Carbohydrate antigen CA19-9 assay kit and detection method using the same
A carbohydrate antigen and kit technology, applied in the direction of biological testing, material inspection products, etc., can solve the problems of high price, unfavorable promotion and use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
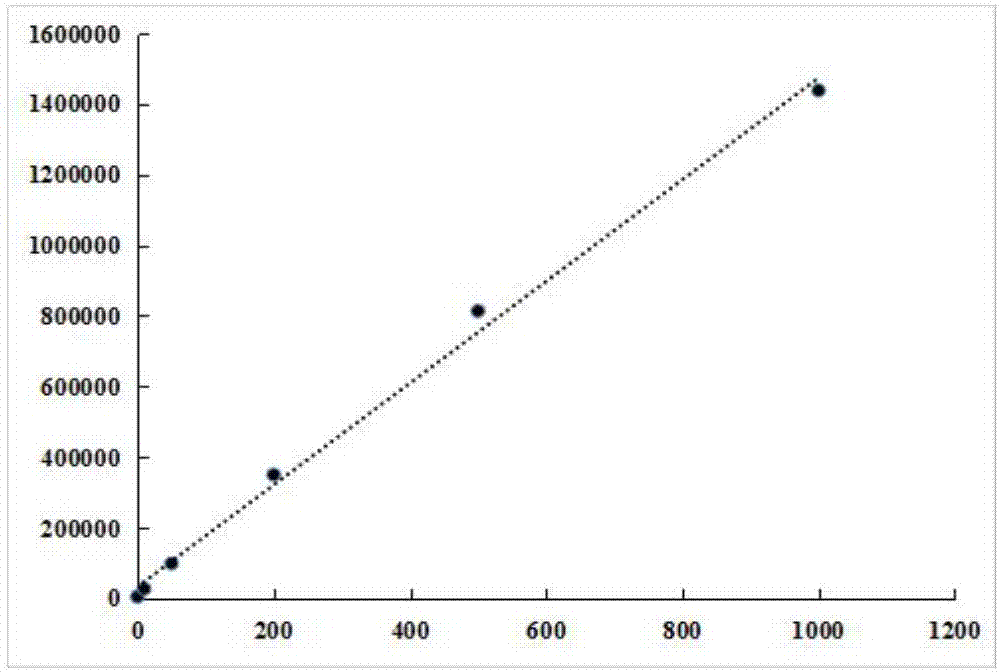
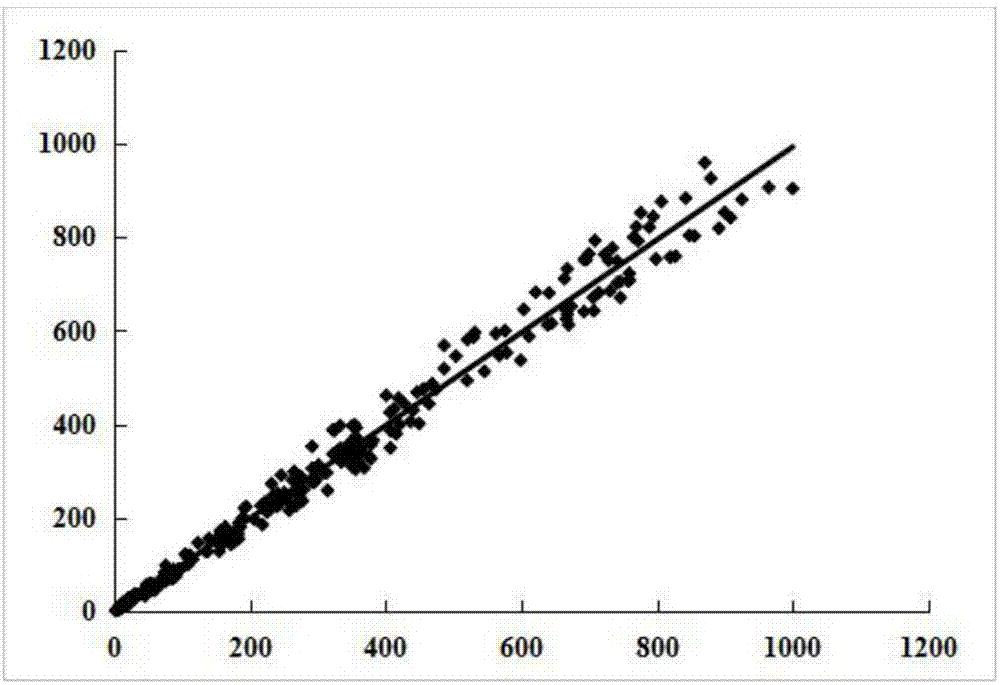
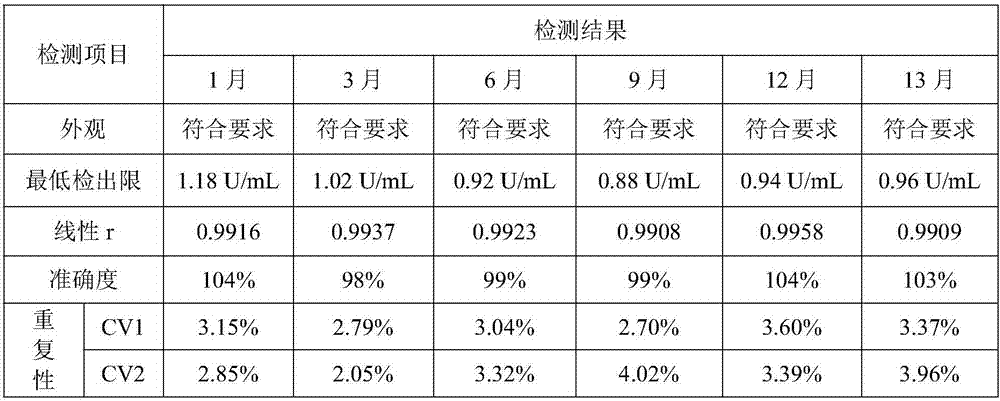
Image
Examples
Embodiment 1
[0077] The various buffers involved in the kit preparation process and detection process are as follows:
[0078] 1. Preparation of magnetic bead buffer, prepare 1L, the specific operation steps are as follows:
[0079] (1). Weigh 800 g of purified water, 7.27 g of Tris, 0.6 g of PROCLIN 3000, and 4.38 g of methyl cellulose ether into a beaker with a capacity of 1 L, and stir thoroughly for 2 hours until completely dissolved.
[0080] (2). Weigh 3.15g of Tween-20, 1.12g of neomycin sulfate, 35mg of tetracycline, and 5.18g of bovine serum albumin (BSA) in the solution obtained in step (1), stir for 1h until completely dissolved, and adjust the pH meter to measure Its pH value is adjusted with dilute HCl and dilute NaOH solution so that its pH value is 8.0±0.05.
[0081] (3). Quantify the solution obtained in step (2) to 1000g, verify the pH value, filter it with a 0.2μm filter into a 1L glass bottle, label it and store it in a cold storage at 2-8°C. The validity period is one ...
Embodiment 2
[0102] Implementation 2: Preparation of Carbohydrate Antigen CA19-9 Assay Kit
[0103] 1. Preparation of magnetic separation reagent R1:
[0104] (1). Use a pipette gun to take 250 μL of magnetic beads into a 5 mL glass vial, absorb on a magnetic plate, discard the supernatant, measure 500 μL of 100 mM MES pH 5.0 buffer solution and wash twice; add 1250 μL of buffer solution, mix well; ultrasonically disperse for 20 s , power 40% (4 times x 5s / time).
[0105] (2). Weigh 20mg of Sulfo-NHS into a centrifuge tube, add 800μL buffer solution 100mM MES pH 5.0, the concentration is 25mg / ml, add 575μL to the solution obtained in step (1). (Use within 2 minutes after preparation)
[0106] (3). Weigh 10 mg of EDC, add 500 μL buffer solution 100 mM MES pH 5.0, the concentration is 20 mg / ml, add 250 μL to the solution obtained in step (2). (Use within 2 minutes after preparation)
[0107] (4). Add buffer solution 100mM MES pH 5.0 to 2.5ml (supplement 425μL) to the solution obtained in...
Embodiment 3
[0137] Implementation 3. The steps of measuring CA19-9 with the carbohydrate antigen CA19-9 assay kit
[0138] The carbohydrate antigen CA19-9 assay kit prepared by the present invention is used to measure CA19-9, and the detection steps are as follows:
[0139] (1). Adding samples: Take 9 test tubes and add 30 μL each of CA19-9 calibrator, quality control product and sample to be tested.
[0140] (2). Add 60 μL of magnetic separation reagent R1 and 60 μL of labeling reagent R2 to the test tubes in step (1), mix with a multi-tube screw mixer for 30 seconds, and incubate in a 37°C water bath for 15 minutes.
[0141] (3). After the incubation, put the test tube on the magnetic separator and let it stand for adsorption for 3 minutes, discard the supernatant, and pat the bottom of the magnetic separator to separate the supernatant as clean as possible.
[0142] (4). Add 300 μL of lotion to the test tube obtained in step (3), place it on a multi-tube spiral mixer to mix, then plac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com