Mycobacterium tuberculosis antibody rapid diagnosis reagent kit and detecting method thereof
A technology for rapid diagnosis of Mycobacterium tuberculosis, which is applied to measurement devices, analytical materials, instruments, etc., can solve the problems of few effective antigens, low labeling efficiency, complex cleavage antigen components, etc., and achieves the effect of good specificity and easy promotion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
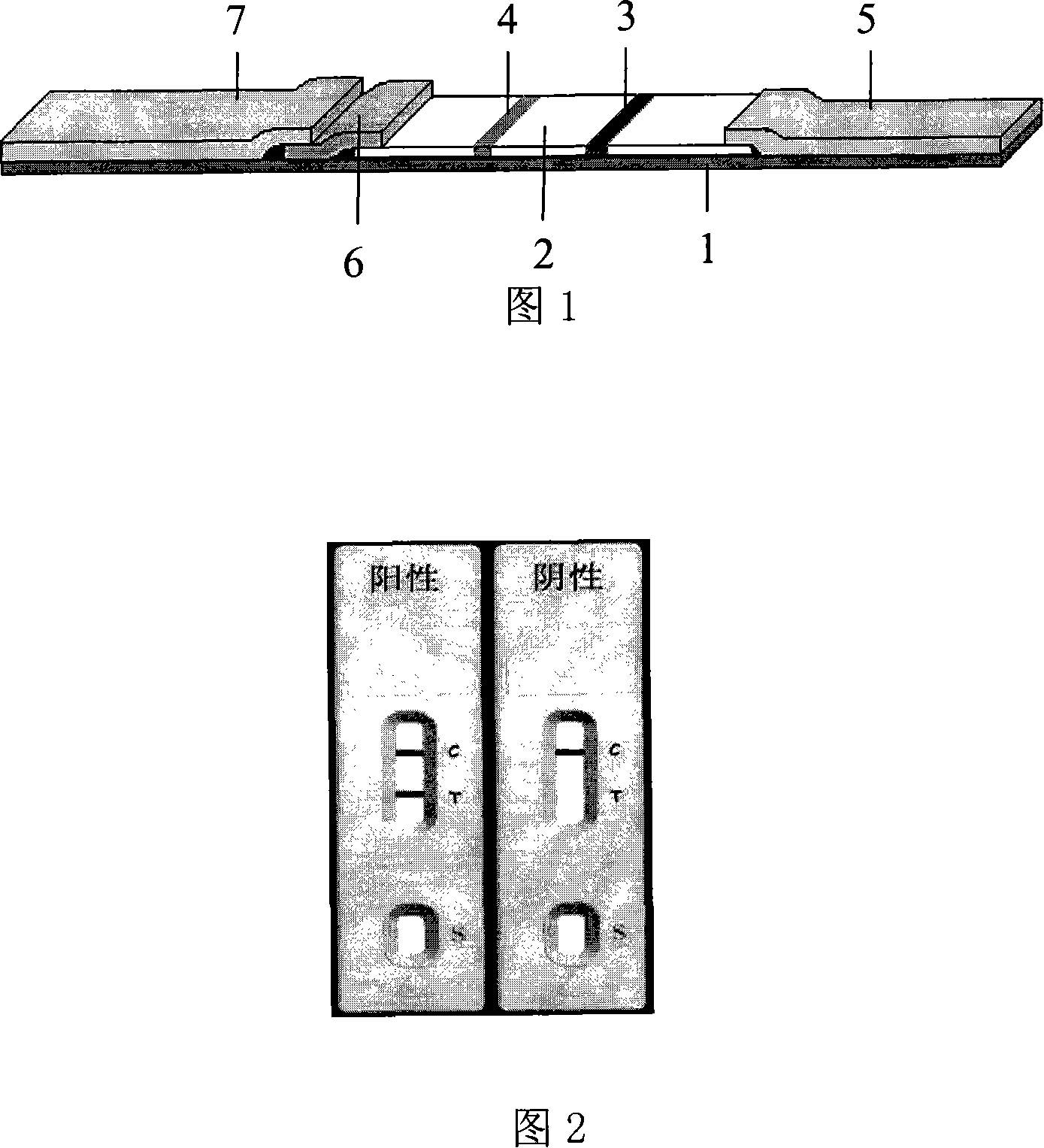
[0031] The Mycobacterium tuberculosis (TB) antibody rapid diagnostic reagent strip of the present invention is shown in Fig. 1, uses the indirect immunochromatographic technique to realize the anti-TB antibody detection in the tested serum or plasma. The preparation method of the kit is briefly described as follows, including: test strips for detecting blood antibodies and the bottom and cover of the plastic box for placing the test strips for detecting blood antibodies. It is attached to the lower part of the NC membrane 2 to form a test line (T) line 4, and goat anti-mouse IgG is coated on the upper part of the NC membrane to form a quality control line (C). Line 3 uses anti-human IgG (mouse anti-human IgG, goat anti- Human IgG or SPA) label nano-scale chromogenic particles (colloidal gold particles or latex particles or nano-magnetic beads) to prepare marker pads 6; NC membrane, colloidal gold pads 6, sample loading pads 7 and sample suction pads 5 are pasted and assembled ...
Embodiment 1
[0043] Embodiment 1: Overview of Product Development
[0044] The selection of biological raw materials is one of the key points of product development. When designing this product, it is planned to use TB 38KD and 16KD antigens as coating antigens, and mouse anti-human IgG as labeling antibodies. During the development, the corresponding antigens and antibodies were purchased from several companies. After testing, the TB antigen and mouse anti-human IgG antibody of Jinhua (Tianjin) Trading Co., Ltd. were selected as the biological raw materials for the development of this product. NC membrane is another key material in product development. After testing the products of several companies, Germany Sartorius CN140 was selected as the solid phase carrier of this kit. Other auxiliary materials such as sample pads, absorbent paper, aluminum foil bags and plastic cards are purchased from relevant qualified companies, and corresponding quality control standards have been established ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com