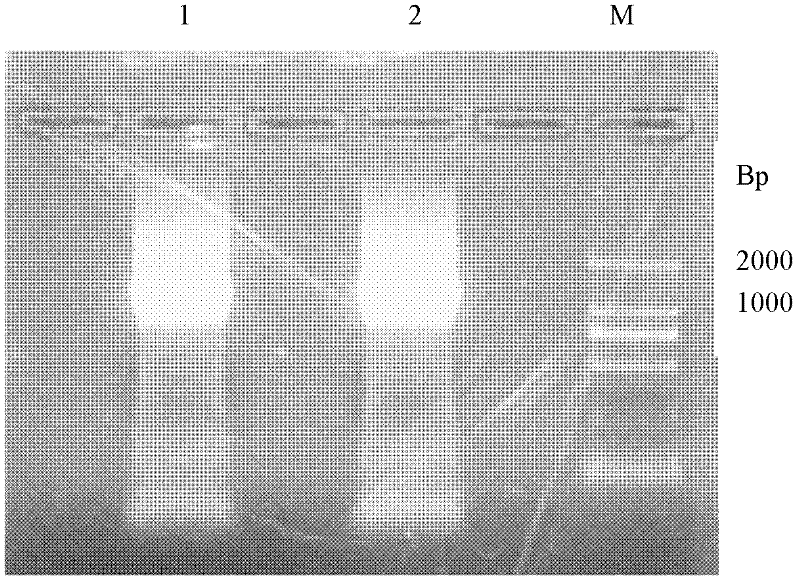
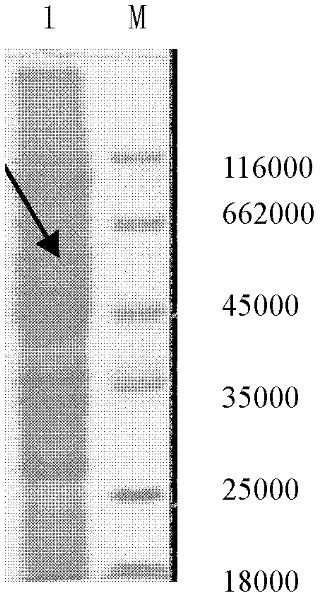
Reagent kit for detecting antiuninuclear cell proliferation Listeria bacteria by colloidal gold Hly gene monoclonal antibodies
A technology of Listeria monocytogenes and monoclonal antibody, applied in the field of hygiene inspection and medical inspection, to achieve the effect of high titer, improved sensitivity and accuracy, and pure antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Process conditions:
[0059] The first step is cloning and prokaryotic expression of the L. monocytogenes gene.
[0060] Main equipment:
[0061] Drugs and reagents: strains and plasmids Listeria monocytogenes strain (C54002 strain), pMD18-T, pGEX-6P vector plasmids are products of TAKARA company. Competent cells Escherichia coli JM109, BL21 recipient bacteria are products of Promega. Enzymes and related reagents Ex Taq polymerase, restriction endonucleases BamH Ⅰ, Xho Ⅰ and IPTG are products of Dalian Bao Biological Engineering Co., Ltd. T4 DNA ligase is a product of Promega. DNA Rapid Purification and Recovery Kit and Gel Rapid Purification and Recovery Kit are products of Dalian Bao Biological Engineering Co., Ltd.
[0062] experimental method:
[0063] 1. Genomic DNA preparation
[0064] Cultivate Listeria monocytogenes according to conventional methods, DNA rapid purification and recovery kit
[0065] Two. Containing the construction of L.monocytogenes Hly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com