantoic fluid, and determining the epitope recognized by the monoclonal antibody. By virtue of the method, the specific epitope can be clearly screened; the method is simple, accurate and short in screening period.
Owner:YANGZHOU UNIV
Calprotectin combined with lactoferrin antigen test strip and preparation method thereof
PendingCN109682979AHigh sensitivityHigh speedDisease diagnosisBiological testingCalprotectinProtein detection
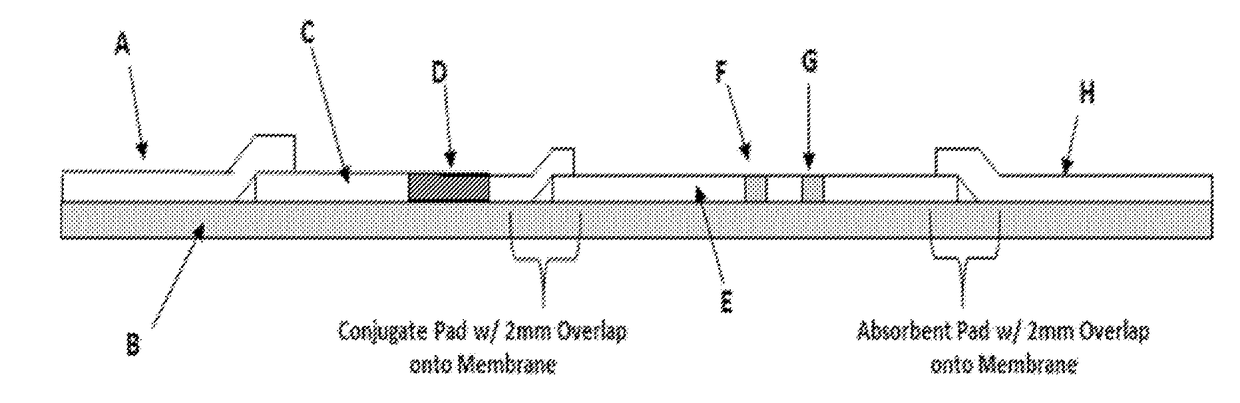
The invention discloses a calprotectin combined with lactoferrin antigen test strip and a preparation method thereof, and aims to provide a calprotectin combined with lactoferrin test strip with highdetection speed, simple operation and high sensitivity and also provides a preparation method of the calprotectin combined with lactoferrin test strip with simple process, good repeatability, stable finished structure and good performance. The calprotectin combined with lactoferrin antigen test strip comprises a bottom plate, a sample pad, a marker pad, a test pad and an absorbent pad. The preparation method comprises the steps that firstly the sample pad, the marker pad and the test pad are prepared, and then the sample pad, the marker pad, the test pad and the absorbent pad are in lap jointon the bottom plate in sequence. The calprotectin combined with lactoferrin antigen test strip is applied to the technical field of intestinal inflammatory disease marker protein detection test strips.
Owner:ZHUHAI ENCODE MEDICAL ENG
Immune Agglutination Reagent Kit and Method of Measuring Antigen
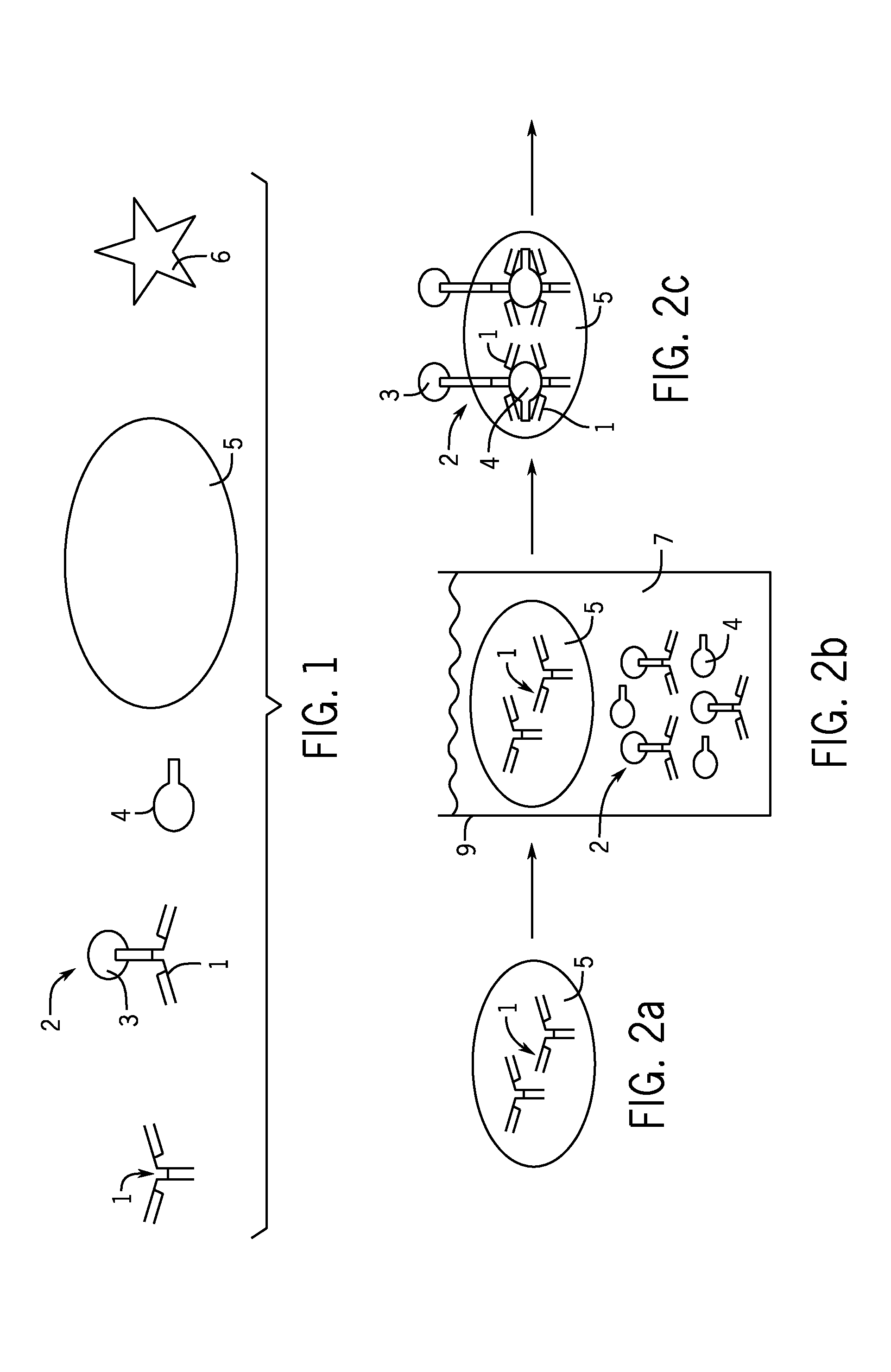
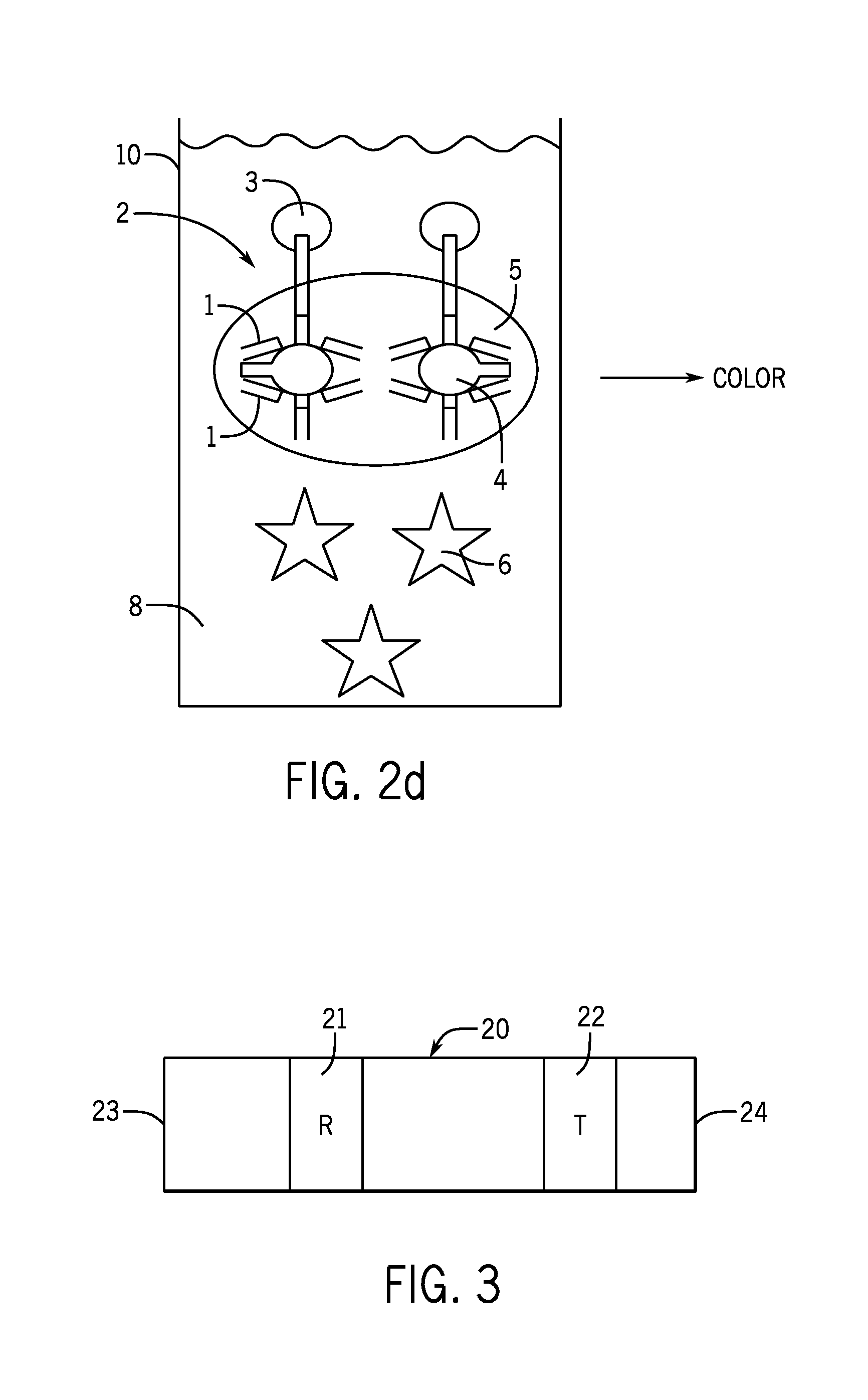
InactiveUS20090117668A1Improve accuracyIncrease rangeImmunoglobulins against animals/humansFermentationEpitopeAntiendomysial antibodies
In cases of poor reaction efficiency with the antibody due to inadequate number of epitopes or steric hindrance caused by epitopes being close to each other, this invention provides a direct method of efficiently measuring antigen in a given specimen without the need of pretreating it. Two types of monoclonal antibodies that recognize different epitopes are individually sensitized on separate latex. The specimen and one latex sensitized monoclonal antibody reagent are reacted to produce a reaction solution which is then reacted with the other latex sensitized monoclonal antibody reagent. The antigen can thus be directly measured in an efficient and highly sensitive way without pretreating it.
Owner:NISSUI PHARMA
Device and method for in-line blood testing using biochips
InactiveUS7785782B2Large fractionBioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acid amplification techniqueDisease
A device for in-line blood screening and testing using biochips is disclosed. The screening methods include nucleic acid amplification techniques and antibody / antigen assays to detect target molecules and agents indicative of infectious diseases or metabolic diseases.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Human blood type test kit and preparation method thereof
The invention discloses a human blood type test kit which comprises a test strip. The test strip comprises a gold conjugate pad and a reaction membrane, and the gold conjugate pad is coated with a colloidal gold-labelled mouse anti-human A antigen monoclonal antibody, a colloidal gold-labelled mouse anti-human B antigen monoclonal antibody and a colloidal gold-labelled mouse anti-human D antigen monoclonal antibody; the reaction membrane comprises a B antigen test line coated with the mouse anti-human B antigen monoclonal antibody, an A antigen test line coated with the mouse anti-human A antigen monoclonal antibody, an Rh test line coated with the mouse anti-human D antigen monoclonal antibody and a quality control line coated with a goat anti-mouse IgG polyclonal antibody. The invention further provides a preparation method of the kit. The preparation method comprises the steps of reaction membrane preparation, gold conjugate pad preparation, cutting assembly and the like. The human blood type test kit can simultaneously test A, B, O and Rh blood types, is intuitive in test result, can be used for testing hemolysis samples and has the high sensitivity, specificity and stability.
Owner:北京中检安泰诊断科技有限公司
Saliva liquefied sugar chain antigen determination kit and preparation method thereof
PendingCN111856016AImprove accuracyHigh precisionBiological material analysisAgainst vector-borne diseasesMicrosphereMedicine
The invention discloses a saliva liquefied sugar chain antigen determination kit, which comprises a latex reagent, and the latex reagent comprises a first KL-6 monoclonal antibody latex reagent and asecond KL-6 monoclonal antibody latex reagent; latex microspheres are activated by an activation buffer solution with a pH value of 5.5-6.0, then the pH value is adjusted to 7.0-7.4 to obtain a firstactivated microsphere solution, and the first activated microsphere solution is coupled with a first KL-6 monoclonal antibody to obtain a first coupling reaction solution; latex microspheres are activated with an activation buffer solution with the pH value of 6.5-7.0, then the pH value is adjusted to 7.5-8.0 to obtain a second activated microsphere solution, and the second activated microsphere solution is coupled with the second KL-6 monoclonal antibody to obtain a second coupling reaction solution; and the first coupling reaction solution and the second coupling reaction solution are respectively sealed, and solid-liquid separation is carried out to obtain solid resuspension, so as to obtain the first KL-6 monoclonal antibody latex reagent and the second KL-6 monoclonal antibody latex reagent. The kit is high in accuracy, wide in linear range and beneficial to large-scale production.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Method for detecting trichinella circulating antigen by utilizing IgY-McAb sandwich ELISA (enzyme-linked immuno sorbent assay)
InactiveCN102331501AEnable early detectionIncreased sensitivityMaterial analysisTrichinella speciesZoology
The invention discloses a method for detecting a trichinella circulating antigen by utilizing IgY-McAb sandwich ELISA (enzyme-linked immnuo sorbent assay). With an anti-trichinella muscle larval ES antigen egg yolk antibody IgY as a capture antibody and an anti-trichinella ES antigen McAb as a detection antibody, the preparation method of the IgY comprises the following steps of: adding the trichinella muscle larval into a culture medium to carry out sterile culture, purifying and dialyzing supernate and then concentrating and drying to obtain the ES antiagen, determining protein concentration and then immunizing roman legehenne by utilizing the ES antigen, collecting the IgY from the produced egg yolk by adopting a saturate ammonium sulphate precipitation method, purifying and determining concentration, and sub-packaging for later use; and the preparation method of the McAb comprises the following steps of: establishing a hybridoma cell strain secreting the anti-trichinella muscle larval ES antigen, cloning by adopting a limiting dilution method, purifying McAb by applying an octanoic acid-ammonia sulphate method, determining the concentration and sub-packaging for later use. The invention has the advantage that a new method which has high sensitivity and specificity and has wide application prospect is provided for early detection of trichinella CAg in blood serum of an infected animal.
Owner:ZHENGZHOU UNIV
Saliva liquefied sugar chain antigen determination kit and detection method thereof
InactiveCN112285359ANo increase in R&D costsLarge diameterDisease diagnosisBiological testingActive agentAntigen testing
The invention relates to the technical field of in-vitro diagnostic reagents, and particularly relates to a saliva liquefied sugar chain antigen determination kit and a detection method thereof. The saliva liquefied sugar chain antigen detection kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 includes 5-50g / L of a stabilizer and 0.05-2g / L of a preservative, and a pH value is adjusted to 2-9 by utilizing a buffer solution; and the reagent R2 includes 5-30 g / L of a surfactant, 5-30 g / L of a stabilizer, 0.1-2 g / L of an anti-human saliva liquefied sugar chain antigen antibody latex particle conjugate and 0.05-2 g / L of a preservative, and the pH value is adjusted to 2-9 by using the buffer solution. The kit is high in sensitivity, the lowest detection can reach 50.0 U / mL, alinear range is wide and can reach 50-10000 U / mL, and accurate detection of the saliva liquefied sugar chain antigen can be achieved.
Owner:广州市伊川生物科技有限公司
A novel coronavirus antigen detection test strip
ActiveCN112557654BAvoid stickinessHigh detection specificityBiological material analysisBiological testingBlood filmFiltration membrane
A novel coronavirus antigen detection test strip provided by the present invention comprises: a substrate, and a sample pad, a colloidal gold adsorption pad, a blood filter membrane, an antibody-carrying membrane and a water-absorbent pad are sequentially overlapped and overlapped on the substrate; The antibody carrying membrane is provided with a detection line and a quality control line at intervals, the detection line is close to the colloidal gold adsorption pad, and the quality control line is close to the water absorption pad; the coating of the colloidal gold adsorption pad Colloidal gold-labeled novel coronavirus monoclonal antibody B and colloidal gold-labeled mouse IgG antibody; the detection line is coated with novel coronavirus monoclonal antibody A; the quality control line is coated with anti-mouse IgG polyclonal antibody; novel coronavirus The virus monoclonal antibody B is different from the new coronavirus monoclonal antibody A; by setting a blood filter membrane, it can prevent the sample to be tested from being viscous and not running, and can also filter out other macromolecular interfering substances to improve specificity.
Owner:NANTONG EGENS BIOTECH CO LTD
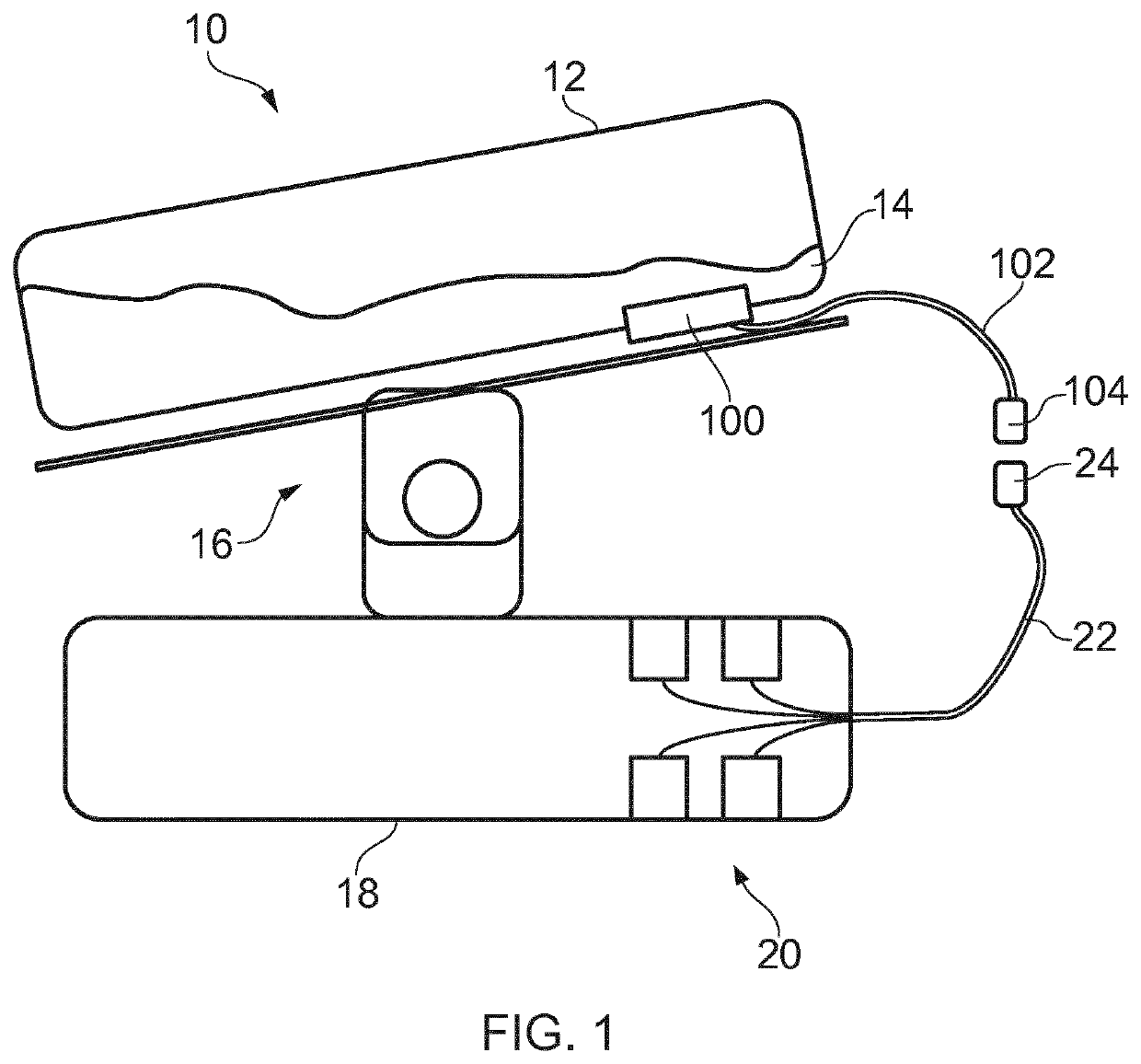
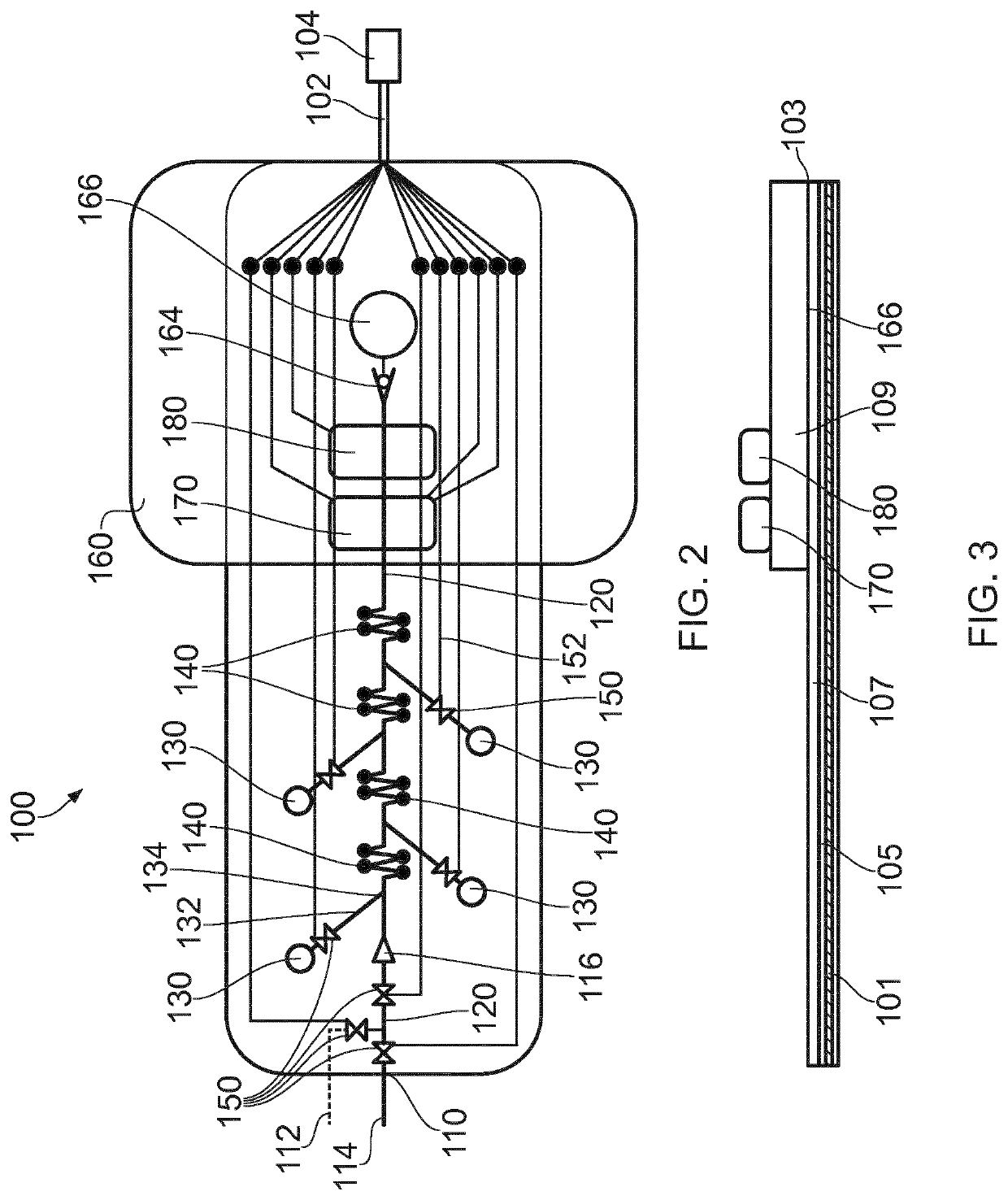
Microfluidic Device for Cell Culture Monitoring
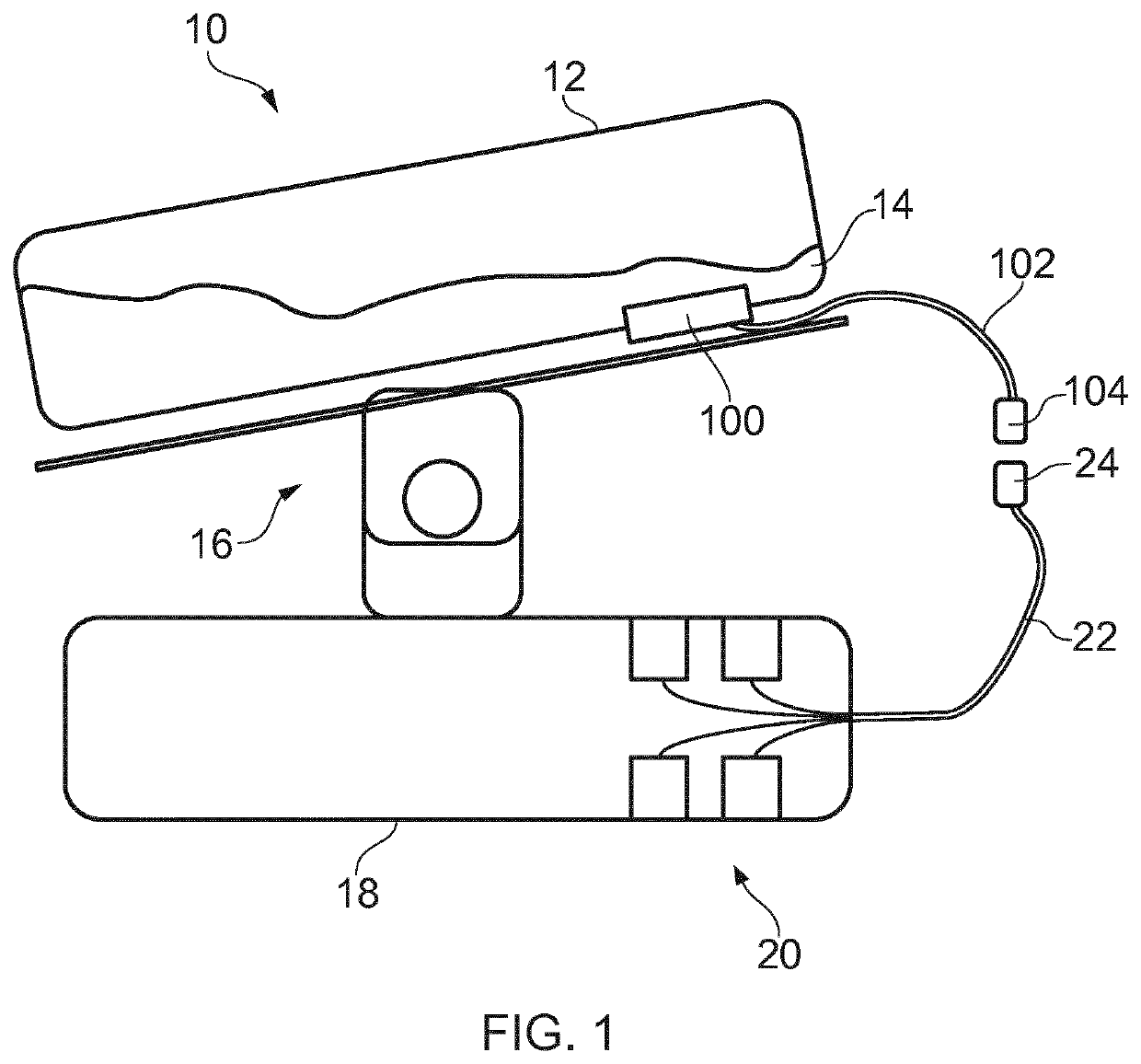
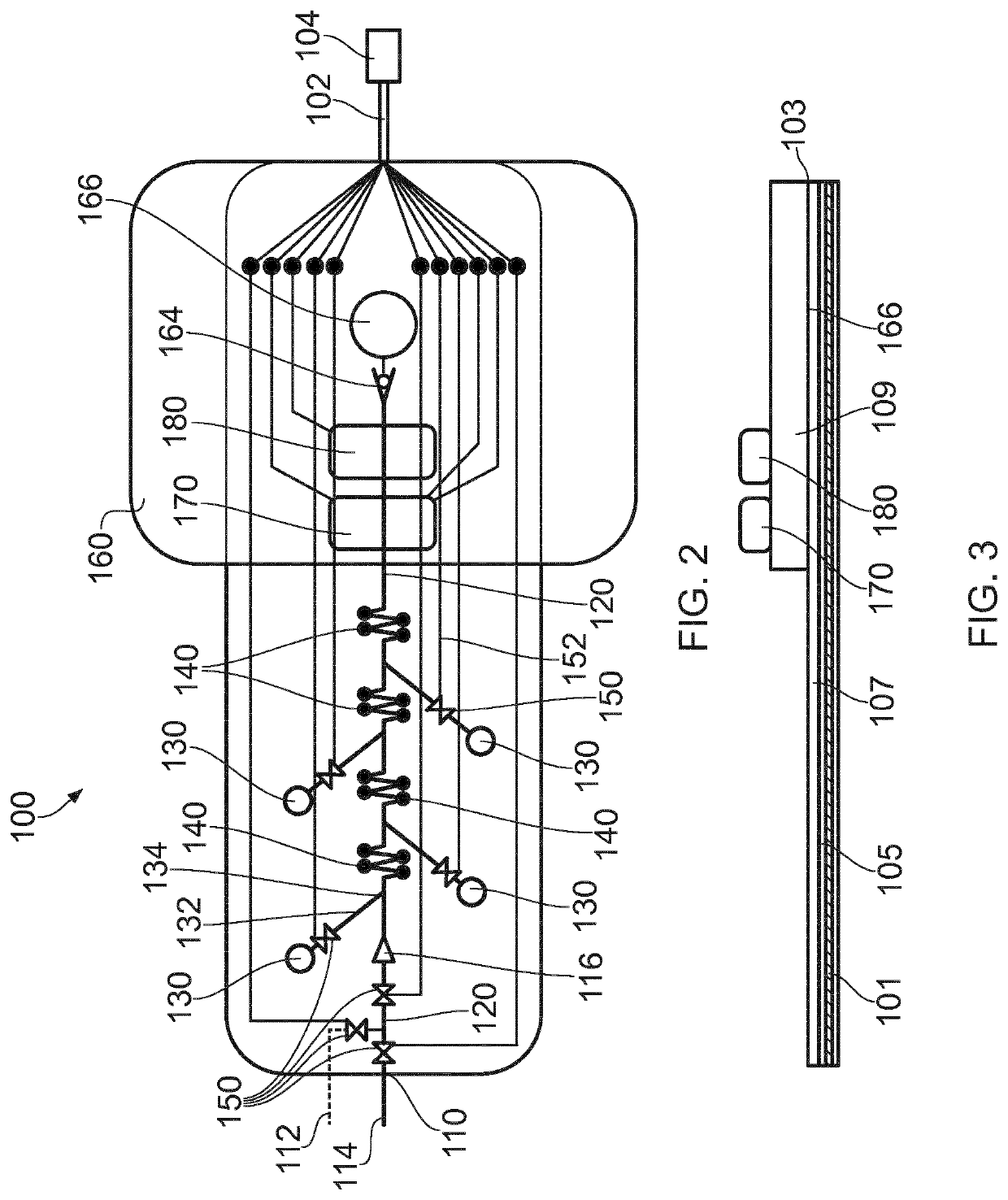
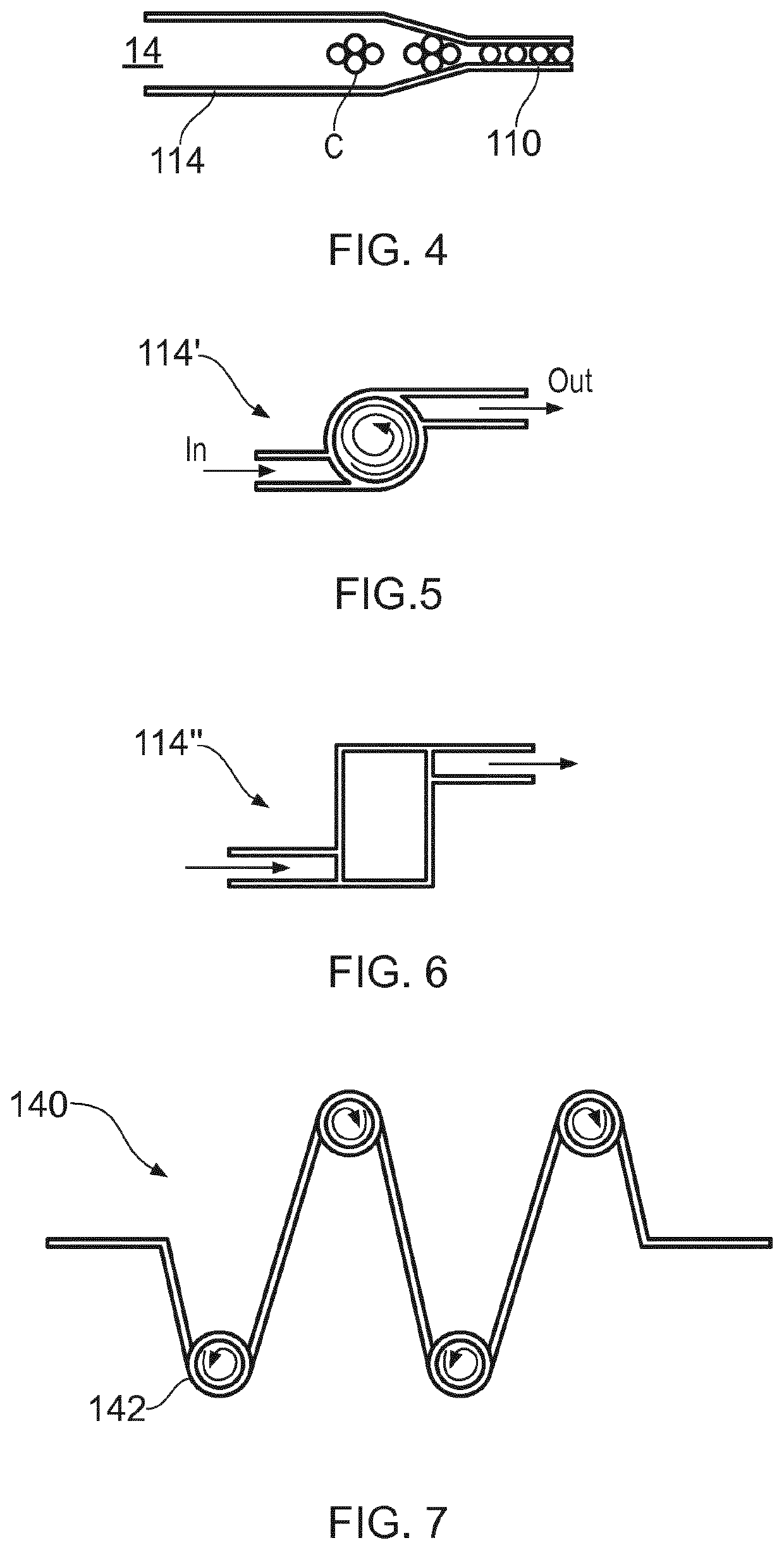
ActiveUS20200181560A1Readily apparentBioreactor/fermenter combinationsBiological substance pretreatmentsEpitopeMetabolite
A microfluidic device (100) is disclosed for in-process monitoring of cell culture conditions including for example one or more of: cell density; cell viability; secreted proteins; protein analysis; epitope markers; concentrations of metabolites or nutrients and antigenic determinations; the device comprising: a cell inlet path (120); plural fluid reservoirs (130) in fluid communication with the cell input path, a cell analysis area (160) in fluid communication with the path and reservoirs, and a waste storage volume (166) also in fluid communication with the cell analysis area, the device being operable to cause a primary fluid flow along the inlet path to the analysis area, and to selectively cause secondary fluid flow(s) into the path from none, one or more of the selected reservoirs to combine, if one or more of the reservoirs are selected, with the primary fluid flow from the cell inlet path, in each case for analysis at the cell analysis area, the device being further operable to cause a fluid flow of the primary and any combined secondary flows from the cell analysis area into the waste storage volume.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
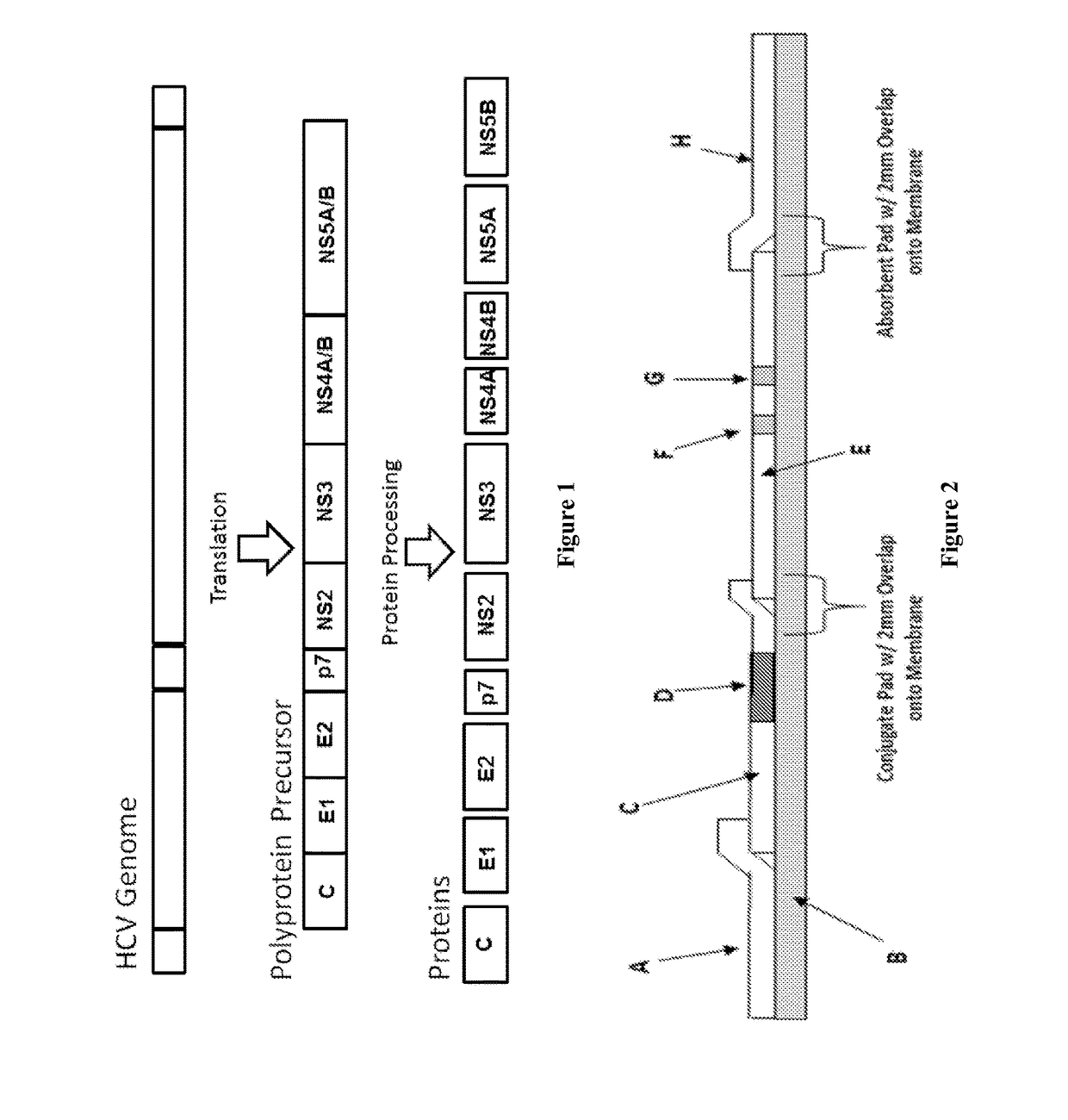
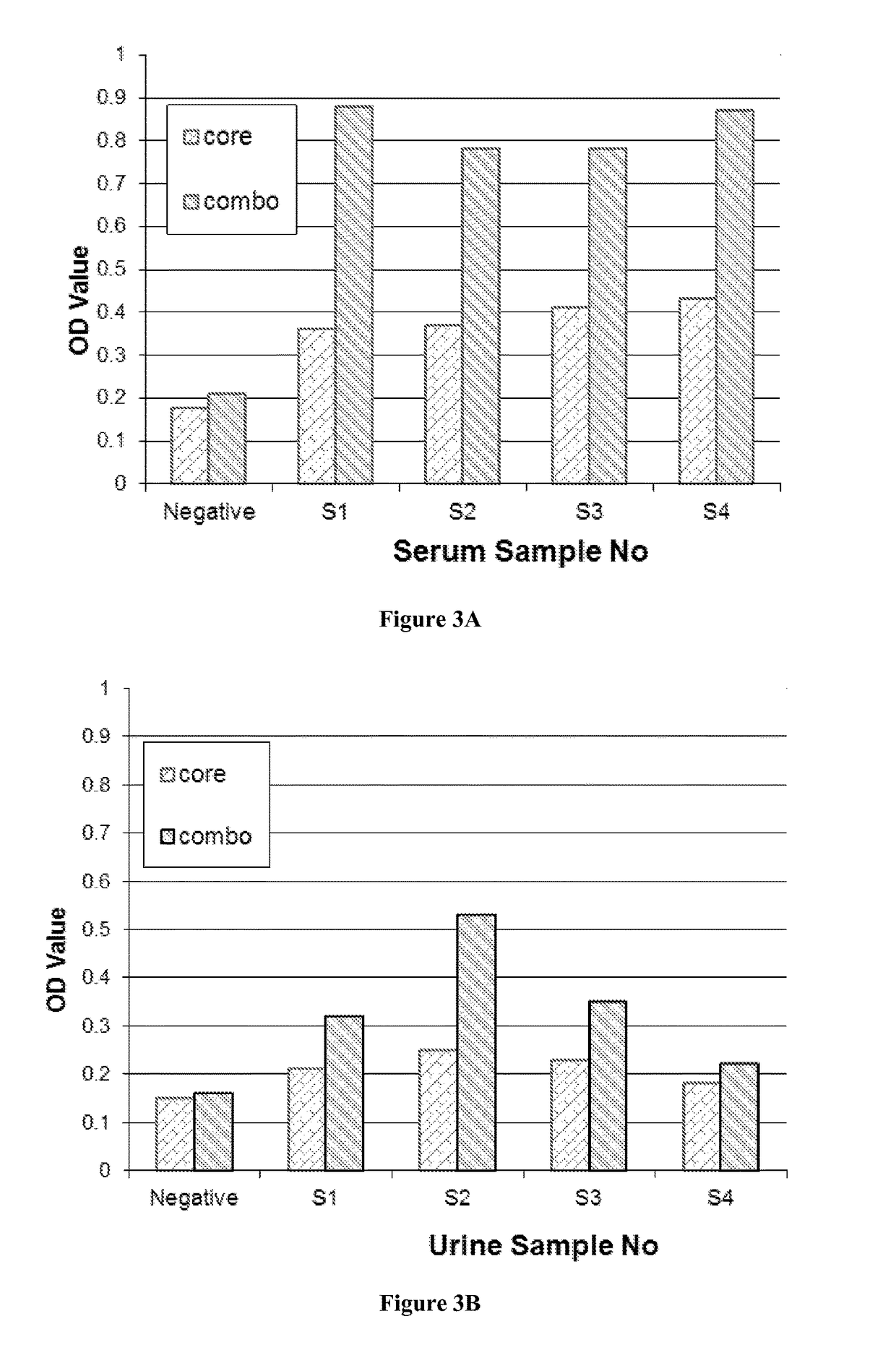
Combo-Hepatitis Antigen Assays and Kits for Detection of Active Hepatitis Virus Infections
InactiveUS20170242010A1Microbiological testing/measurementDisease diagnosisActive hepatitisAntigen assays
Owner:RGT UNIV OF CALIFORNIA
Rapid Lyme antigen test for detection of Lyme disease
ActiveUS9500648B1Detect presenceEarly diagnosisBiological material analysisAntigen assaysCapture antibody
A method for detecting B. burgdorferi antigens in body fluid samples, such as urine. Polyclonal antibodies are used that bind to 31, 34, and 39 kDa B. burgdorferi antigens, wherein the polyclonal antibodies function as immobilized capture antibodies. Detection antibodies are used, having an enzyme linked thereto, which also bind to the B. burgdorferi antigens. A body fluid sample is reacted with the detection antibodies to form complexes between the detection antibodies and the B. burgdorferi antigens in the body fluid sample. The complexes are reacted with the immobilized capture antibodies, wherein the complexes become linked to the capture antibodies. A substrate is added to the complexes linked to the capture antibodies, wherein the substrate is converted by the enzyme to a visual and / or detectable product if B. burgdorferi antigens are present in the body fluid sample.
Owner:TATE JR ROBERT M
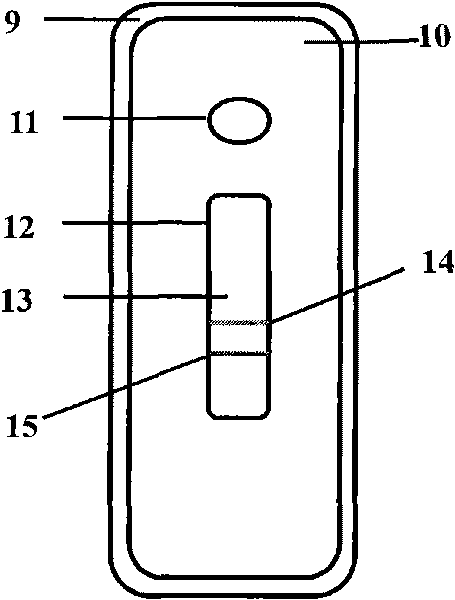
Antigen test kit suitable for self-service detection and data acquisition system
PendingCN114778837AGuaranteed correctnessRealize centralized managementCo-operative working arrangementsBiological testingPsa testAntigen assays
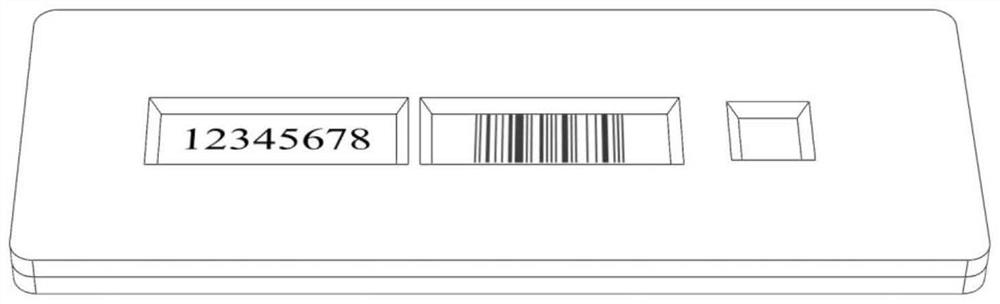
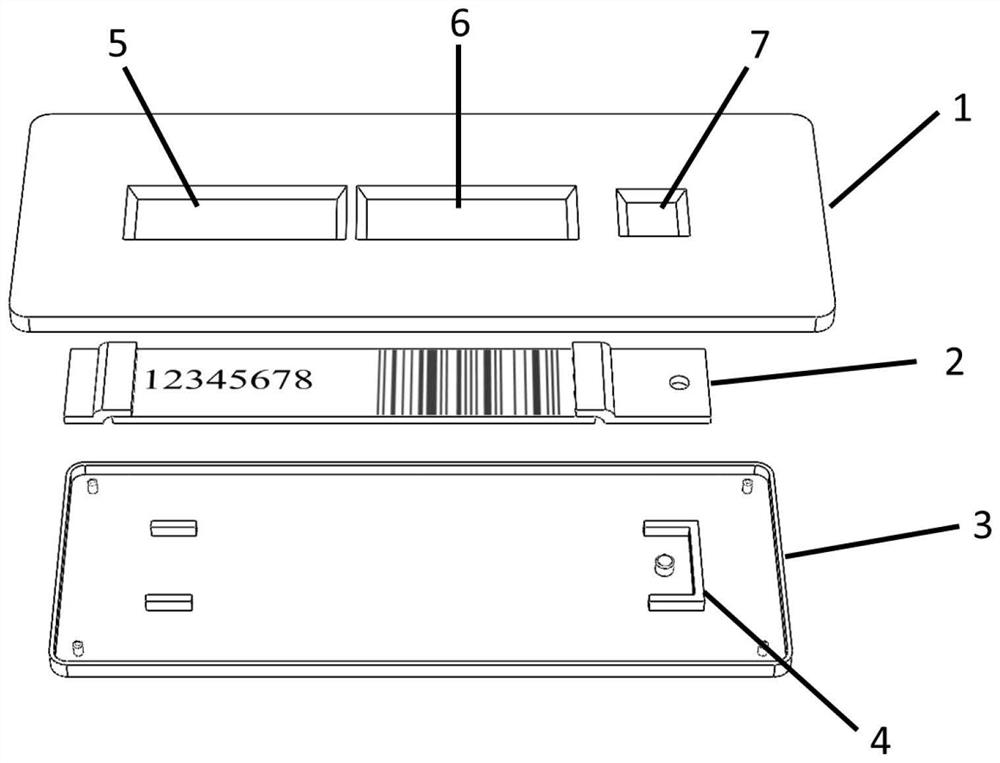
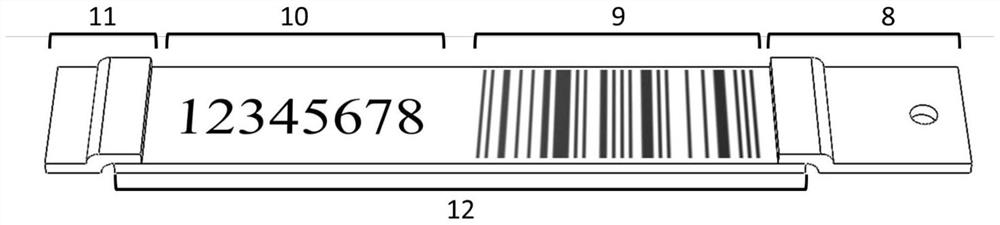
The invention discloses an antigen test kit suitable for self-service detection and a data acquisition system. A chromatography detection card of the antigen test kit is arranged in a test kit card shell; a serial number window, a result observation window and a sample adding opening are sequentially formed in the top surface of the test kit clamping shell upper cover; the sample pad and the water absorption pad are arranged at the two ends of the reaction pad, and the sample pad, the water absorption pad and the reaction pad are fixedly arranged between the test kit clamping shell upper cover and the test kit clamping shell bottom cover. And a background database of the antigen test data acquisition system stores a serial number of an antigen test kit, text information of a detection bar code and a corresponding detection result. The detection result is displayed in an encrypted pattern mode, the pattern or the decrypted text cannot be used for judging the detection result, the detection result can be obtained only by matching the pattern or the decrypted text with the serial number in a data acquisition system, detection is accurate and rapid, the device is simple and easy to popularize, and accurate and rapid centralized acquisition of the detection result can be achieved.
Owner:倪晟
Microfluidic device for cell culture monitoring
ActiveUS11359176B2Bioreactor/fermenter combinationsBiological substance pretreatmentsEpitopeMetabolite
A microfluidic device (100) is disclosed for in-process monitoring of cell culture conditions including for example one or more of: cell density; cell viability; secreted proteins; protein analysis; epitope markers; concentrations of metabolites or nutrients and antigenic determinations; the device comprising: a cell inlet path (120); plural fluid reservoirs (130) in fluid communication with the cell input path, a cell analysis area (160) in fluid communication with the path and reservoirs, and a waste storage volume (166) also in fluid communication with the cell analysis area, the device being operable to cause a primary fluid flow along the inlet path to the analysis area, and to selectively cause secondary fluid flow(s) into the path from none, one or more of the selected reservoirs to combine, if one or more of the reservoirs are selected, with the primary fluid flow from the cell inlet path, in each case for analysis at the cell analysis area, the device being further operable to cause a fluid flow of the primary and any combined secondary flows from the cell analysis area into the waste storage volume.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
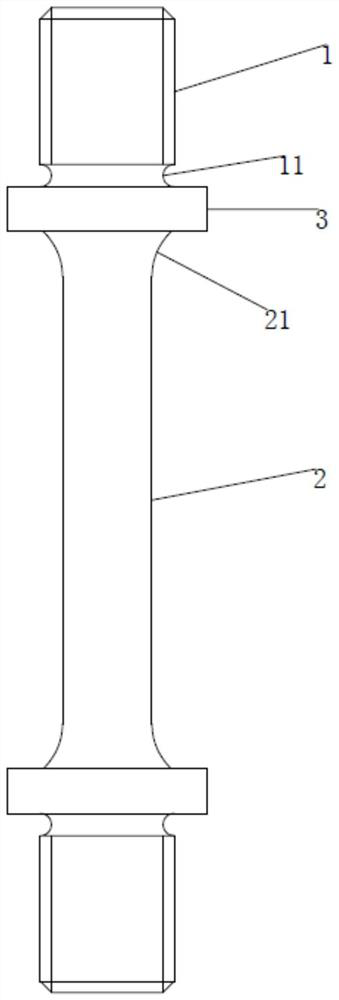
A metal material uniaxial creep resistance test sample, fixture and method
ActiveCN109030196BEasy to fixEasy to testMaterial strength using tensile/compressive forcesTest sampleAntigen assays
The invention relates to the technical field of metal material mechanical property test, more specifically, it relates to a metal material uniaxial creep resistance test sample, fixture and method. The key points of its technical solution are: it includes the testing part and the clamping part integrally formed with both ends of the testing part, the connection between the testing part and the clamping part is surrounded by a boss, the central axes of the testing part, the clamping part and the boss coincide, and The diameter of the cross-section increases sequentially; the clamping part is provided with a thread that is threadedly connected with the test fixture. The clamp fixes the clamping part, and when the tensile creep of the sample needs to be tested, the threaded connection is better for clamping, and it is not easy to slide during the test, so the test result is more accurate; the compressive creep of the sample needs to be tested The threaded connection can also play a very good role in fixing, the clamping and clamping of the boss is more stable, the boss has a certain centering effect, and the test results are more accurate; Determination of compressive creep and tensile creep with high test efficiency.
Owner:深圳阿尔泰克轻合金技术有限公司
Antigen measuring method and measuring apparatus
PendingUS20220260558A1Material analysis by observing effect on chemical indicatorBiological material analysisAntigen assaysHydrogen peroxide
There is provided a method for measuring an antigen, comprising: providing a solution containing an antigen; providing a first antibody that specifically recognizes the antigen and is bound to a magnetic carrier; providing a second antibody that specifically recognizes the antigen and is modified with an oxidase; providing (a substrate liquid including) a substrate which reacts with the oxidase; allowing the first antibody to recognize the antigen; allowing the second antibody to recognize the antigen; using a magnetic field to capture an antigen-antibody complex of the antigen recognized by the first antibody and the second antibody in the magnetic field; washing the antigen-antibody complex while it is captured in the magnetic field; reacting the substrate with the antigen-antibody complex to produce hydrogen peroxide; and measuring the hydrogen peroxide.
Owner:PROVIGATE INC
Three-in-one colloidal gold chromatography test strip for detecting thiamphenicol, chloramphenicol and florfenicol and its preparation method
ActiveCN104237521BAchieving Simultaneous DetectionRapid Field DetectionMaterial analysisCellulosePolyvinyl chloride
The invention provides a three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol and a preparation method thereof, and belongs to the technical field of immunological detection. The preparation method comprises two parts, namely, the preparation of a monoclonal antibody and the preparation of a colloidal gold chromatographic test strip, wherein the monoclonal antibody can be used for recognizing thiamphenicol, chloramphenicol and florfenicol at the same time and is high in sensitivity; the colloidal gold chromatographic test strip comprises a polyvinyl chloride backing; a sample pad is arranged at the front end of the polyvinyl chloride backing and is connected with the front end of a nitrocellulose membrane; the rear end of the nitrocellulose membrane is connected with a water absorbing pad; the monoclonal antibody marked by colloidal gold is used as a combining pad; the nitrocellulose membrane is sequentially wrapped with a chloramphenicol sodium succinate-BSA (Bovine Serum Albumin) antigen test ray T and a goat-anti-mouse IgG control ray C. The three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol is fast to detect, and the detection needs only 3 to 5 minutes; the test strip is convenient to carry, and suitable for detection on site; the operation is simple and convenient and does not need a professional technical person.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com